Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications
Abstract
1. Introduction
2. Type of Stem Cells for Cancer Treatment
2.1. Pluripotent Stem Cells (PSCs)
2.2. Adult Stem Cells (ASCs)
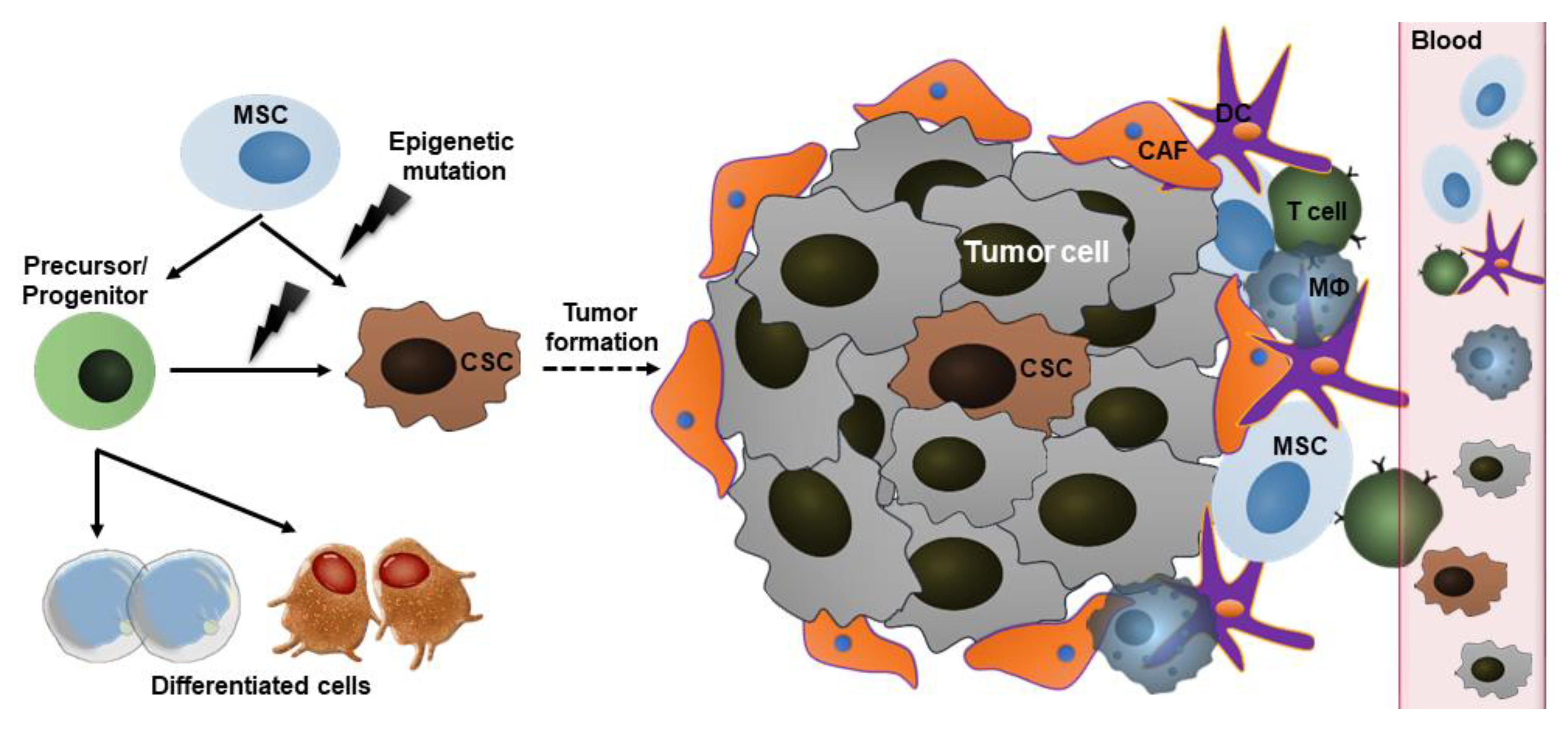
2.3. Cancer Stem Cells (CSCs)
3. Mechanisms Underlying the Action of Stem Cells in Cancer
3.1. Homing to Bone Marrow
3.2. Tumor-Tropic Effect
3.3. Paracrine Factor Secretion and Differentiation Capacity
3.4. Signaling in CSCs
4. The Potential Applications of Stem Cell Therapy in Cancer
4.1. HSC Transplantation
4.2. MSC Transplantation After Cancer Treatment
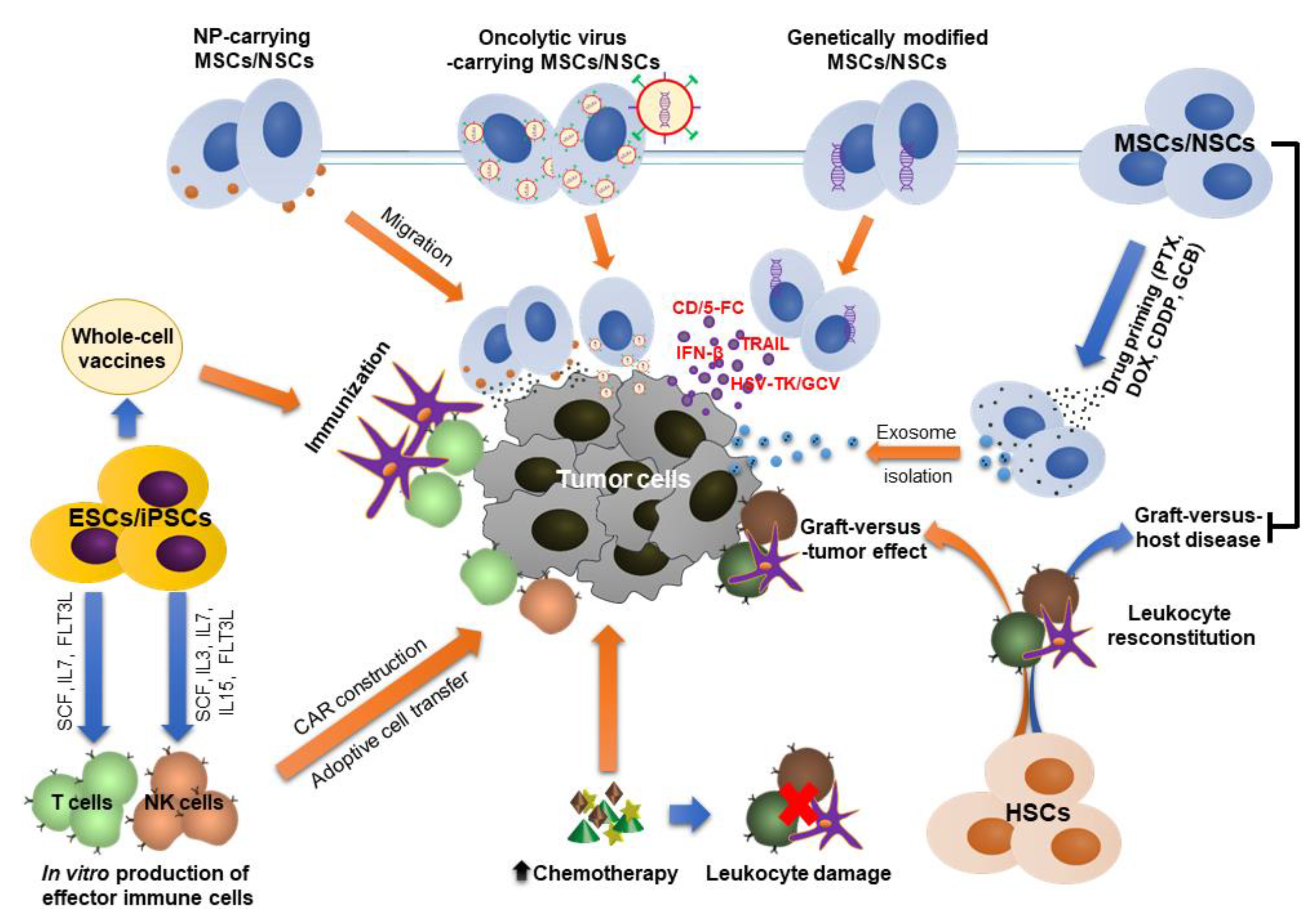
4.3. Stem Cells as Potential Therapeutic Carriers
4.3.1. Genetically Modified Stem Cells
4.3.2. Nanoparticles (NPs)-Carrying Stem Cells
4.3.3. Stem Cells as Carriers for Oncolytic Viruses (OVs)
4.3.4. Stem Cell-Derived Exosomes as Therapeutic Carriers
4.4. Stem Cell Source for Production of Immune Cells
4.5. Stem Cell-Based Anti-Cancer Vaccines
5. Side Effects and Potential Risks of Stem Cell Therapy
5.1. Tumorigenesis
5.2. Adverse Events in Allogeneic HSC Transplantation
5.3. Drug Toxicity and Drug Resistance
5.4. Increased Immune Responses and Autoimmunity
5.5. Viral Infection
6. Conclusions and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CXCR | CXC-chemokine receptor |
| SDF-1 | stromal cell-derived factor 1 |
| ATP | adenosine triphosphate |
| UTP | uridine triphosphate |
| LFA-1 | lymphocyte function-associated antigen 1 |
| VLA-4/5 | very late antigen-4/5 |
| CD44 | cluster of differentiation 44 |
| MMP-2/9 | matrix metalloproteinase-2/9 |
| ECM | extracellular matrix |
| CCL-25 | C-C motif 25 |
| IL | interleukin |
| TNF-α | tumor necrosis factor α |
| TGF | transforming growth factor |
| IDO | indoleamine 2,3-dioxygenase |
| iNOS | induced nitric oxide synthase |
| HLA-G | histocompatibility antigen type G |
| TSG-6 | TNF-α stimulated gene-6 |
| PGE-2 | prostaglandin E2 |
| STC-1 | stanniocalcin-1 |
| VEGF | vascular endothelial growth factor |
| HO-1 | heme oxygenase-1 |
| IGF-1 | insulin-like growth factor-1 |
| PI3K | phosphoinositide 3-kinase |
| PTEN | phosphatase and tensin homolog |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| Lgf5 | leucine-rich repeat-containing G-protein coupled receptor 5 |
| EpCAM | epithelial cell adhesion molecule |
| IFN | interferon |
| PLA | poly lactic acid |
| PLGA | poly (lactic-co-glycolic) acid |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Vasievich, E.A.; Huang, L. The Suppressive Tumor Microenvironment: A Challenge in Cancer Immunotherapy. Mol. Pharm. 2011, 8, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Quandt, Z.; Bluestone, J.A. The Balancing Act between Cancer Immunity and Autoimmunity in Response to Immunotherapy. Cancer Immunol. Res. 2018, 6, 1445–1452. [Google Scholar] [CrossRef]
- Gomes, J.P.; Assoni, A.F.; Pelatti, M.; Coatti, G.; Okamoto, O.K.; Zatz, M. Deepening a Simple Question: Can MSCs Be Used to Treat Cancer? Anticancer Res. 2017, 37, 4747–4758. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Knorr, D.A.; Ni, Z.; Hermanson, D.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.; Lee, D.-J.; Kaufman, D.S. Clinical-Scale Derivation of Natural Killer Cells From Human Pluripotent Stem Cells for Cancer Therapy. Stem Cells Transl. Med. 2013, 2, 274–283. [Google Scholar] [CrossRef]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef]
- Ruella, M.; Kenderian, S.S. Next-Generation Chimeric Antigen Receptor T-Cell Therapy: Going off the Shelf. BioDrugs 2017, 31, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, F.; Velghe, I.; Vanwalleghem, L.; De Smedt, M.; Van Coppernolle, S.; Taghon, T.; Moore, H.D.; Leclercq, G.; Langerak, A.W.; Kerre, T.; et al. Generation of T Cells from Human Embryonic Stem Cell-Derived Hematopoietic Zones. J. Immunol. 2009, 182, 6879–6888. [Google Scholar] [CrossRef] [PubMed]
