ASK1 Mediates Nur77 Expression in T-Cell Receptor Mediated Thymocyte Apoptosis
Abstract
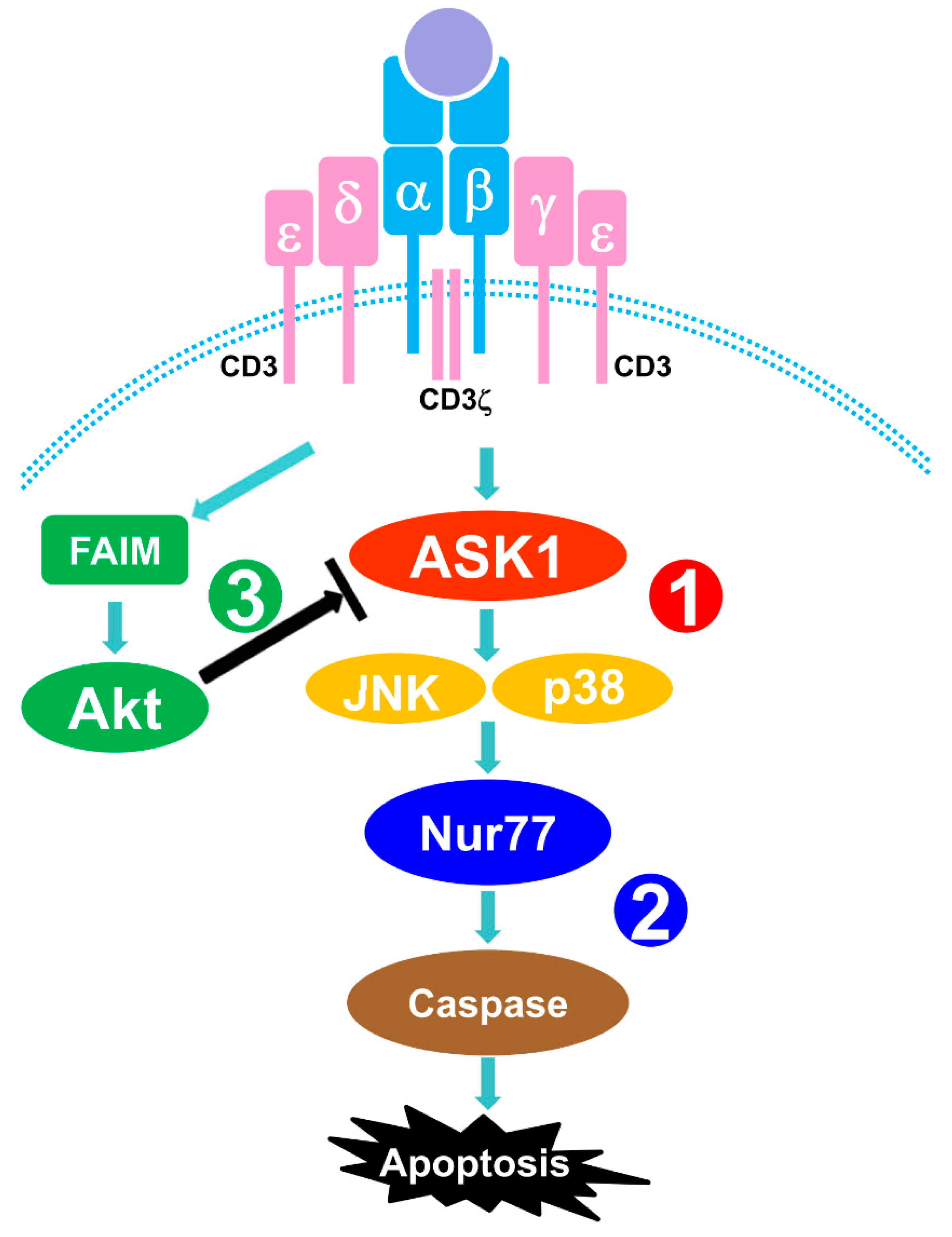
1. Introduction
2. Materials and Methods
2.1. Mouse
2.2. Antibodies and Reagents
2.3. Cell Viability Analysis
2.4. Immunoblotting
2.5. Statistical Analysis
3. Results
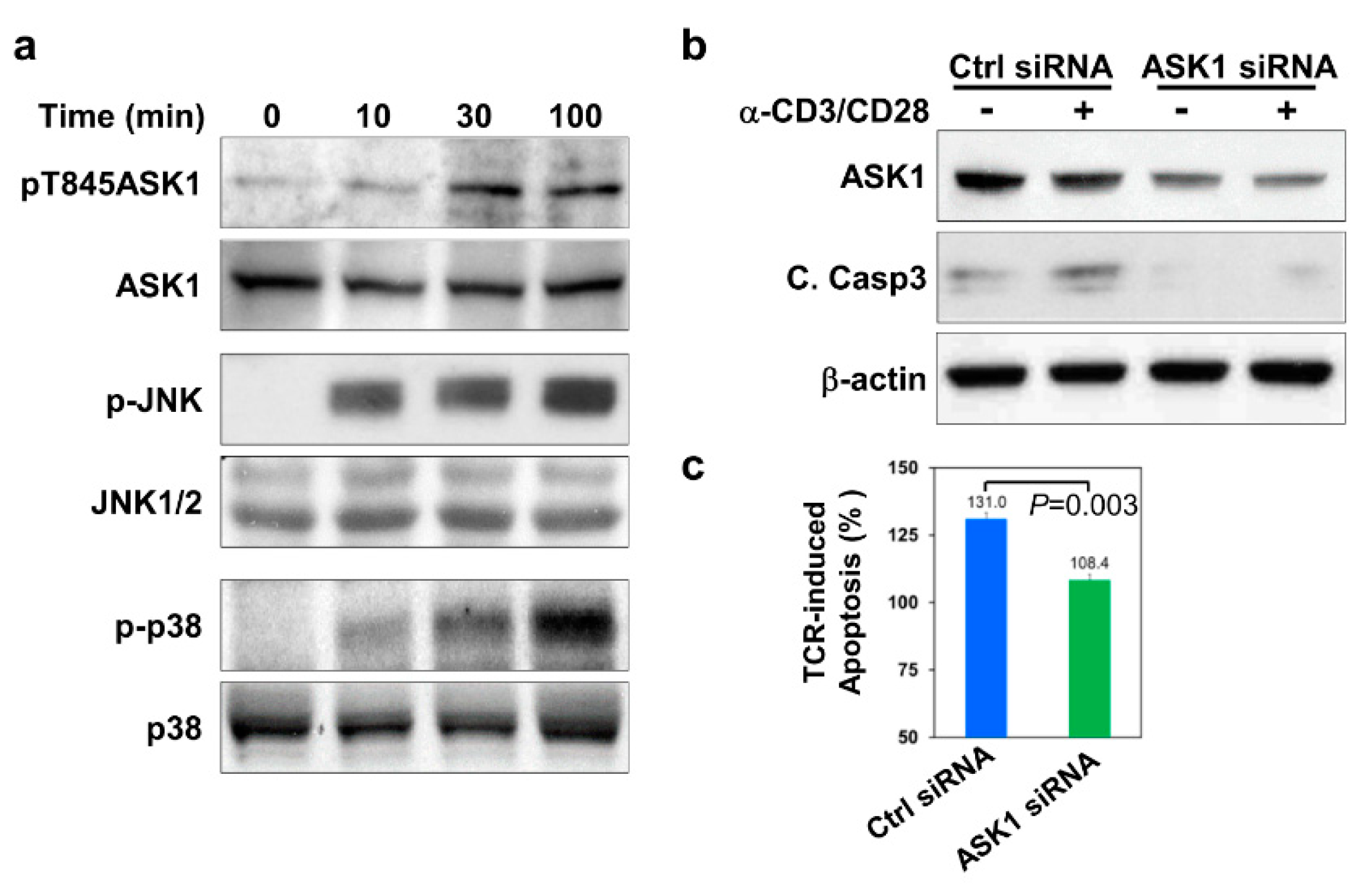
3.1. ASK1 Is Activated and Required for Thymocyte Apoptosis upon TCR Engagement
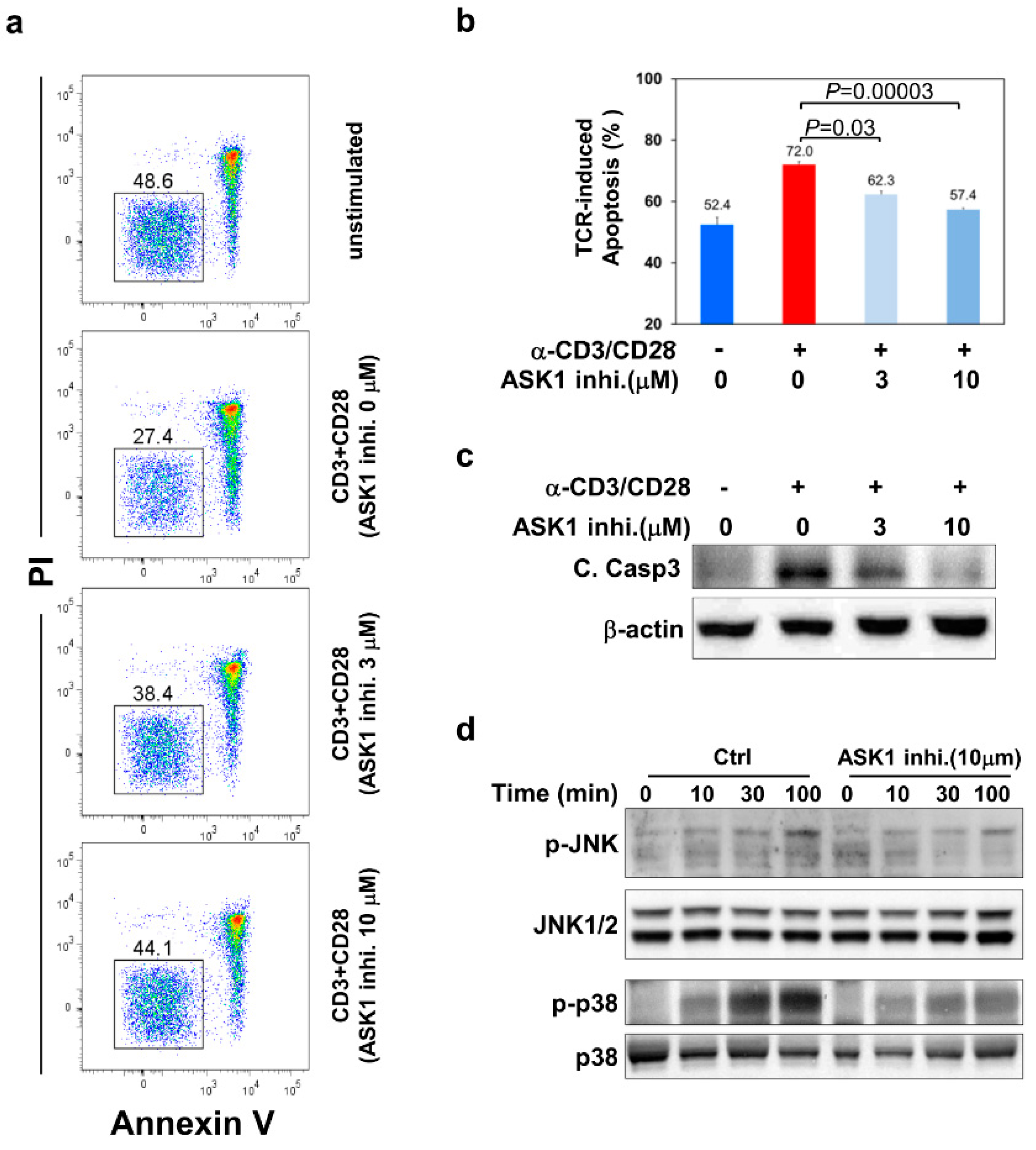
3.2. ASK1- JNK/p38 Pathways Are Important for TCR-Induced Apoptosis of Thymocytes
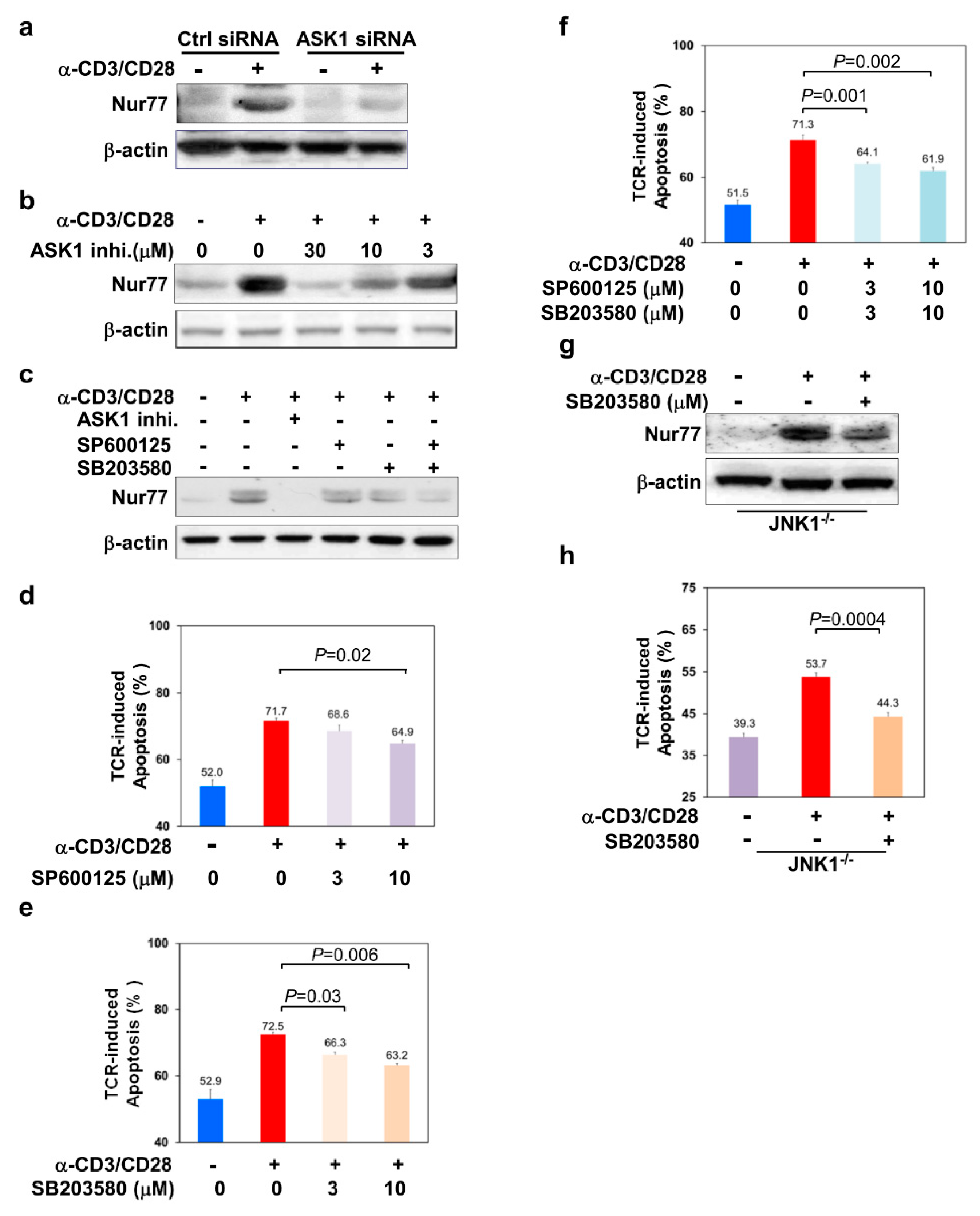
3.3. ASK1-JNK/p38 Signaling Axes Regulate the Level of Nur77 in TCR-Stimulated Thymocytes
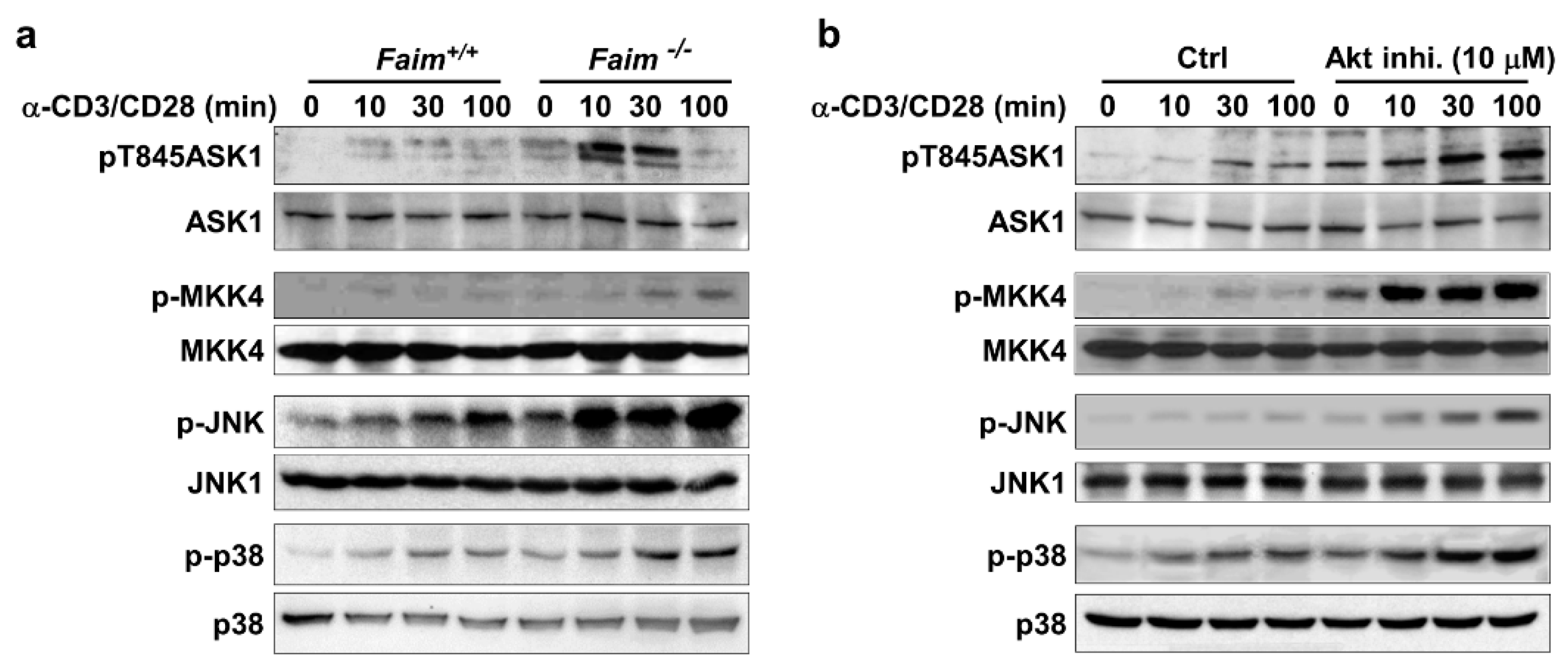
3.4. Akt Negatively Regulates ASK1-MKK4-JNK/p38-Nur77 Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Gascoigne, N.R.J.; Palmer, E. Signaling in thymic selection. Curr. Opin. Immunol. 2011, 23, 207–212. [Google Scholar] [CrossRef]
- Morris, G.P.; Allen, P.M. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat. Immunol. 2012, 13, 121–128. [Google Scholar] [CrossRef]
- Rathmell, J.C.; Thompson, C.B. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 2002, 109, S97–S107. [Google Scholar] [CrossRef]
- Sohn, S.J.; Rajpal, A.; Winoto, A. Apoptosis during lymphoid development. Curr. Opin. Immunol. 2003, 15, 209–216. [Google Scholar] [CrossRef]
