NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate
Abstract
1. Introduction
2. Materials and Methods
2.1. Reprogramming, Validation and Culture of Human Induced Pluripotent Stem Cells
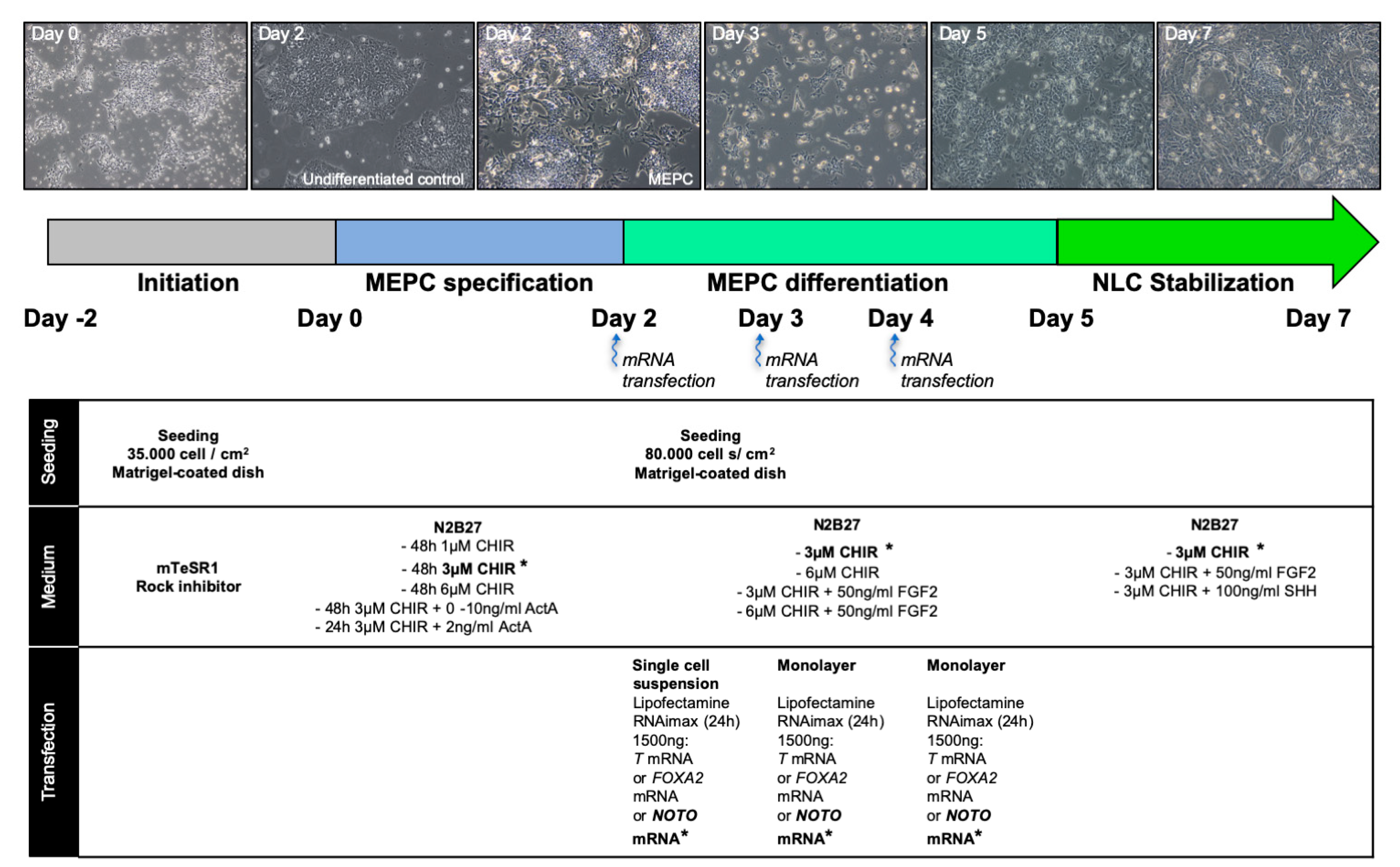
2.2. Differentiation of Human Induced Pluripotent Stem Cells
2.3. RNA Extraction and RT-qPCR
2.4. Immunostainings
2.5. cDNA Libraries, 3′ Digital Gene Expression RNA-Sequencing (DGE-seq), and Bioinformatic Analyses
2.6. Statistical Analysis
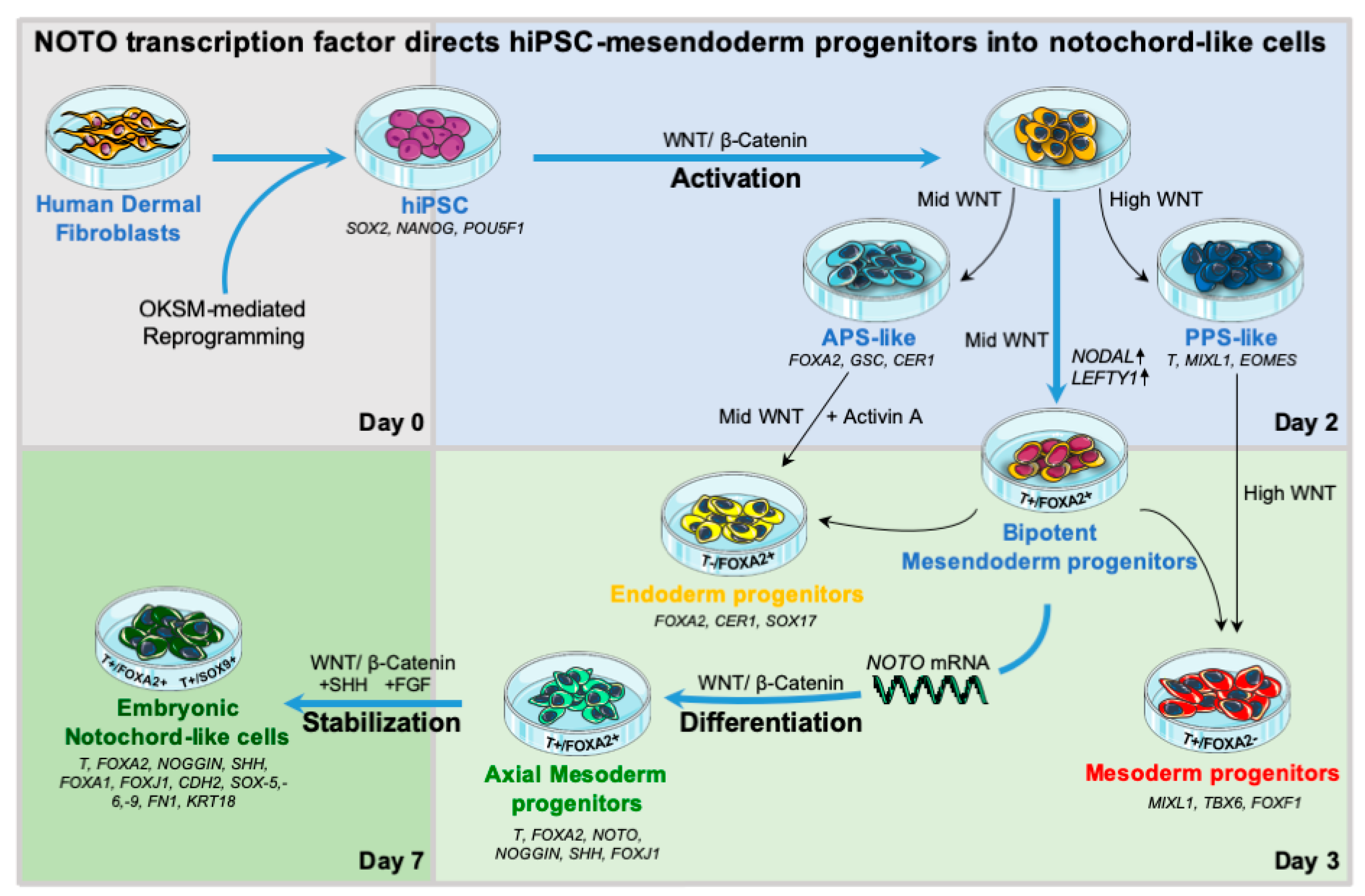
3. Results
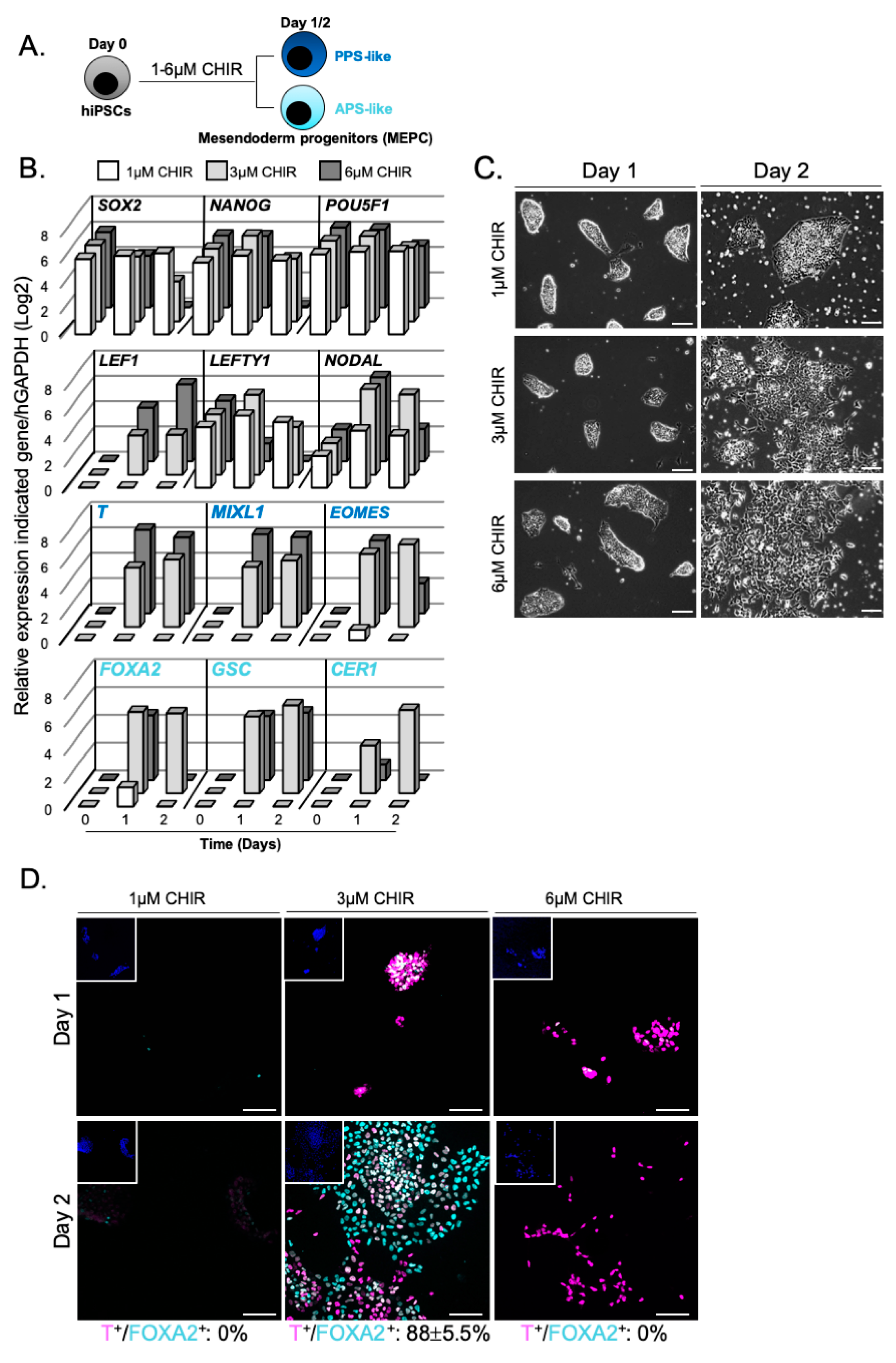
3.1. WNT Activity Induces High Levels of Mesendoderm Progenitors
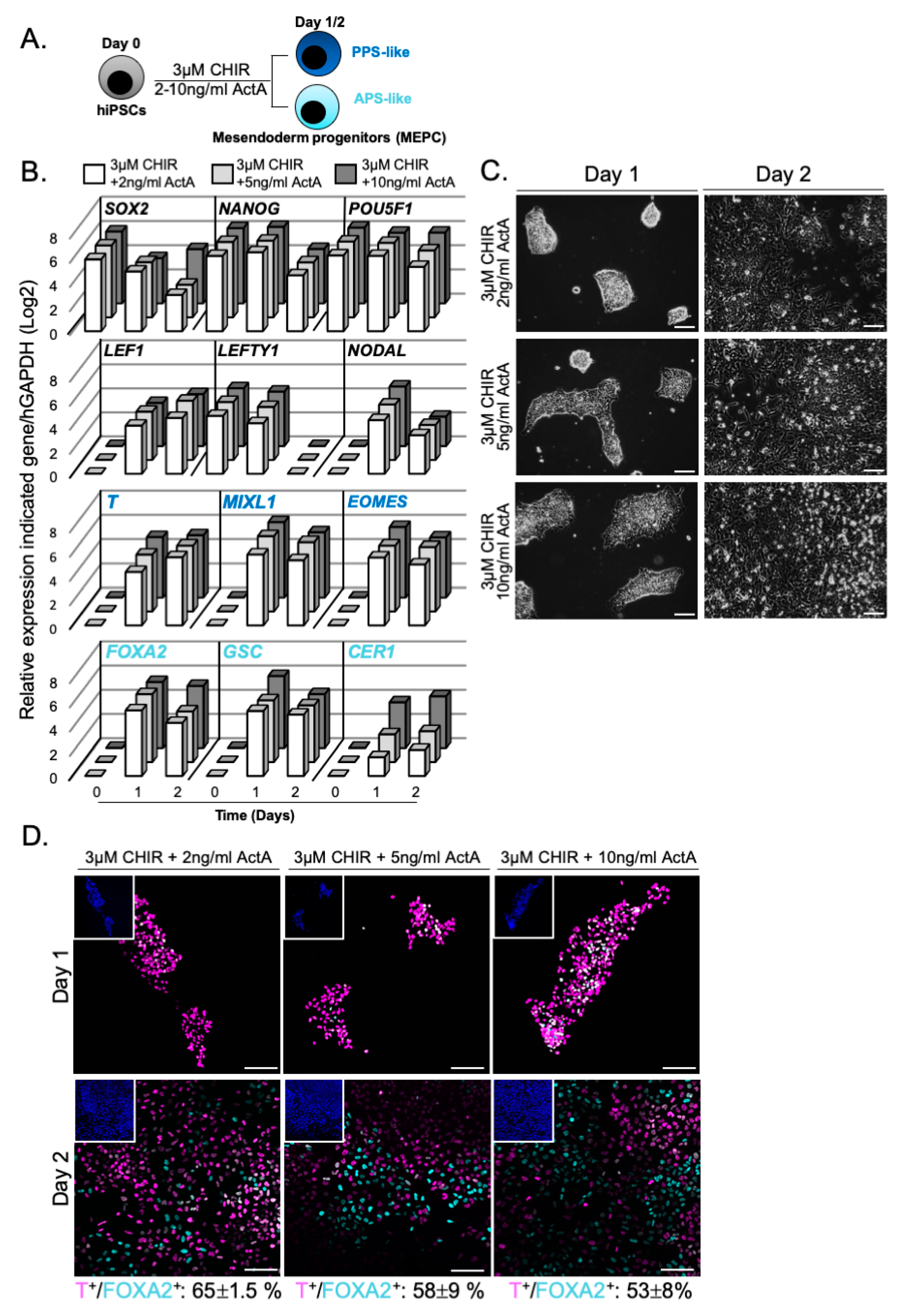
3.2. WNT and ACTIVIN/NODAL Activities Induces Mesendoderm Progenitors with Distinct Lineage Competencies
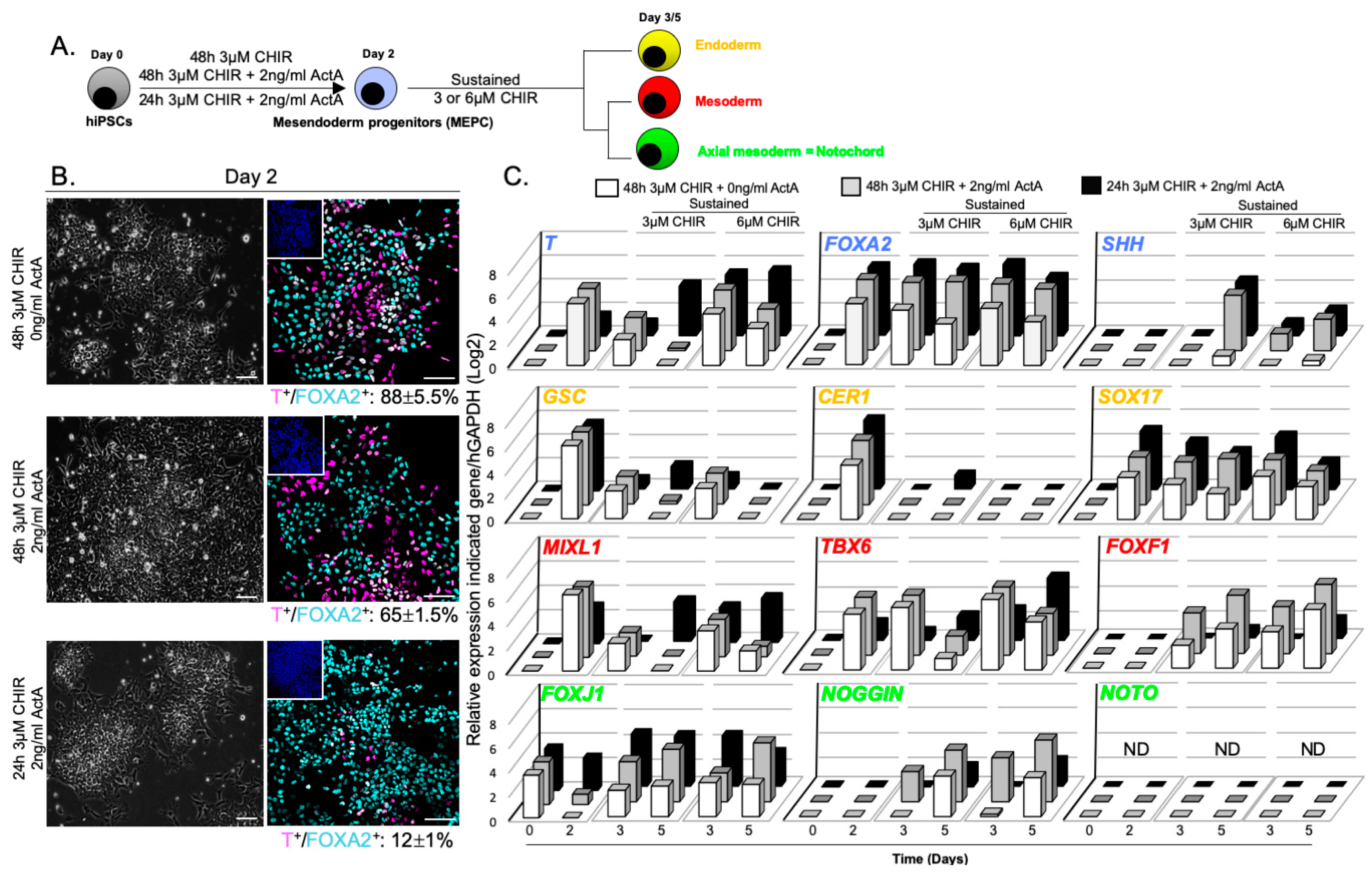
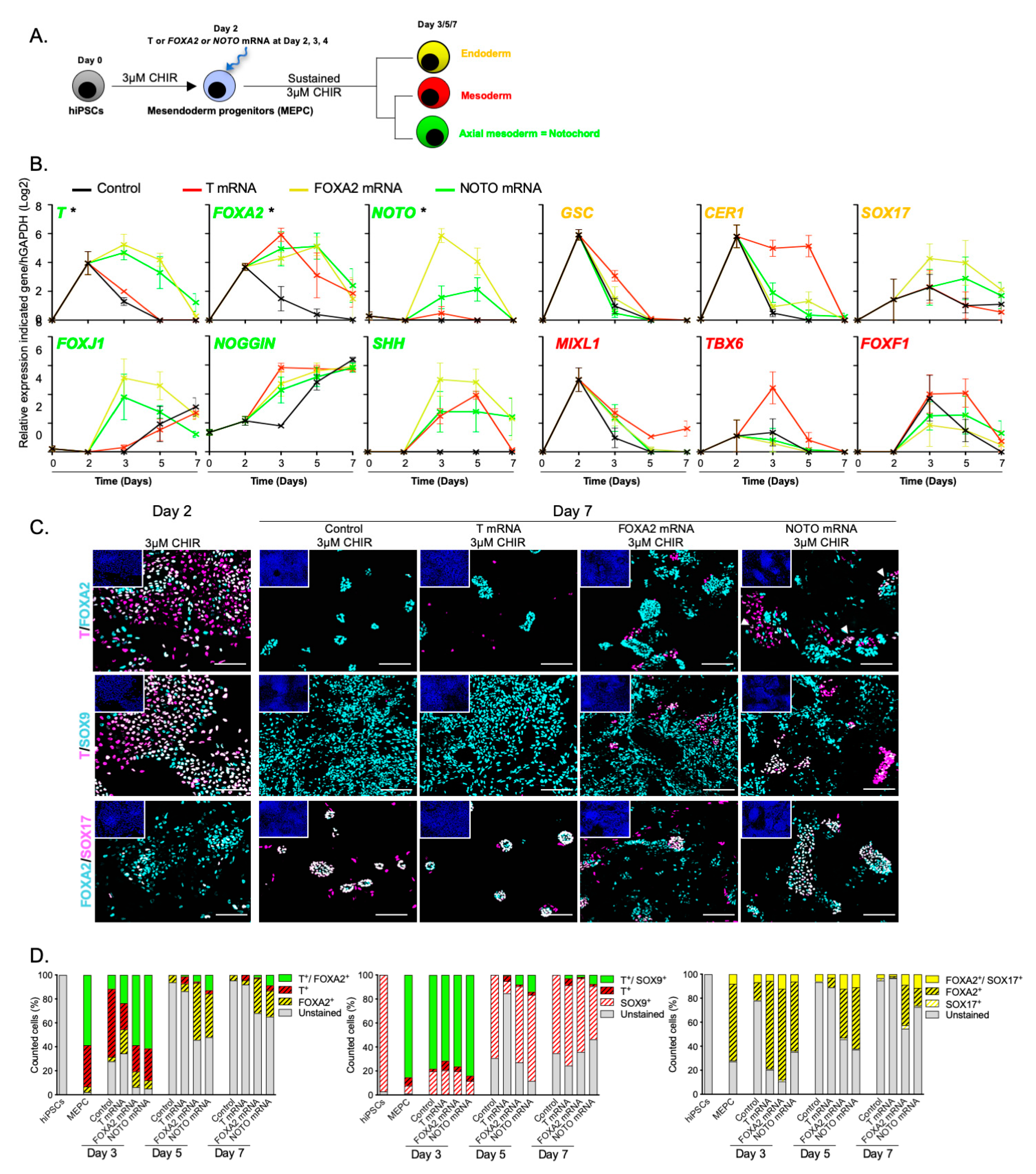
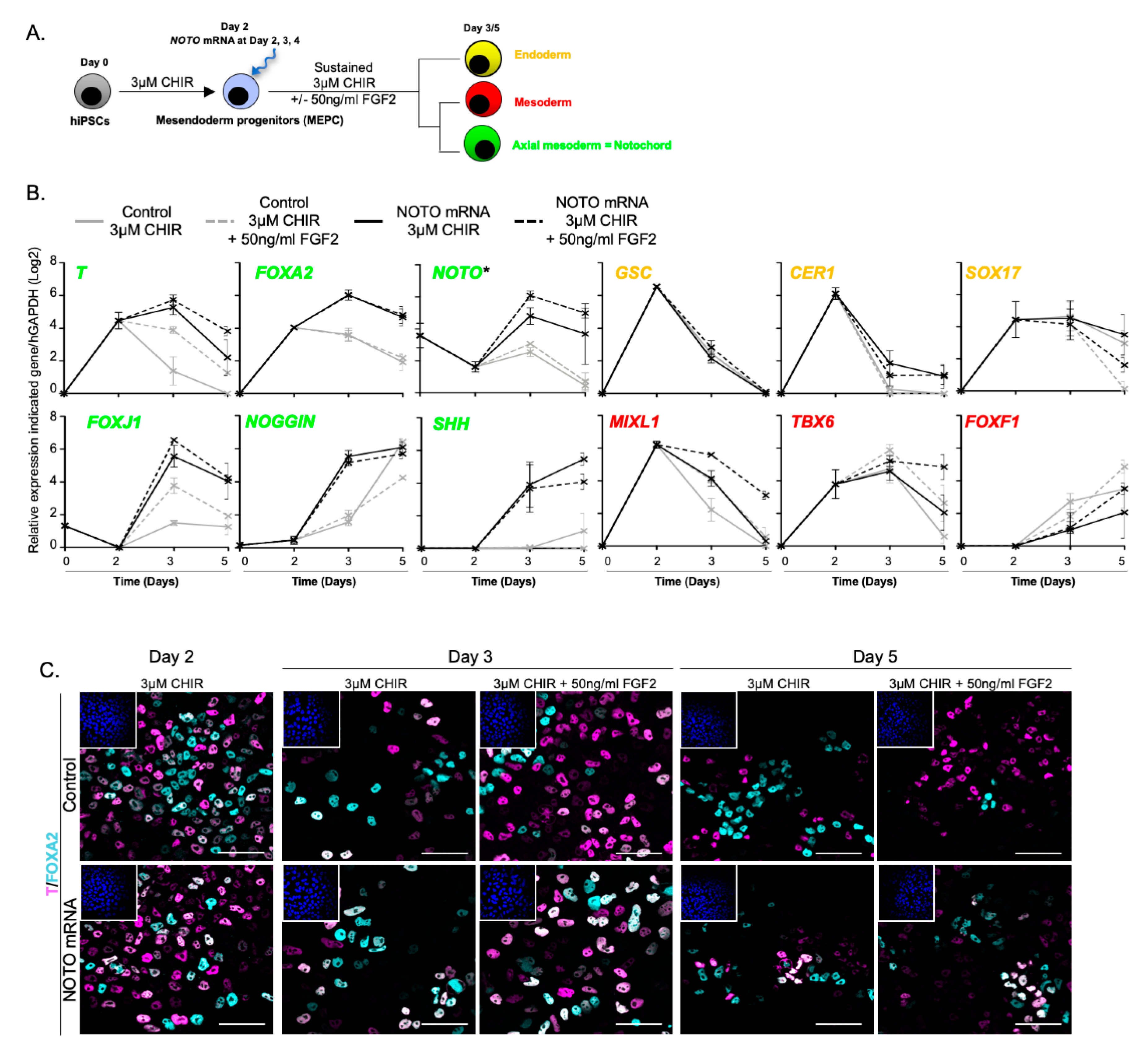
3.3. NOTO Transcription Factor Triggers MEPC Commitment toward Notochordal Fate
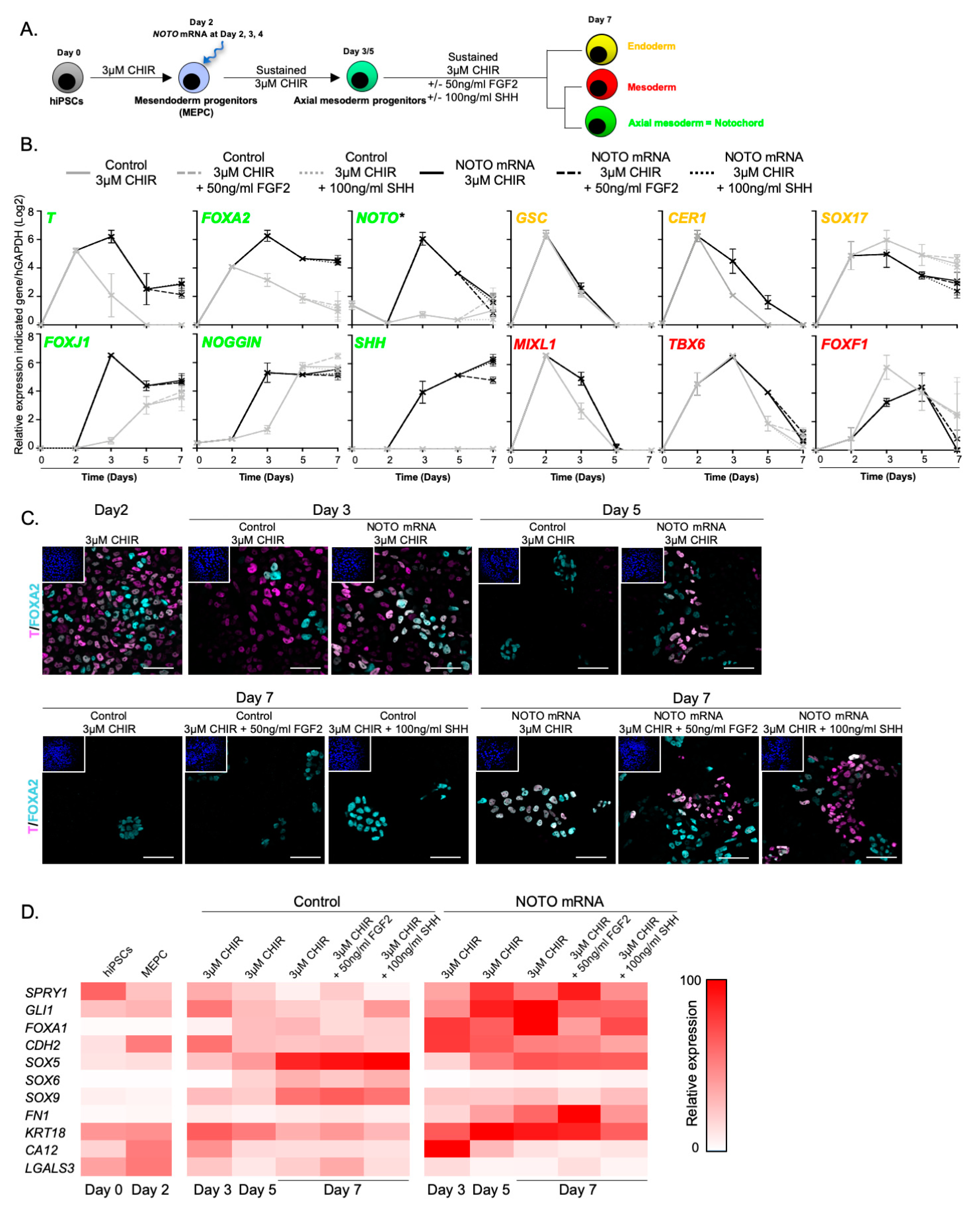
3.4. NOTO mRNA Transfection and WNT Signalling Activity Are Sufficient to Induce a Stable Notochord Population
3.5. Molecular Characterization of NOTO- and FOXA2-Directed MEPC Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Culture Condition | 48 h 3 µM CHIR Sustained 3 µM CHIR | 48 h 3 µM CHIR Sustained 6 µM CHIR | 48 h 3 µM CHIR + 2 ng/mlActA Sustained 3 µM CHIR | 48 h 3 µM CHIR + 2 ng/mlActA Sustained 6 µM CHIR | 24 h 3 µM CHIR + 2 ng/mlActA Sustained 3 µM CHIR | 24 h 3 µM CHIR + 2 ng/mlActA Sustained 6 µM CHIR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Name | Time (Days) | Mean RQ Value | SEM | Mean RQ Value | SEM | Mean RQ Value | SEM | Mean RQ Value | SEM | Mean RQ Value | SEM | Mean RQ Value | SEM |
| T | 0 | −4.68 | 0.50 | −4.68 | 0.50 | −4.68 | 0.50 | −4.68 | 0.50 | −4.68 | 0.50 | −4.68 | 0.50 |
| 2 | 5.24 | 0.36 | 5.24 | 0.36 | 5.27 | 0.16 | 5.27 | 0.16 | 1.62 | 0.39 | 1.62 | 0.39 | |
| 3 | 2.19 | 0.71 | 4.38 | 0.20 | 2.80 | 0.66 | 5.16 | 0.37 | 0.99 | 0.35 | 5.18 | 0.44 | |
| 5 | −2.39 | 1.64 | 3.14 | 1.50 | 0.20 | 1.33 | 3.54 | 1.22 | 4.23 | 0.62 | 5.45 | 0.43 | |
| FOXA2 | 0 | −1.71 | 0.28 | −1.71 | 0.28 | −1.71 | 0.28 | −1.71 | 0.28 | −1.71 | 0.28 | −1.71 | 0.28 |
| 2 | 4.78 | 0.20 | 4.78 | 0.20 | 5.53 | 0.25 | 5.53 | 0.25 | 5.31 | 0.18 | 5.31 | 0.18 | |
| 3 | 4.65 | 0.30 | 4.89 | 0.51 | 5.78 | 0.22 | 5.72 | 0.27 | 6.03 | 0.36 | 6.12 | 0.32 | |
| 5 | 3.48 | 0.55 | 3.74 | 0.59 | 5.84 | 0.38 | 5.29 | 0.23 | 5.64 | 0.42 | 4.94 | 0.46 | |
| SHH | 0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | ND | ND | ND | −0.32 | 0.87 | −0.32 | 0.87 | |
| 3 | −2.55 | 0.81 | ND | ND | −0.40 | 0.96 | 1.44 | 1.28 | −0.51 | 0.97 | 1.04 | 1.20 | |
| 5 | 0.80 | 1.93 | 0.40 | 1.68 | 4.71 | 0.73 | 2.67 | 0.79 | 4.57 | 0.92 | 2.11 | 1.26 | |
| GSC | 0 | −3.07 | 0.36 | −3.07 | 0.36 | −3.07 | 0.36 | −3.07 | 0.36 | −3.07 | 0.36 | −3.07 | 0.36 |
| 2 | 6.16 | 0.22 | 6.16 | 0.22 | 6.16 | 0.26 | 6.16 | 0.26 | 5.59 | 1.03 | 5.59 | 1.03 | |
| 3 | 2.39 | 0.17 | 2.60 | 0.14 | 2.37 | 0.23 | 2.65 | 0.20 | 0.74 | 0.28 | 1.00 | 0.25 | |
| 5 | −0.71 | 0.37 | −1.03 | 0.10 | 0.25 | 0.32 | −0.53 | 0.33 | 1.95 | 0.84 | −1.06 | 0.46 | |
| CER1 | 0 | −2.57 | 0.26 | −2.57 | 0.26 | −2.57 | 0.26 | −2.57 | 0.26 | −2.57 | 0.26 | −2.57 | 0.26 |