- Kooreman, N.G.; Kim, Y.; De Almeida, P.; Termglinchan, V.; Diecke, S.; Shao, N.; Wei, T.; Yi, H.; Dey, D.; Nelakanti, R.; et al. Autologous iPSC-Based Vaccines Elicit Anti-tumor Responses In Vivo. Cell Stem Cell 2018, 22, 501–513.e7. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Telli, M.L.; Wu, J.C. Induced Pluripotent Stem Cell-Based Cancer Vaccines. Front. Immunol. 2019, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Copelan, E.A. Hematopoietic Stem-Cell Transplantation. N. Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Goulielmaki, M.; Devetzi, M.; Panayiotidis, M.I.; Koliakos, G.; Zoumpourlis, V. Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Res. Ther. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, L.; Li, Y.; Fang, B.; Li, G.; He, W.; Xu, L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. BioMed Res. Int. 2019, 2019, 2820853-12. [Google Scholar] [CrossRef] [PubMed]
- Kanojia, D.; Balyasnikova, I.V.; Morshed, R.A.; Frank, R.T.; Yu, D.; Zhang, L.; Spencer, E.A.; Kim, J.W.; Han, Y.; Yu, D.; et al. Neural Stem Cells Secreting Anti-HER2 Antibody Improve Survival in a Preclinical Model of HER2 Overexpressing Breast Cancer Brain Metastases. Stem Cells 2015, 33, 2985–2994. [Google Scholar] [CrossRef][Green Version]
- Lee, H.J.; Doo, S.W.; Kim, D.H.; Cha, Y.J.; Kim, J.H.; Song, Y.S.; Kim, S.U. Cytosine deaminase-expressing human neural stem cells inhibit tumor growth in prostate cancer-bearing mice. Cancer Lett. 2013, 335, 58–65. [Google Scholar] [CrossRef]
- Yi, B.-R.; Kim, S.U.; Choi, K.-C. Co-treatment with therapeutic neural stem cells expressing carboxyl esterase and CPT-11 inhibit growth of primary and metastatic lung cancers in mice. Oncotarget 2014, 5, 12835–12848. [Google Scholar] [CrossRef]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95 (Suppl. 1). [Google Scholar] [CrossRef]
- Seitz, G.; Boehmler, A.M.; Kanz, L.; Möhle, R. The Role of Sphingosine 1-Phosphate Receptors in the Trafficking of Hematopoietic Progenitor Cells. Ann. N. Y. Acad. Sci. 2005, 1044, 84–89. [Google Scholar] [CrossRef]
- Massberg, S.; Von Andrian, U.H. Novel trafficking routes for hematopoietic stem and progenitor cells. Ann. N. Y. Acad. Sci. 2009, 1176, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Juarez, J.G.; Harun, N.; Thien, M.; Welschinger, R.; Baraz, R.; Pena, A.D.; Pitson, S.M.; Rettig, M.; DiPersio, J.F.; Bradstock, K.F.; et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood 2012, 119, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Manfredini, R.; Bertolini, F.; Ferrari, D.; Fogli, M.; Zini, R.; Salati, S.; Salvestrini, V.; Gulinelli, S.; Adinolfi, E.; et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood 2006, 109, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.B.; Chabner, K.T.; Alley, I.R.; Olson, D.P.; Szczepiorkowski, Z.M.; Poznansky, M.C.; Kos, C.H.; Pollak, M.R.; Brown, E.M.; Scadden, D.T. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 2005, 439, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Okajima, F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell. Signal. 2013, 25, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Dar, A.; Kollet, O. How do stem cells find their way home? Blood 2005, 106, 1901–1910. [Google Scholar] [CrossRef]
- Wang, H.; Cao, F.; De, A.; Cao, Y.; Contag, C.H.; Gambhir, S.S.; Wu, J.C.; Chen, X. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells 2009, 27, 1548–1558. [Google Scholar] [CrossRef]
- Pattabiraman, D.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef]
