Abstract
Neurodegenerative diseases are a large group of neurological disorders with diverse etiological and pathological phenomena. However, current therapeutics rely mostly on symptomatic relief while failing to target the underlying disease pathobiology. G-protein-coupled receptors (GPCRs) are one of the most frequently targeted receptors for developing novel therapeutics for central nervous system (CNS) disorders. Many currently available antipsychotic therapeutics also act as either antagonists or agonists of different GPCRs. Therefore, GPCR-based drug development is spreading widely to regulate neurodegeneration and associated cognitive deficits through the modulation of canonical and noncanonical signals. Here, GPCRs’ role in the pathophysiology of different neurodegenerative disease progressions and cognitive deficits has been highlighted, and an emphasis has been placed on the current pharmacological developments with GPCRs to provide an insight into a potential therapeutic target in the treatment of neurodegeneration.
1. Introduction
G-protein-coupled receptors (GPCRs) constitute the largest family of membrane receptors in humans, and ~34% of marketed drugs target this family. Since their discovery, this receptor family has been both pharmacologically and biologically important in the treatment of different pathological conditions. GPCRs contain a common pattern in their basic structures. They consist of seven membrane-spanning domain structures, which they use to sense different signals and stimuli, including light, stress, hormones, peptides, and so forth [1].
Neurodegenerative diseases (NDDs) are most common and prevalent in elderly people worldwide [2] and cause progressive neuronal dysfunction, toxicities, and death. These diseases lead to an irreversible weakening of all brain functions. Around 30 million individuals worldwide are affected by NDDs [3,4]. Alzheimer’s disease (AD), vascular dementia (VaD), frontotemporal dementia (FTD), Parkinson’s disease (PD), and Huntington’s disease (HD) [5] are the prevalent NDDs commonly diagnosed in aged people. The cognitive function in patients is seriously affected during these disease’s progression. Therefore, the age-related decline in cognitive function is a leading challenge in mental health research [6]. Although some symptomatic treatments are available for NDDs, no specific treatments have yet been found [7]. Among the various NDDs, AD, which causes impaired cognitive function and memory loss, is the most extensively discussed and researched [8]. Amyloid β (Aβ) plaque formation and the hyperphosphorylation of tau proteins in the neurofibrillary tangles of the frontal and temporal lobe and other regions of the neocortex are observed during the progression of AD. VaD is another common cause of dementia, in which patients often complain of disturbances in frontal executive function [1] and multiple cerebrovascular pathologies, including vessel occlusion, arteriosclerosis, hypertension, aneurysms, and various forms of arteritis [8]. FTD can be diagnosed in people less than 65 years old [9] and is characterised by neuropsychiatric symptoms and behavioural, motor, and cognitive impairments [10]. Abnormal depositions of tau proteins are observed in different regions of an FTD patient’s brain, such as the hippocampus, frontal cortex, and striatum [11].
Extensive research is ongoing in an attempt to clearly understand the pathobiologies of these NDDs. Within this context, several pieces of evidence strongly suggest that GPCRs are involved in the pathogenesis of several NDDs, including AD, PD, HD, and MS [8,12]. Around 90% of the ~370 nonsensory GPCRs have been found to express in the brain, and they play important roles in regulating mood, appetite, pain, vision, immune responses, cognition, and synaptic transmissions [8]. GPCRs are now the most common target used to develop novel therapeutics. In this article, we present GPCRs and their involvement in the pathophysiology of NDDs and discuss the changes in GPCR expression in different neurodegenerative conditions in light of the recent research in that field. The aim is to provide a potential and novel target for the development of novel therapeutics for NDD treatment.
2. GPCRs’ Relevance in Cognition and Neurodegenerative Disorders
GPCRs comprise the largest family of transmembrane receptors, with over 800 members present in humans [13,14]. Slightly over half of the 800 receptors have sensory functions that mediate olfaction (400), taste (33), light perception (10), and pheromone signalling (5) [15]. More than 90% (370) of the remaining receptors are expressed in the brain. These nonsensory GPCRs mediate signalling from multiple ligand types and regulate several physiological processes throughout the human organism, mainly endocrine and neurological processes [8,13,16] (Figure 1). GPCRs are currently the most common therapeutic drug target [13]. In 2017, Hauser and coworkers accumulated data from the Center Watch Drugs in Clinical Trials database and, by cross-referencing with public sources, reported 475 approved drugs that target GPCRs, representing 34% of all FDA-approved drugs [13,17]. This report also indicates that about 20% to 50% of drugs have GPCRs as a target, with discrepancies probably resulting from the varying definitions of ‘drug target’ [16,18,19]. As new functions for GPCRs are discovered, especially in the case of the 100 orphan GPCRs for which no endogenous ligand or clearly defined function are currently known, the number of drugs targeting GPCRs is expected to increase [14,19]. Therefore, the relevance of the different types of GPCRs in neurodegeneration and age-related disease progression is the focus of this article.