| 2 | 4.63 | 0.28 | 4.63 | 0.28 | 5.47 | 0.29 | 5.47 | 0.29 | 5.96 | 1.48 | 5.96 | 1.48 | |
| 3 | −1.83 | 0.74 | −3.68 | 0.55 | −2.43 | 0.44 | −3.09 | 0.35 | −2.57 | 0.39 | −1.86 | 0.15 | |
| 5 | −6.17 | 0.52 | −6.51 | 0.46 | −5.57 | 0.47 | −6.50 | 0.20 | 1.24 | 1.24 | −3.35 | 1.14 | |
| SOX17 | 0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2 | 3.60 | 0.98 | 3.60 | 0.98 | 4.10 | 0.39 | 4.10 | 0.39 | 5.03 | 0.70 | 5.03 | 0.70 | |
| 3 | 3.00 | 0.68 | 3.71 | 0.99 | 3.68 | 0.33 | 3.87 | 0.34 | 4.03 | 0.51 | 4.55 | 0.40 | |
| 5 | 2.21 | 0.90 | 2.82 | 1.19 | 3.95 | 0.54 | 2.91 | 0.46 | 3.21 | 0.87 | 2.31 | 0.70 | |
| MIXL1 | 0 | −3.47 | 0.19 | −3.47 | 0.19 | −3.47 | 0.19 | −3.47 | 0.19 | −3.47 | 0.19 | −3.47 | 0.19 |
| 2 | 6.27 | 0.15 | 6.27 | 0.15 | 5.79 | 0.28 | 5.79 | 0.28 | 2.96 | 0.58 | 2.96 | 0.58 | |
| 3 | 2.35 | 0.50 | 3.86 | 0.14 | 2.01 | 0.61 | 3.59 | 0.29 | −0.45 | 0.47 | 3.34 | 0.34 | |
| 5 | −2.46 | 1.16 | 1.94 | 1.48 | −0.28 | 0.99 | 1.01 | 0.84 | 3.38 | 0.99 | 4.23 | 0.41 | |
| TBX6 | 0 | −0.12 | 0.18 | −0.12 | 0.18 | −0.12 | 0.18 | −0.12 | 0.18 | −0.12 | 0.18 | −0.12 | 0.18 |
| 2 | 4.77 | 0.74 | 4.77 | 0.74 | 4.95 | 0.45 | 4.95 | 0.45 | 1.44 | 0.37 | 1.44 | 0.37 | |
| 3 | 5.34 | 0.63 | 6.03 | 0.32 | 5.13 | 0.49 | 5.83 | 0.39 | 0.68 | 0.30 | 2.54 | 0.31 | |
| 5 | 0.98 | 1.07 | 4.10 | 1.30 | 1.64 | 0.51 | 3.57 | 0.64 | 2.02 | 0.39 | 5.31 | 0.44 | |
| FOXF1 | 0 | −3.30 | 0.63 | −3.30 | 0.63 | −3.30 | 0.63 | −3.30 | 0.63 | −3.30 | 0.63 | −3.30 | 0.63 |
| 2 | −3.10 | 1.08 | −3.10 | 1.08 | −1.31 | 0.97 | −1.31 | 0.97 | −5.69 | 1.00 | −5.69 | 1.00 | |
| 3 | 1.96 | 0.87 | 3.06 | 1.41 | 3.46 | 0.34 | 3.95 | 1.07 | −1.11 | 0.53 | −1.65 | 0.24 | |
| 5 | 3.35 | 1.37 | 4.93 | 1.24 | 4.98 | 0.20 | 5.81 | 0.79 | 0.75 | 0.75 | 1.63 | 0.87 | |
| FOXJ1 | 0 | 3.49 | 0.30 | 3.49 | 0.30 | 3.49 | 0.30 | 3.49 | 0.30 | 3.49 | 0.30 | 3.49 | 0.30 |
| 2 | −1.43 | 0.33 | −1.43 | 0.33 | 0.83 | 0.52 | 0.83 | 0.52 | 2.74 | 0.69 | 2.74 | 0.69 | |
| 3 | 2.24 | 0.14 | 2.91 | 0.84 | 3.42 | 0.54 | 2.47 | 0.70 | 4.53 | 0.29 | 4.38 | 0.43 | |
| 5 | 2.60 | 0.46 | 2.75 | 0.57 | 4.48 | 0.36 | 5.04 | 0.51 | 4.36 | 0.42 | 2.91 | 0.87 | |
| NOGGIN | 0 | −0.32 | 0.86 | −0.32 | 0.86 | −0.32 | 0.86 | −0.32 | 0.86 | −0.32 | 0.86 | −0.32 | 0.86 |
| 2 | −1.45 | 0.76 | −1.45 | 0.76 | −1.49 | 0.72 | −1.49 | 0.72 | −5.13 | 0.67 | −5.13 | 0.67 | |
| 3 | −0.19 | 0.69 | 0.20 | 0.72 | 2.58 | 0.92 | 3.76 | 1.22 | −1.50 | 0.26 | −0.81 | 0.24 | |
| 5 | 3.46 | 0.45 | 3.32 | 0.53 | 4.41 | 0.39 | 5.28 | 1.76 | 1.64 | 0.77 | 2.22 | 0.77 | |
| NOTO | 0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 3 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 5 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
Appendix B
| Gene | MEPC | Day 2 | Day 5 | |
|---|---|---|---|---|
| Sustained 3 μM CHIR | Sustained 6 μM CHIR | |||
| T | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | >0.999 | >0.999 | 0.485 |
| 48 h 3 µM CHIR vs. 24 h 3 µM CHIR + 2 ng/mL ActA | 0.001 * | 0.167 | 0.085 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24 h 3 µM CHIR + 2 ng/mL ActA | 0.001 * | 0.039 * | 0.012 * | |
| FOXA2 | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | 0.379 | 0.106 | 0.169 |