- Flier, J.S.; Underhill, L.H.; Dvorak, H.F. Tumors: Wounds That Do Not Heal. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Jiang, Y.; Wells, A.; Sylakowski, K.; Clark, A.; Ma, B. Adult Stem Cell Functioning in the Tumor Micro-Environment. Int. J. Mol. Sci. 2019, 20, 2566. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Zhao, R.C. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 2014, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013, 4, 1795. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, Y.; Hsu, J.-M.; Mishra, P.J.; Glod, J.; Banerjee, D. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp. Cell Res. 2010, 316, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-T.; Bian, Z.-Y.; Fan, Q.-M.; Li, G.; Tang, T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009, 281, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Menu, E.; De Becker, A.; Van Camp, B.; Vanderkerken, K.; Van Riet, I. Bone Marrow-Derived Mesenchymal Stromal Cells are Attracted by Multiple Myeloma Cell-Produced Chemokine CCL25 and Favor Myeloma Cell Growth in Vitro and In Vivo. Stem Cells 2012, 30, 266–279. [Google Scholar] [CrossRef]
- Sullivan, C.B.; Porter, R.M.; Evans, C.H.; Ritter, T.; Shaw, G.; Barry, F.P.; Murphy, M. TNFα and IL-1β influence the differentiation and migration of murine MSCs independently of the NF-κB pathway. Stem Cell Res. Ther. 2014, 5, 104. [Google Scholar] [CrossRef]
- Uchibori, R.; Tsukahara, T.; Mizuguchi, H.; Saga, Y.; Urabe, M.; Mizukami, H.; Kume, A.; Ozawa, K. NF-κB Activity Regulates Mesenchymal Stem Cell Accumulation at Tumor Sites. Cancer Res. 2012, 73, 364–372. [Google Scholar] [CrossRef]
- Vakhshiteh, F.; Atyabi, F.; Ostad, S.N. Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. Int. J. Nanomed. 2019, 14, 2847–2859. [Google Scholar] [CrossRef]
- Lee, R.H.; Oh, J.Y.; Choi, H.; Bazhanov, N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J. Cell. Biochem. 2011, 112, 3073–3078. [Google Scholar] [CrossRef]
- Reddy, R.L. Mobilization and collection of peripheral blood progenitor cells for transplantation. Transfus. Apher. Sci. 2005, 32, 63–72. [Google Scholar] [CrossRef]
- Matsui, W.H. Cancer stem cell signaling pathways. Medicine 2016, 95, S8–S19. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; Kreso, A.; Jamieson, C.H.M. Cancer Stem Cells and Self-renewal. Clin. Cancer Res. 2010, 16, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Cojoc, M.; Mäbert, K.; Muders, M.H.; Dubrovska, A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin. Cancer Boil. 2015, 31, 16–27. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Codd, A.S.; Kanaseki, T.; Torigo, T.; Tabi, Z. Cancer stem cells as targets for immunotherapy. Immunology 2017, 153, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Guzmán, M.E.; Bigoni-Ordóñez, G.D.; Ibáñez-Hernández, M.; Ortiz-Sánchez, E. Cancer stem cell impact on clinical oncology. World J. Stem Cells 2018, 10, 183–195. [Google Scholar]
- Al-Hajj, M. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Croker, A.; Goodale, D.; Chu, J.; Postenka, C.; Hedley, B.D.; Hess, D.A.; Allan, A. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 2008, 13, 2236–2252. [Google Scholar] [CrossRef] [PubMed]
- Casper, J.; Wolff, D.; Knauf, W.; Blau, I.W.; Ruutu, T.; Volin, L.; Wandt, H.; Schäfer-Eckart, K.; Holowiecki, J.; Giebel, S.; et al. Allogeneic Hematopoietic Stem-Cell Transplantation in Patients With Hematologic Malignancies After Dose-Escalated Treosulfan/Fludarabine Conditioning. J. Clin. Oncol. 2010, 28, 3344–3351. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Ringdén, O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med. 2007, 262, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.; Riminucci, M.; et al. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids Can Organize a Hematopoietic Microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.; Lira, S.A.; Scadden, D.T.; Ma’Ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; De La Mata, C.; Ruiz-García, A.; López-Fernández, E.; Espinosa, O.; Remigia, M.J.; Moratalla, L.; Goterris, R.; García-Martín, P.; Ruiz-Cabello, F.; et al. Adipose tissue-derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: A phase I/II study. Cytotherapy 2017, 19, 927–936. [Google Scholar] [CrossRef]