- Werlen, G.; Hausmann, B.; Naeher, D.; Palmer, E. Signaling life and death in the thymus: Timing is everything. Science 2003, 15, 209–216. [Google Scholar] [CrossRef]
- Sohn, S.J.; Thompson, J.; Winoto, A. Apoptosis during negative selection of autoreactive thymocytes. Curr. Opin. Immunol. 2007, 19, 510–515. [Google Scholar] [CrossRef]
- Rincón, M.; Whitmarsh, A.; Yang, D.D.; Weiss, L.; Dérijard, B.; Jayaraj, P.; Davis, R.J.; Flavell, R.A. The JNK pathway regulates the in vivo deletion of immature CD4 + CD8 + thymocytes. J. Exp. Med. 1998, 188, 1817–1830. [Google Scholar] [CrossRef]
- Mohit, A.A.; Martin, J.H.; Miller, C.A. p493F12 kinase: A novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron 1995, 14, 67–78. [Google Scholar] [CrossRef]
- Sabapathy, K.; Hu, Y.; Kallunki, T.; Schreiber, M.; David, J.P.; Jochum, W.; Wagner, E.F.; Karin, M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr. Biol. 1999, 9, 116–125. [Google Scholar] [CrossRef]
- Sabapathy, K.; Kallunki, T.; David, J.P.; Graef, I.; Karin, M.; Wagner, E.F. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J. Exp. Med. 2001, 193, 317–328. [Google Scholar] [CrossRef]
- Tanaka, N.; Kamanaka, M.; Enslen, H.; Dong, C.; Wysk, M.; Davis, R.J.; Flavell, R.A. Differential involvement of p38 mitogen-activated protein kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep. 2002, 3, 785–791. [Google Scholar] [CrossRef]