| 48 h 3 µM CHIR vs. 24 h 3 µM CHIR + 2 ng/mL ActA | 0.363 | 0.156 | 0.379 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24 h 3 µM CHIR + 2 ng/mL ActA | >0.999 | 0.999 | 0.831 | |
| SHH | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | x | 0.037 | 0.332 |
| 48 h 3 µM CHIR vs. 24 h 3 µM CHIR + 2 ng/mL ActA | x | 0.076 | 0.892 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24 h 3 µM CHIR + 2 ng/mL ActA | x | 0.999 | 0.231 | |
| GSC | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | >0.999 | 0.498 | 1.000 |
| 48 h 3 µM CHIR vs. 24 h 3 µM CHIR + 2 ng/mL ActA | 0.716 | 0.386 | 0.967 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24 h 3 µM CHIR + 2 ng/mL ActA | 0.748 | 0.852 | 0.245 | |
| CER1 | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | 0.444 | 0.950 | >0.999 |
| 48 h 3 µM CHIR vs. 24h 3 µM CHIR + 2 ng/mL ActA | >0.999 | 0.033 * | 0.999 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.985 | 0.052 | >0.999 | |
| SOX17 | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | 0.894 | 0.408 | 0.827 |
| 48 h 3 µM CHIR vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.831 | 0.963 | 0.986 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.997 | 0.828 | 0.565 | |
| MIXL1 | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | 0.512 | 0.999 | 0.908 |
| 48 h 3 µM CHIR vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.007 * | 0.274 | 0.066 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.012 * | 0.110 | 0.008 * | |
| TBX6 | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | 0.998 | 0.999 | 0.926 |
| 48 h 3 µM CHIR vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.089 | >0.999 | 0.188 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.002 * | 0.949 | 0.002 * | |
| FOXF1 | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | x | 0.702 | 0.687 |
| 48 h 3 µM CHIR vs. 24h 3 µM CHIR + 2 ng/mL ActA | x | 0.455 | 0.751 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24h 3 µM CHIR + 2 ng/mL ActA | x | 0.005 * | 0.027 * | |
| FOXJ1 | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | 0.095 | 0.072 | 0.071 |
| 48 h 3 µM CHIR vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.005 * | 0.122 | >0.999 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.122 | 0.999 | 0.350 | |
| NOGGIN | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | 0.987 | 0.391 | >0.999 |
| 48 h 3 µM CHIR vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.229 | 0.225 | 0.688 | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24h 3 µM CHIR + 2 ng/mL ActA | 0.412 | 0.131 | 0.774 | |
| NOTO | 48 h 3 µM CHIR vs. 48 h 3 µM CHIR + 2 ng/mL ActA | x | x | x |
| 48 h 3 µM CHIR vs. 24h 3 µM CHIR + 2 ng/mL ActA | x | x | x | |
| 48 h 3 µM CHIR + 2 ng/mL ActA vs. 24h 3 µM CHIR + 2 ng/mL ActA | x | x | x | |
References