- Muroi, K.; Miyamura, K.; Okada, M.; Yamashita, T.; Murata, M.; Ishikawa, T.; Uike, N.; Hidaka, M.; Kobayashi, R.; Imamura, M.; et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: A phase II/III study. Int. J. Hematol. 2015, 103, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Battiwalla, M.; Ito, S.; Feng, X.; Chinian, F.; Melenhorst, J.J.; Koklanaris, E.; Sabatino, M.; Stroncek, D.; Samsel, L.; et al. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: Correlation of biological markers with clinical responses. Stem Cells 2014, 32, 1278–1288. [Google Scholar] [CrossRef]
- Baron, F.; Lechanteur, C.; Willems, E.; Bruck, F.; Baudoux, E.; Seidel, L.; Vanbellinghen, J.-F.; Hafraoui, K.; Lejeune, M.; Gothot, A.; et al. Cotransplantation of Mesenchymal Stem Cells Might Prevent Death from Graft-versus-Host Disease (GVHD) without Abrogating Graft-versus-Tumor Effects after HLA-Mismatched Allogeneic Transplantation following Nonmyeloablative Conditioning. Boil. Blood Marrow Transplant. 2010, 16, 838–847. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, M.; Bian, C.; Sun, Z.; Yang, Z.; Zeng, Y.; Ai, H.-S.; Zhao, R.C. Efficacy of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Sclerodermatous Chronic Graft-versus-Host Disease: Clinical Report. Boil. Blood Marrow Transplant. 2010, 16, 403–412. [Google Scholar] [CrossRef]
- Sage, E.; Thakrar, R.M.; Janes, S.M. Genetically modified mesenchymal stromal cells in cancer therapy. Cytotherapy 2016, 18, 1435–1445. [Google Scholar] [CrossRef]
- Malekshah, O.M.; Chen, X.; Nomani, A.; Sarkar, S.; Hatefi, A. Enzyme/Prodrug Systems for Cancer Gene Therapy. Curr. Pharmacol. Rep. 2016, 2, 299–308. [Google Scholar] [CrossRef]
- Kucerova, L.; Matuskova, M.; Pastorakova, A.; Tyciakova, S.; Jakubikova, J.; Bohovic, R.; Altanerova, V.; Altaner, C. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J. Gene Med. 2008, 10, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.-Y.; Yoo, S.-W.; Hong, Y.; Kim, S.; Kim, S.J.; Yoon, S.-H.; Cho, K.-G.; Paek, S.H.; Lee, Y.-D.; Kim, S.-S.; et al. The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int. J. Cancer 2010, 127, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Gutova, M.; Goldstein, L.; Metz, M.; Hovsepyan, A.; Tsurkan, L.G.; Tirughana, R.; Tsaturyan, L.; Annala, A.; Synold, T.W.; Wan, Z.; et al. Optimization of a Neural Stem-Cell-Mediated Carboxylesterase/Irinotecan Gene Therapy for Metastatic Neuroblastoma. Mol. Ther. Oncolytics 2016, 4, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Yoon, K.; Choi, S.-A.; Yoon, S.-B.; Kim, S.U.; Lee, H.J. Tumor-specific gene therapy for pancreatic cancer using human neural stem cells encoding carboxylesterase. Oncotarget 2016, 7, 75319–75327. [Google Scholar] [CrossRef][Green Version]
- Von Einem, J.C. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the Phase 1/2 TREAT-ME-1 Trial. Int. J. Cancer 2019. [Google Scholar] [CrossRef]
- Jobst, C. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells-TREAT-ME-1-a phase I, first in human, first in class trial. Oncotarget 2017, 8, 80156. [Google Scholar]
- Kauer, T.M.; Figueiredo, J.-L.; Hingtgen, S.; Shah, K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat. Neurosci. 2011, 15, 197–204. [Google Scholar] [CrossRef]