- Ono, K.; Han, J. The p38 signal transduction pathway Activation and function. Cell. Signal. 2000, 12, 1–13. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Shirakabe, K.; Shibuya, H.; Irie, K.; Oishi, I.; Ueno, N.; Taniguchi, T.; Nishida, E.; Matsumoto, K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995, 270, 2008–2011. [Google Scholar] [CrossRef]
- Liu, H.H.; Xie, M.; Schneider, M.D.; Chen, Z.J. Essential role of TAK1 in thymocyte development and activation. Proc. Natl. Acad. Sci. USA 2006, 103, 11677–11682. [Google Scholar] [CrossRef]
- Ichijo, H.; Nishida, E.; Irie, K.; Ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef]
- Chang, H.Y.; Nishitoh, H.; Yang, X.; Ichijo, H.; Baltimore, D. Activation of Apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 1998, 281, 1860–1863. [Google Scholar] [CrossRef]
- Pleinis, J.M.; Davis, C.W.; Cantrell, C.B.; Qiu, D.Y.; Zhan, X. Purification, auto-activation and kinetic characterization of apoptosis signal-regulating kinase I. Protein Expr. Purif. 2017, 14, 34–43. [Google Scholar] [CrossRef]
- Takeda, K.; Noguchi, T.; Naguro, I.; Ichijo, H. Apoptosis Signal-Regulating Kinase 1 in Stress and Immune Response. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 199–225. [Google Scholar] [CrossRef]