- Colombier, P.; Camus, A.; Lescaudron, L.; Clouet, J.; Guicheux, J. Intervertebral disc regeneration: A great challenge for tissue engineers. Trends Biotechnol. 2014, 32, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Ludwinski, F.E.; Gnanalingham, K.K.; Atkinson, R.A.; Freemont, A.J.; Hoyland, J.A. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci. Rep. 2017, 7, 1501. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.J.; Johnson, S.L.; Oegema, T.R. Notochordal cells interact with nucleus pulposus cells: Regulation of proteoglycan synthesis. Exp. Cell Res. 1999, 246, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Erwin, W.M.; Inman, R.D. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine 2006, 31, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Séguin, C. Notochord Cells in Intervertebral Disc Development and Degeneration. JDB 2016, 4, 3. [Google Scholar] [CrossRef]
- Sakai, D.; Nakamura, Y.; Nakai, T.; Mishima, T.; Kato, S.; Grad, S.; Alini, M.; Risbud, M.V.; Chan, D.; Cheah, K.S.E.; et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 2012, 3, 1264. [Google Scholar] [CrossRef]
- Boyd, L.M.; Chen, J.; Kraus, V.B.; Setton, L.A. Conditioned medium differentially regulates matrix protein gene expression in cells of the intervertebral disc. Spine 2004, 29, 2217–2222. [Google Scholar] [CrossRef]
- Abbott, R.D.; Purmessur, D.; Monsey, R.D.; Iatridis, J.C. Regenerative potential of TGFβ3 + Dex and notochordal cell conditioned media on degenerated human intervertebral disc cells. J. Orthop. Res. 2012, 30, 482–488. [Google Scholar] [CrossRef]
- Bach, F.C.; de Vries, S.A.H.; Krouwels, A.; Creemers, L.B.; Ito, K.; Meij, B.P.; Tryfonidou, M.A. The species-specific regenerative effects of notochordal cell-conditioned medium on chondrocyte-like cells derived from degenerated human intervertebral discs. Eur. Cell Mater. 2015, 30, 132–147. [Google Scholar] [CrossRef]
- Mehrkens, A.; Matta, A.; Karim, M.Z.; Kim, S.; Fehlings, M.G.; Schaeren, S.; Mark Erwin, W. Notochordal cell-derived conditioned medium protects human nucleus pulposus cells from stress-induced apoptosis. Spine J. 2017, 17, 579–588. [Google Scholar] [CrossRef]
- Matta, A.; Karim, M.Z.; Isenman, D.E.; Erwin, W.M. Molecular Therapy for Degenerative Disc Disease: Clues from Secretome Analysis of the Notochordal Cell-Rich Nucleus Pulposus. Sci. Rep. 2017, 7, 45623. [Google Scholar] [CrossRef]
- Liebscher, T.; Haefeli, M.; Wuertz, K.; Nerlich, A.G.; Boos, N. Age-related variation in cell density of human lumbar intervertebral disc. Spine 2011, 36, 153–159. [Google Scholar] [CrossRef]
- Clouet, J.; Fusellier, M.; Camus, A.; Le Visage, C.; Guicheux, J. Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies. Adv. Drug Deliv. Rev. 2018, 146, 306–324. [Google Scholar] [CrossRef]
- Choi, K.-S.; Cohn, M.J.; Harfe, B.D. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: Implications for disk degeneration and chordoma formation. Dev. Dyn. 2008, 237, 3953–3958. [Google Scholar] [CrossRef]
- McCann, M.R.; Tamplin, O.J.; Rossant, J.; Séguin, C.A. Tracing notochord-derived cells using a Noto-cre mouse: Implications for intervertebral disc development. Dis. Model. Mech. 2012, 5, 73–82. [Google Scholar] [CrossRef]
- Camus, A.; Davidson, B.P.; Billiards, S.; Khoo, P.; Rivera-Pérez, J.A.; Wakamiya, M.; Behringer, R.R.; Tam, P.P. The morphogenetic role of midline mesendoderm and ectoderm in the development of the forebrain and the midbrain of the mouse embryo. Development 2000, 127, 1799–1813. [Google Scholar]
- Lawson, L.Y.; Harfe, B.D. Developmental mechanisms of intervertebral disc and vertebral column formation. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6. [Google Scholar] [CrossRef]