- Yi, B.-R.; Hwang, K.-A.; Aboody, K.S.; Jeung, E.-B.; Kim, S.U.; Choi, K.-C. Selective antitumor effect of neural stem cells expressing cytosine deaminase and interferon-beta against ductal breast cancer cells in cellular and xenograft models. Stem Cell Res. 2014, 12, 36–48. [Google Scholar] [CrossRef]
- Yi, B.-R.; Kim, S.U.; Choi, K.-C. Synergistic effect of therapeutic stem cells expressing cytosine deaminase and interferon-beta via apoptotic pathway in the metastatic mouse model of breast cancer. Oncotarget 2015, 7, 5985–5999. [Google Scholar] [CrossRef][Green Version]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.; Dreaden, E.; Brown, D.; Alkilany, A.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Roger, M.; Clavreul, A.; Venier-Julienne, M.-C.; Passirani, C.; Sindji, L.; Schiller, P.; Montero-Menei, C.; Menei, P. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials 2010, 31, 8393–8401. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-Engineered Mesenchymal Stem Cells Increase Therapeutic Efficacy of Anticancer Drug Through True Active Tumor Targeting. Mol. Cancer Ther. 2018, 17, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs Improve Myocardial Infarction in Mice because Cells Embolized in Lung Are Activated to Secrete the Anti-inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Zeng, X.; Guo, W.; Jin, Y.; Wang, S.; Tian, R.; Han, Y.; Guo, L.; Han, J.; et al. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm. Sin. B 2018, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Moku, G.; Layek, B.; Trautman, L.; Putnam, S.; Panyam, J.; Prabha, S. Improving Payload Capacity and Anti-Tumor Efficacy of Mesenchymal Stem Cells Using TAT Peptide Functionalized Polymeric Nanoparticles. Cancers 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Kastrup, C.J.; Ramanathan, R.; Siegwart, D.J.; Ma, M.; Bogatyrev, S.R.; Xu, Q.; Whitehead, K.A.; Langer, R.; Anderson, D.G. Nanoparticulate Cellular Patches for Cell-Mediated Tumoritropic Delivery. ACS Nano 2010, 4, 625–631. [Google Scholar] [CrossRef]
- Sarkar, D.; Vemula, P.K.; Teo, G.S.L.; Spelke, D.; Karnik, R.; Wee, L.Y.; Karp, J.M. Chemical Engineering of Mesenchymal Stem Cells to Induce a Cell Rolling Response. Bioconjugate Chem. 2008, 19, 2105–2109. [Google Scholar] [CrossRef]
- Stephan, M.T.; Moon, J.J.; Um, S.H.; Bershteyn, A.; Irvine, D.J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010, 16, 1035–1041. [Google Scholar] [CrossRef]
- Stephan, M.T.; Irvine, D.J. Enhancing cell therapies from the outside in: Cell surface engineering using synthetic nanomaterials. Nano Today 2011, 6, 309–325. [Google Scholar] [CrossRef]
- Layek, B.; Sehgal, D.; Argenta, P.A.; Panyam, J.; Prabha, S. Nanoengineering of Mesenchymal Stem Cells via Surface Modification for Efficient Cancer Therapy. Adv. Ther. 2019, 2, 1900043. [Google Scholar] [CrossRef]
- Suryaprakash, S.; Lao, Y.-H.; Cho, H.-Y.; Li, M.; Ji, H.Y.; Shao, D.; Hu, H.; Quek, C.H.; Huang, D.; Mintz, R.L.; et al. Engineered Mesenchymal Stem Cell/Nanomedicine Spheroid as an Active Drug Delivery Platform for Combinational Glioblastoma Therapy. Nano Lett. 2019, 19, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword against Cancer. Front. Immunol. 2018, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A.L.; Thaci, B.; Auffinger, B.; Rincón, E.; Balyasnikova, I.V.; Kim, C.K.; Han, Y.; Zhang, L.; Aboody, K.S.; Ahmed, A.U.; et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl. Med. 2013, 2, 655–666. [Google Scholar] [CrossRef]
- Ong, H.-T.; Federspiel, M.J.; Guo, C.M.; Ooi, L.L.; Russell, S.J.; Peng, K.-W.; Hui, K.M. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J. Hepatol. 2013, 59, 999–1006. [Google Scholar] [CrossRef]