- Kim, A.H.; Khursigara, G.; Sun, X.; Franke, T.F.; Chao, M.V. Akt Phosphorylates and Negatively Regulates Apoptosis Signal-Regulating Kinase 1. Mol. Cell. Biol. 2001, 21, 893–901. [Google Scholar] [CrossRef]
- Tobiume, K.; Matsuzawa, A.; Takahashi, T.; Nishitoh, H.; Morita, K.I.; Takeda, K.; Minowa, O.; Miyazono, K.; Noda, T.; Ichijo, H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001, 2, 222–228. [Google Scholar] [CrossRef]
- Woronicz, J.D.; Calnan, B.; Ngo, V.; Winoto, A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature 1994, 367, 277–281. [Google Scholar] [CrossRef]
- Liu, Z.G.; Smith, S.W.; McLaughlin, K.A.; Schwartz, L.M.; Osborne, B.A. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature 1994, 367, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Xu, S.; Lam, K.P. Fas apoptosis inhibitory molecule regulates T cell receptor-mediated apoptosis of thymocytes by modulating akt activation and Nur77 expression. J. Biol. Chem. 2010, 285, 11827–11835. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, W.; Xie, G.; Huang, M.; Hu, M.; Jiang, X.; Zeng, D.; Liu, J.; Zhou, H.; Chen, H.; et al. Induction of Nur77-dependent apoptotic pathway by a coumarin derivative through activation of JNK and p38 MAPK. Carcinogenesis 2014, 35, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Xu, S.; Guo, K.; Zeng, Q.; Lam, K.P. Genetic deletion of faim reveals its role in modulating c-FLIP expression during CD95-mediated apoptosis of lymphocytes and hepatocytes. Cell Death Differ. 2009, 16, 1062–1070. [Google Scholar] [CrossRef]