- Williams, S.; Alkhatib, B.; Serra, R. Development of the axial skeleton and intervertebral disc. Curr. Top. Dev. Biol. 2019, 133, 49–90. [Google Scholar]
- Winzi, M.K.; Hyttel, P.; Dale, J.K.; Serup, P. Isolation and characterization of node/notochord-like cells from mouse embryonic stem cells. Stem Cells Dev. 2011, 20, 1817–1827. [Google Scholar] [CrossRef]
- Liu, Y.; Rahaman, M.N.; Bal, B.S. Modulating notochordal differentiation of human induced pluripotent stem cells using natural nucleus pulposus tissue matrix. PLoS ONE 2014, 9, e100885. [Google Scholar] [CrossRef]
- Tang, R.; Jing, L.; Willard, V.P.; Wu, C.-L.; Guilak, F.; Chen, J.; Setton, L.A. Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Czichos, S.; Kaps, C.; Bächner, D.; Mayer, H.; Kurkalli, B.G.; Zilberman, Y.; Turgeman, G.; Pelled, G.; Gross, G.; et al. The T-box transcription factor Brachyury mediates cartilage development in mesenchymal stem cell line C3H10T1/2. J. Cell Sci. 2002, 115, 769–781. [Google Scholar] [PubMed]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat. Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef]
- Vincent, S.D.; Dunn, N.R.; Hayashi, S.; Norris, D.P.; Robertson, E.J. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003, 17, 1646–1662. [Google Scholar] [CrossRef]
- Tamplin, O.J.; Kinzel, D.; Cox, B.J.; Bell, C.E.; Rossant, J.; Lickert, H. Microarray analysis of Foxa2 mutant mouse embryos reveals novel gene expression and inductive roles for the gastrula organizer and its derivatives. BMC Genom. 2008, 9, 511. [Google Scholar] [CrossRef]
- Balmer, S.; Nowotschin, S.; Hadjantonakis, A.-K. Notochord morphogenesis in mice: Current understanding & open questions. Dev. Dyn. 2016, 245, 547–557. [Google Scholar]
- Wymeersch, F.J.; Skylaki, S.; Huang, Y.; Watson, J.A.; Economou, C.; Marek-Johnston, C.; Tomlinson, S.R.; Wilson, V. Transcriptionally dynamic progenitor populations organised around a stable niche drive axial patterning. Development 2019, 146. [Google Scholar] [CrossRef]
- Tamplin, O.J.; Cox, B.J.; Rossant, J. Integrated microarray and ChIP analysis identifies multiple Foxa2 dependent target genes in the notochord. Dev. Biol. 2011, 360, 415–425. [Google Scholar] [CrossRef]
- Alten, L.; Schuster-Gossler, K.; Eichenlaub, M.P.; Wittbrodt, B.; Wittbrodt, J.; Gossler, A. A novel mammal-specific three partite enhancer element regulates node and notochord-specific Noto expression. PLoS ONE 2012, 7, e47785. [Google Scholar] [CrossRef][Green Version]
- Lolas, M.; Valenzuela, P.D.T.; Tjian, R.; Liu, Z. Charting Brachyury-mediated developmental pathways during early mouse embryogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, 4478–4483. [Google Scholar] [CrossRef]
- Abdelkhalek, H.B.; Beckers, A.; Schuster-Gossler, K.; Pavlova, M.N.; Burkhardt, H.; Lickert, H.; Rossant, J.; Reinhardt, R.; Schalkwyk, L.C.; Müller, I.; et al. The mouse homeobox gene Not is required for caudal notochord development and affected by the truncate mutation. Genes Dev. 2004, 18, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Beckers, A.; Alten, L.; Viebahn, C.; Andre, P.; Gossler, A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc. Natl. Acad. Sci. USA 2007, 104, 15765–15770. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Tamplin, O.J.; Beckers, A.; Gossler, A.; Rossant, J. Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms. Dev. Cell 2007, 13, 884–896. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Ward, L.; Humphreys, M.; Zeef, L.A.H.; Berry, A.; Hanley, K.P.; Hanley, N.; Richardson, S.M.; Hoyland, J.A. Human notochordal cell transcriptome unveils potential regulators of cell function in the developing intervertebral disc. Sci. Rep. 2018, 8, 12866. [Google Scholar] [CrossRef]
- Gaignerie, A.; Lefort, N.; Rousselle, M.; Forest-Choquet, V.; Flippe, L.; Francois-Campion, V.; Girardeau, A.; Caillaud, A.; Chariau, C.; Francheteau, Q.; et al. Urine-derived cells provide a readily accessible cell type for feeder-free mRNA reprogramming. Sci. Rep. 2018, 8, 14363. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kilens, S.; Meistermann, D.; Moreno, D.; Chariau, C.; Gaignerie, A.; Reignier, A.; Lelièvre, Y.; Casanova, M.; Vallot, C.; Nedellec, S.; et al. Parallel derivation of isogenic human primed and naive induced pluripotent stem cells. Nat. Commun 2018, 9, 360s. [Google Scholar] [CrossRef]
- Gadue, P.; Huber, T.L.; Paddison, P.J.; Keller, G.M. Wnt and TGF-beta signalling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16806–16811. [Google Scholar] [CrossRef]
- Sumi, T.; Tsuneyoshi, N.; Nakatsuji, N.; Suemori, H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signalling. Development 2008, 135, 2969–2979. [Google Scholar] [CrossRef]
- Costello, I.; Nowotschin, S.; Sun, X.; Mould, A.W.; Hadjantonakis, A.-K.; Bikoff, E.K.; Robertson, E.J. Lhx1 functions together with Otx2, Foxa2, and Ldb1 to govern anterior mesendoderm, node, and midline development. Genes Dev. 2015, 29, 2108–2122. [Google Scholar] [CrossRef]
- Arnold, S.J.; Robertson, E.J. Making a commitment: Cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 2009, 10, 91–103. [Google Scholar] [CrossRef]
- Brennan, J.; Lu, C.C.; Norris, D.P.; Rodriguez, T.A.; Beddington, R.S.; Robertson, E.J. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 2001, 411, 965–969. [Google Scholar] [CrossRef]
- Besser, D. Expression of nodal, lefty-a, and lefty-B in undifferentiated human embryonic stem cells requires activation of Smad2/3. J. Biol. Chem. 2004, 279, 45076–45084. [Google Scholar] [CrossRef]
- Lickert, H.; Kutsch, S.; Kanzler, B.; Tamai, Y.; Taketo, M.M.; Kemler, R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev. Cell 2002, 3, 171–181. [Google Scholar] [CrossRef]
- Ukita, K.; Hirahara, S.; Oshima, N.; Imuta, Y.; Yoshimoto, A.; Jang, C.-W.; Oginuma, M.; Saga, Y.; Behringer, R.R.; Kondoh, H.; et al. Wnt signalling maintains the notochord fate for progenitor cells and supports the posterior extension of the notochord. Mech. Dev. 2009, 126, 791–803. [Google Scholar] [CrossRef]
- Rudloff, S.; Kemler, R. Differential requirements for β-catenin during mouse development. Development 2012, 139, 3711–3721. [Google Scholar] [CrossRef]
- Smits, P.; Lefebvre, V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development 2003, 130, 1135–1148. [Google Scholar] [CrossRef]
- Barrionuevo, F.; Taketo, M.M.; Scherer, G.; Kispert, A. Sox9 is required for notochord maintenance in mice. Dev. Biol. 2006, 295, 128–140. [Google Scholar] [CrossRef]