- Duebgen, M.; Martinez-Quintanilla, J.; Tamura, K.; Hingtgen, S.; Redjal, N.; Shah, K.; Wakimoto, H. Stem Cells Loaded With Multimechanistic Oncolytic Herpes Simplex Virus Variants for Brain Tumor Therapy. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Mader, E.K.; Butler, G.W.; Dowdy, S.C.; Mariani, A.; Knutson, K.L.; Federspiel, M.J.; Russell, S.J.; Galanis, E.; Dietz, A.B.; Peng, K.-W. Optimizing patient derived mesenchymal stem cells as virus carriers for a Phase I clinical trial in ovarian cancer. J. Transl. Med. 2013, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Miska, J.; Lesniak, M.S. Neural Stem Cell Carriers for the Treatment of Glioblastoma Multiforme. EBioMedicine 2015, 2, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Serio, A.; Mazo, M.M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.; Schiffelers, R.M.; Zarovni, N.; Vago, R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol. Res. 2016, 111, 487–500. [Google Scholar] [CrossRef]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface Functionalization of Exosomes Using Click Chemistry. Bioconjugate Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Zhang, L.; Ban, L.; Chen, P.; Du, W.; Feng, X.; Liu, B.-F. Chemically Edited Exosomes with Dual Ligand Purified by Microfluidic Device for Active Targeted Drug Delivery to Tumor Cells. ACS Appl. Mater. Interfaces 2017, 9, 27441–27452. [Google Scholar] [CrossRef]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Greco, K.A.; Franzen, C.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G. PLK-1 Silencing in Bladder Cancer by siRNA Delivered With Exosomes. Urology 2016, 91, 241.e1–241.e7. [Google Scholar] [CrossRef]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Bonomi, A.; Steimberg, N.; Benetti, A.; Berenzi, A.; Alessandri, G.; Pascucci, L.; Boniotti, J.; Coccè, V.; Sordi, V.; Pessina, A.; et al. Paclitaxel-releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. Hematol. Oncol. 2016, 35, 693–702. [Google Scholar] [CrossRef]
- Pessina, A. Mesenchymal stromal cells primed with P aclitaxel attract and kill leukaemia cells, inhibit angiogenesis and improve survival of leukaemia-bearing mice. Br. J. Haematol. 2013, 160, 766–778. [Google Scholar] [CrossRef]
- Coccè, V.; Farronato, D.; Brini, A.T.; Masia, C.; Gianni’, A.; Piovani, G.; Sisto, F.; Alessandri, G.; Angiero, F.; Pessina, A. Drug Loaded Gingival Mesenchymal Stromal Cells (GinPa-MSCs) Inhibit In Vitro Proliferation of Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 9376. [Google Scholar] [CrossRef]
- Miliotou, A.; Papadopoulou, L.C.; Androulla, M.N.; Lefkothea, P.C. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr. Pharm. Biotechnol. 2018, 19, 5–18. [Google Scholar] [CrossRef]
- Dolnikov, A.; Sylvie, S.; Xu, N.; O’Brien, T. Stem Cell Approach to Generate Chimeric Antigen Receptor Modified Immune Effector Cells to Treat Cancer. Blood 2014, 124, 2437. [Google Scholar] [CrossRef]
- Iriguchi, S.; Kaneko, S. Toward the development of true “off-the-shelf” synthetic T-cell immunotherapy. Cancer Sci. 2019, 110, 16–22. [Google Scholar] [CrossRef] [PubMed]
- La Motte-Mohs, R.N.; Herer, E.; Zúñiga-Pflücker, J.C. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood 2005, 105, 1431–1439. [Google Scholar] [CrossRef]
- Katsukawa, M.; Nakajima, Y.; Fukumoto, A.; Doi, D.; Takahashi, J.; Katsukawa, M.M. Fail-Safe Therapy by Gamma-Ray Irradiation Against Tumor Formation by Human-Induced Pluripotent Stem Cell-Derived Neural Progenitors. Stem Cells Dev. 2016, 25, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Minami, K.; Ito, E.; Imaizumi, H.; Mori, S.; Koizumi, M.; Fukushima, S.; Miyagawa, S.; Sawa, Y.; Matsuura, N. Irradiation strongly reduces tumorigenesis of human induced pluripotent stem cells. J. Radiat. Res. 2017, 58, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Chen, X.-H.; Chang, X.-H.; Ye, X.; Yi, L.; Cui, H. Human embryonic stem cells—A potential vaccine for ovarian cancer. Asian Pac. J. Cancer Prev. 2012, 13, 4295–4300. [Google Scholar] [CrossRef]