- Xu, S.; Huo, J.; Chew, W.-K.; Hikida, M.; Kurosaki, T.; Lam, K.-P. Phospholipase Cγ2 Dosage Is Critical for B Cell Development in the Absence of Adaptor Protein BLNK. J. Immunol. 2006, 176, 4690–4698. [Google Scholar] [CrossRef]
- Werlen, G.; Hausmann, B.; Palmer, E. A motif in the αβ T-cell receptor controls positive selection by modulating ERK activity. Nature 2000, 406, 422–426. [Google Scholar] [CrossRef]
- Hatai, T.; Matsuzawa, A.; Inoshita, S.; Mochida, Y.; Kuroda, T.; Sakamaki, K.; Kuida, K.; Yonehara, S.; Ichijo, H.; Takeda, K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J. Biol. Chem. 2000, 275, 26576–26581. [Google Scholar] [CrossRef]
- Lin, B.; Kolluri, S.K.; Lin, F.; Liu, W.; Han, Y.H.; Cao, X.; Dawson, M.I.; Reed, J.C.; Zhang, X.K. Conversion of Bcl-2 from Protector to Killer by Interaction with Nuclear Orphan Receptor Nur77/TR3. Cell 2004, 116, 527–540. [Google Scholar] [CrossRef]
- Van Den Brink, M.R.M.; Kapeller, R.; Pratt, J.C.; Chang, J.H.; Burakoff, S.J. The extracellular signal-regulated kinase pathway is required for activation-induced cell death of T cells. J. Biol. Chem. 1999, 274, 11178–11185. [Google Scholar] [CrossRef]