- Maier, J.A.; Lo, Y.; Harfe, B.D. Foxa1 and Foxa2 are required for formation of the intervertebral discs. PLoS ONE 2013, 8, e55528. [Google Scholar] [CrossRef]
- Bagheri-Fam, S.; Barrionuevo, F.; Dohrmann, U.; Günther, T.; Schüle, R.; Kemler, R.; Mallo, M.; Kanzler, B.; Scherer, G. Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Dev. Biol. 2006, 291, 382–397. [Google Scholar] [CrossRef]
- Spence, J.R.; Lange, A.W.; Lin, S.-C.J.; Kaestner, K.H.; Lowy, A.M.; Kim, I.; Whitsett, J.A.; Wells, J.M. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev. Cell 2009, 17, 62–74. [Google Scholar] [CrossRef]
- Korostylev, A.; Mahaddalkar, P.U.; Keminer, O.; Hadian, K.; Schorpp, K.; Gribbon, P.; Lickert, H. A high-content small molecule screen identifies novel inducers of definitive endoderm. Mol. Metab 2017, 6, 640–650. [Google Scholar] [CrossRef]
- Ciruna, B.; Rossant, J. FGF signalling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 2001, 1, 37–49. [Google Scholar] [CrossRef]
- Choi, K.-S.; Harfe, B.D. Hedgehog signalling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc. Natl. Acad. Sci. USA 2011, 108, 9484–9489. [Google Scholar] [CrossRef]
- Omlor, G.W.; Nerlich, A.G.; Tirlapur, U.K.; Urban, J.P.; Guehring, T. Loss of notochordal cell phenotype in 3D-cell cultures: Implications for disc physiology and disc repair. Arch. Orthop Trauma Surg. 2014, 134, 1673–1681. [Google Scholar] [CrossRef]
- Hwang, P.Y.; Jing, L.; Michael, K.W.; Richardson, W.J.; Chen, J.; Setton, L.A. N-Cadherin-Mediated Signaling Regulates Cell Phenotype for Nucleus Pulposus Cells of the Intervertebral Disc. Cell Mol. Bioeng 2015, 8, 51–62. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Z.-X.; Cai, F.; Sinkemani, A.; Xie, Z.-Y.; Shi, R.; Wei, J.-N.; Wu, X.-T. Formation, function, and exhaustion of notochordal cytoplasmic vacuoles within intervertebral disc: Current understanding and speculation. Oncotarget 2017, 8, 57800–57812. [Google Scholar] [CrossRef]
- Resutek, L.; Hsieh, A.H. The vacuolated morphology of chordoma cells is dependent on cytokeratin intermediate filaments. J. Cell. Physiol. 2018, 110, 115. [Google Scholar] [CrossRef]
- Tsankov, A.M.; Gu, H.; Akopian, V.; Ziller, M.J.; Donaghey, J.; Amit, I.; Gnirke, A.; Meissner, A. Transcription factor binding dynamics during human ES cell differentiation. Nature 2015, 518, 344–349. [Google Scholar] [CrossRef]
- Sharon, N.; Mor, I.; Golan-lev, T.; Fainsod, A.; Benvenisty, N. Molecular and functional characterizations of gastrula organizer cells derived from human embryonic stem cells. Stem Cells 2011, 29, 600–608. [Google Scholar] [CrossRef]
- Martyn, I.; Kanno, T.Y.; Ruzo, A.; Siggia, E.D.; Brivanlou, A.H. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature 2018, 558, 132–135. [Google Scholar] [CrossRef]
- Vallier, L.; Touboul, T.; Chng, Z.; Brimpari, M.; Hannan, N.; Millan, E.; Smithers, L.E.; Trotter, M.; Rugg-Gunn, P.; Weber, A.; et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS ONE 2009, 4, e6082. [Google Scholar] [CrossRef]