- Dong, W.; Du, J.; Shen, H.; Gao, D.; Li, Z.; Wang, G.; Mu, X.; Liu, Q. Administration of embryonic stem cells generates effective antitumor immunity in mice with minor and heavy tumor load. Cancer Immunol. Immunother. 2010, 59, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Malta, T.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Kaminska, B.; Huelsken, J.; Gevaert, O.; Colaprico, A.; Czerwinska, P.; et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018, 173, 338–354.e15. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
- Røsland, G.V.; Svendsen, A.; Torsvik, A.; Sobala, E.; Mc Cormack, E.; Immervoll, H.; Mysliwietz, J.; Tonn, J.-C.; Goldbrunner, R.; Lonning, P.E.; et al. Long-term Cultures of Bone Marrow–Derived Human Mesenchymal Stem Cells Frequently Undergo Spontaneous Malignant Transformation. Cancer Res. 2009, 69, 5331–5339. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.J.; Counts, G.W.; Appelbaum, F.R.; Lee, S.J.; Sanders, J.E.; Deeg, H.J.; Flowers, M.E.; Syrjala, K.L.; Hansen, J.A.; Storb, R.F.; et al. Life Expectancy in Patients Surviving More Than 5 Years After Hematopoietic Cell Transplantation. J. Clin. Oncol. 2010, 28, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Mohty, B.; Mohty, M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: An update. Blood Cancer J. 2011, 1, e16. [Google Scholar] [CrossRef]
- Narimatsu, H.; Miyakoshi, S.; Yamaguchi, T.; Kami, M.; Matsumura, T.; Yuji, K.; Murashige, N.; Kusumi, E.; Kodama, Y.; Komatsu, T.; et al. Chronic graft-versus-host disease following umbilical cord blood transplantation: Retrospective survey involving 1072 patients in Japan. Blood 2008, 112, 2579–2582. [Google Scholar] [CrossRef] [PubMed]
- Albarenque, S.M.; Zwacka, R.; Mohr, A. Both human and mouse mesenchymal stem cells promote breast cancer metastasis. Stem Cell Res. 2011, 7, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Studeny, M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002, 62, 3603–3608. [Google Scholar]
- Kidd, S.; Spaeth, E.L.; Dembinski, J.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C.; et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef]
- Osieka, R. Studies on drug resistance in a human melanoma xenograft system. Cancer Treat. Rev. 1984, 11, 85–98. [Google Scholar] [CrossRef]
- Chaudhary, P.M.; Roninson, I.B. Induction of Multidrug Resistance in Human Cells by Transient Exposure to Different Chemotherapeutic Drugs. J. Natl. Cancer Inst. 1993, 85, 632–639. [Google Scholar] [CrossRef]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A. Anti-Donor Immune Responses Elicited by Allogeneic Mesenchymal Stem Cells and Their Extracellular Vesicles: Are We Still Learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef]
- Eliopoulos, N.; Stagg, J.; Lejeune, L.; Pommey, S.; Galipeau, J. Allogeneic marrow stromal cells are immune rejected by MHC class I– and class II–mismatched recipient mice. Blood 2005, 106, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Ryan, A.; Alagesan, S.; Lohan, P.; Treacy, O.; Ritter, T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol. Cell Boil. 2012, 91, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.A.; Willemze, R.; Fibbe, W.E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006, 108, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Schu, S. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, J.; Ruan, H.; Zhang, X.; Ye, Y.; Shen, S.; Wang, C.; Lu, W.; Cheng, K.; et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat. Biomed. Eng. 2018, 2, 831–840. [Google Scholar] [CrossRef]