- Sakaue, M.; Adachi, H.; Dawson, M.; Jetten, A.M. Induction of Egr-1 expression by the retinoid AHPN in human lung carcinoma cells is dependent on activated ERK1/2. Cell Death Differ. 2001, 8, 411–424. [Google Scholar] [CrossRef]
- Sohn, S.J.; Lewis, G.M.; Winoto, A. Non-redundant function of the MEK5-ERK5 pathway in thymocyte apoptosis. EMBO J. 2008, 27, 1896–1906. [Google Scholar] [CrossRef]
- Fujii, Y.; Matsuda, S.; Takayama, G.; Koyasu, S. ERK5 is involved in TCR-induced apoptosis through the modification of Nur77. Genes to Cells 2008, 13, 411–419. [Google Scholar] [CrossRef]
- Juntilla, M.M.; Koretzky, G.A. Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol. Lett. 2008, 116, 104–110. [Google Scholar] [CrossRef]
- Matsukawa, J.; Matsuzawa, A.; Takeda, K.; Ichijo, H. The ASK1-MAP kinase cascades in mammalian stress response. J. Biochem. 2004, 136, 261–265. [Google Scholar] [CrossRef]
- Liu, H.; Nishitoh, H.; Ichijo, H.; Kyriakis, J.M. Activation of Apoptosis Signal-Regulating Kinase 1 (ASK1) by Tumor Necrosis Factor Receptor-Associated Factor 2 Requires Prior Dissociation of the ASK1 Inhibitor Thioredoxin. Mol. Cell. Biol. 2000, 20, 2198–2208. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Beeson, C.; Wülfing, C.; Tate, K.; Allen, P.M.; Davis, M.M.; McConnell, H.M. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity 1996, 5, 125–135. [Google Scholar] [CrossRef]