- Funa, N.S.; Schachter, K.A.; Lerdrup, M.; Ekberg, J.; Hess, K.; Dietrich, N.; Honoré, C.; Hansen, K.; Semb, H. b-Catenin Regulates Primitive Streak Induction through Collaborative Interactions with SMAD2/SMAD3 and Oct4. Cell Stem Cell 2015, 15, 639–652. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Berry, A.; Piper-Hanley, K.; Hanley, N.; Richardson, S.M.; Hoyland, J.A. Spatiotemporal analysis of putative notochordal cell markers reveals CD24 and keratins 8, 18, and 19 as notochord-specific markers during early human intervertebral disc development. J. Orthop. Res. 2016, 34, 1327–1340. [Google Scholar] [CrossRef]
- Peck, S.H.; McKee, K.K.; Tobias, J.W.; Malhotra, N.R.; Harfe, B.D.; Smith, L.J. Whole Transcriptome Analysis of Notochord-Derived Cells during Embryonic Formation of the Nucleus Pulposus. Sci. Rep. 2017, 7, 10504. [Google Scholar] [CrossRef]
- Tang, S.; Richards, J.; Khan, S.; Hoyland, J.; Gallego Perez, D.; Higuita Castro, N.; Walter, B.; Purmessur, D. Nonviral Transfection with Brachyury Reprograms Human Intervertebral Disc Cells to a Pro-Anabolic Anti-Catabolic/Inflammatory Phenotype: A Proof of Concept Study. J. Orthop. Res. 2019, 37, 2389–2400. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Tawackoli, W.; Zhou, Z.; Salehi, K.; Bez, M.; De Mel, S.; Chan, V.; Roth, J.; Avalos, P.; et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 2019, 9, 7506–7524. [Google Scholar] [CrossRef]
- Faial, T.; Bernardo, A.S.; Mendjan, S.; Diamanti, E.; Ortmann, D.; Gentsch, G.E.; Mascetti, V.L.; Trotter, M.W.B.; Smith, J.C.; Pedersen, R.A. Brachyury and SMAD signalling collaboratively orchestrate distinct mesoderm and endoderm gene regulatory networks in differentiating human embryonic stem cells. Development 2015, 142, 2121–2135. [Google Scholar] [CrossRef]
- Melby, A.E.; Kimelman, D.; Kimmel, C.B. Spatial regulation of floating head expression in the developing notochord. Dev. Dyn. 1997, 209, 156–165. [Google Scholar] [CrossRef]
- Sawada, A.; Nishizaki, Y.; Sato, H.; Yada, Y.; Nakayama, R.; Yamamoto, S.; Nishioka, N.; Kondoh, H.; Sasaki, H. Tead proteins activate the Foxa2 enhancer in the node in cooperation with a second factor. Development 2005, 132, 4719–4729. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombier, P.; Halgand, B.; Chédeville, C.; Chariau, C.; François-Campion, V.; Kilens, S.; Vedrenne, N.; Clouet, J.; David, L.; Guicheux, J.; et al. NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate. Cells 2020, 9, 509. https://doi.org/10.3390/cells9020509
Colombier P, Halgand B, Chédeville C, Chariau C, François-Campion V, Kilens S, Vedrenne N, Clouet J, David L, Guicheux J, et al. NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate. Cells. 2020; 9(2):509. https://doi.org/10.3390/cells9020509
Chicago/Turabian StyleColombier, Pauline, Boris Halgand, Claire Chédeville, Caroline Chariau, Valentin François-Campion, Stéphanie Kilens, Nicolas Vedrenne, Johann Clouet, Laurent David, Jérôme Guicheux, and et al. 2020. "NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate" Cells 9, no. 2: 509. https://doi.org/10.3390/cells9020509
APA StyleColombier, P., Halgand, B., Chédeville, C., Chariau, C., François-Campion, V., Kilens, S., Vedrenne, N., Clouet, J., David, L., Guicheux, J., & Camus, A. (2020). NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate. Cells, 9(2), 509. https://doi.org/10.3390/cells9020509