| Disease & Interventions | Phase | Clinical Outcome | NCT/Ref. |
|---|---|---|---|
| Recent Published/Completed Trials | |||
| - Refractory chronic GVHD - Adipose tissue-MSCs (1–3 × 106/kg) & cyclosporine, prednisone | I/II | 71.4% patients alive, 80% patients achieved complete remission (CR) 100% patients were free of steroids at week 56 No side events related to MSC treatment | [54] |
| - Steroid-refractory grade III or IV acute GVHD - 72–100 × 106 MSCs | II/III | At 24 weeks (primary endpoint), 12/25 (48%) patients achieved CR At 52 weeks, 48% patients receiving MSCs were alive No side events related to MSC treatment | [55] |
| - Refractory acute GVHD - Bone marrow-MSCs (2 × 106 cells/kg), 3 doses for a week | I | 5/7 patients achieved CR with remarkable decrease in inflammatory cytokines Good correlation between clinical responses with decrease in the level of biomarkers (Elafin, CK18, and Reg 3α) | [56] |
| - Refractory chronic GVHD - MSCs & mycophenolate mofetil (15 mg/kg × 3 doses/day × 42 days) & tacrolimus (0.06 mg/kg × 2 doses/day × 180 days) | II | At 100 days (primary endpoint), < 35% nonrelapse mortality (NRM) 19/20 patients achieved sustained engraftment of HSCs At 1 year, 10% NRM, 30% relapse, 80% overall survival, 60% non-relapse survival | NCT00504803/ [57] |
| - Sclerodermatous GVHD - Bone marrow-MSCs (10–20 × 106 cells, infusion into anterosuperior iliac spine) | I | Reduce symptoms in all 4 patients. Dramatic increase in Th1/Th2 cell ratio No side events related to MSC treatment | [58] |
| Ongoing Trial | |||
| Mesenchymoangioblast derived MSCs | I | Ongoing | NCT02923375 |
| Stem Cell Therapy | Drug(s) | Tumor Type | Phase | Trial Number |
|---|---|---|---|---|
| CD-expressing NSCs | 5-FC | Recurrent high-grade gliomas | Phase I, completed | NCT01172964 |
| HSV-TK-expressing MSCs | Ganciclovir | Advanced, recurrent or metastatic gastrointestinal adenocarcinoma | Phase I/II, completed | EudraCT 2012-003741-15 |
| CD-expressing NSCs | 5-FC and leucovorin | Recurrent high-grade gliomas | Phase I, ongoing | NCT02015819 |
| INF-β-expressing MSCs | - | Ovarian cancer | Phase I, completed | NCT02530047 |
| TRAIL-expressing MSCs | - | Adenocarcinoma of lung | Phase I/II, recruiting | NCT03298763 |
| ICOVIR5-infected MSCs | - | Metastatic and refractory solid tumors | Phase I/II, completed | NCT01844661 |
| OMV-infected MSCs | - | Recurrent ovarian cancer | Phase I/II, recruiting | NCT02068794 |
| Ad5-DNX-2401-infected MSCs | - | Recurrent high-grade glioma | Phase I, recruiting | NCT03896568 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, D.-T.; Nguyen, T.T.; Tien, N.L.B.; Tran, D.-K.; Jeong, J.-H.; Anh, P.G.; Thanh, V.V.; Truong, D.T.; Dinh, T.C. Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells 2020, 9, 563. https://doi.org/10.3390/cells9030563
Chu D-T, Nguyen TT, Tien NLB, Tran D-K, Jeong J-H, Anh PG, Thanh VV, Truong DT, Dinh TC. Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells. 2020; 9(3):563. https://doi.org/10.3390/cells9030563
Chicago/Turabian StyleChu, Dinh-Toi, Tiep Tien Nguyen, Nguyen Le Bao Tien, Dang-Khoa Tran, Jee-Heon Jeong, Pham Gia Anh, Vo Van Thanh, Dang Tien Truong, and Thien Chu Dinh. 2020. "Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications" Cells 9, no. 3: 563. https://doi.org/10.3390/cells9030563
APA StyleChu, D.-T., Nguyen, T. T., Tien, N. L. B., Tran, D.-K., Jeong, J.-H., Anh, P. G., Thanh, V. V., Truong, D. T., & Dinh, T. C. (2020). Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells, 9(3), 563. https://doi.org/10.3390/cells9030563