- Dequiedt, F.; Van Lint, J.; Lecomte, E.; Van Duppen, V.; Seufferlein, T.; Vandenheede, J.R.; Wattiez, R.; Kettmann, R. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J. Exp. Med. 2005, 201, 793–804. [Google Scholar] [CrossRef]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef]
- Kanamoto, T.; Mota, M.; Takeda, K.; Rubin, L.L.; Miyazono, K.; Ichijo, H.; Bazenet, C.E. Role of Apoptosis Signal-Regulating Kinase in Regulation of the c-Jun N-Terminal Kinase Pathway and Apoptosis in Sympathetic Neurons. Mol. Cell. Biol. 2000, 20, 196–204. [Google Scholar] [CrossRef]
- Huo, J.; Xu, S.; Lam, K.-P. FAIM: An Antagonist of Fas-Killing and Beyond. Cells 2019, 8, 541. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, J.; Xu, S.; Lam, K.-P. ASK1 Mediates Nur77 Expression in T-Cell Receptor Mediated Thymocyte Apoptosis. Cells 2020, 9, 585. https://doi.org/10.3390/cells9030585
Huo J, Xu S, Lam K-P. ASK1 Mediates Nur77 Expression in T-Cell Receptor Mediated Thymocyte Apoptosis. Cells. 2020; 9(3):585. https://doi.org/10.3390/cells9030585
Chicago/Turabian StyleHuo, Jianxin, Shengli Xu, and Kong-Peng Lam. 2020. "ASK1 Mediates Nur77 Expression in T-Cell Receptor Mediated Thymocyte Apoptosis" Cells 9, no. 3: 585. https://doi.org/10.3390/cells9030585
APA StyleHuo, J., Xu, S., & Lam, K.-P. (2020). ASK1 Mediates Nur77 Expression in T-Cell Receptor Mediated Thymocyte Apoptosis. Cells, 9(3), 585. https://doi.org/10.3390/cells9030585