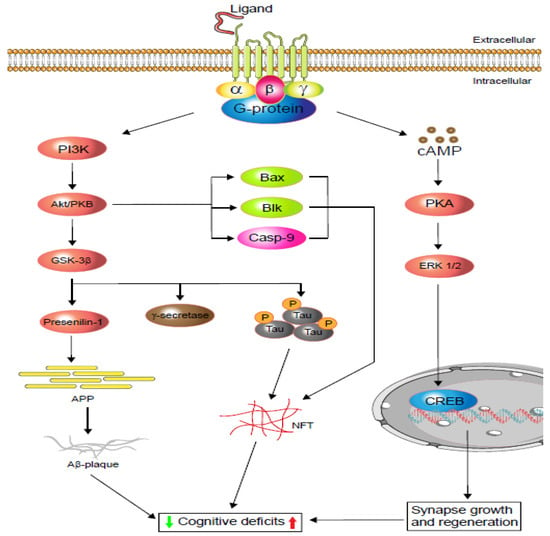
Figure 1.
Schematic display of G-protein-coupled receptors (GPCRs) signalling in cognitive impairment. Depending on the agonist or inverse agonist ligand binding, the PI3/Akt-signalling pathway signals Bax and Casp-9 while increasing neurofibrillary tangle (NFT) formation via phosphorylation. PI3/Akt-signalling hyperphosphorylation also activates glycogen synthase kinase-3β (GSK-3β), which increases either or both tau protein phosphorylation and amyloid precursor protein (APP). Phosphorylated tau protein forms neurofibrillary tangles (NFTs) and regulates cognitive function. Similarly, APP metabolism regulates Aβ-plaque formation and controls cognition. Moreover, neuronal and dendritic plasticity is required for synaptic growth, regeneration, and memory formation, and it depends on extracellular signal-regulated kinase (ERK ½) modulation. Depending on the ligands, GPCRs activate cAMP-response element-binding protein (CREB) via the cAMP/ERK ½ pathway and regulate cognition (based on [20,21]).
2.1. Alzheimer’s Disease
AD is the predominant form of NDD and is most prevalent among people over the age of 65. AD patients exhibit cognitive deficits, memory loss, and changes in personality and behaviour [8,22]. The pathophysiology includes an accumulation of amyloid β (Aβ) plaques and the aggregation of tau proteins in neurofibrillary tangles, which are detected primarily in the frontal and temporal lobes and later slowly progress into other regions of the neocortex [22]. With the progression of this disease, different regions of the brain degenerate, and their neurochemical pathways change, including acetylcholine, serotonin and adenosine homeostasis, leading to cognitive impairments.
Although AD treatment strategies are progressing, they are limited to symptomatic relief only. One of the common features of AD is the gradual downregulation of the acetylcholine level in the brain, which is due to the abundance of acetylcholinesterase [23]. Acetylcholinesterase inhibitors (AChEi), therefore, play a vital role in the symptomatic treatment of AD and also improve cholinergic neurotransmission and cognitive function [24]. However, glutamatergic neurotransmitter overexpression is also reported in the pathogenesis of AD [25,26]. In addition, glutamate receptors are reported to mediate most of the excitatory neurotransmissions in the mammalian brain, and their overstimulation can cause excitotoxicity [25,27]. Metabotropic glutamate receptors (especially mGluR5) have been found to be involved in both cognition and Aβ generation and accumulation. A study showed that genetic deletion of mGluR5 in the mouse (APPswe/PS1ΔE9) ameliorates cognitive deficiencies and minimises Aβ production [28]. The study also indicates that mGluR5 expression is related to the increase in Aβ plaque and mediated by the mechanism of the fragile X mental retardation protein (FMRP) [28]. However, the mGluR5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl]-pyridine (MTEP) has efficiently reversed this condition under the same paradigm [29]. Extensive evidence indicates that a wide-ranging serotonergic denervation of the neocortex and hippocampus occurs in AD patients. For example, activation of the serotonergic 5-HT2A and 5-HT4 receptors improves learning and memory [30,31] through the activation of the extracellular signal-regulated kinase (ERK) mediated by either the G-protein or β-arrestin (Figure 1) [20]. Therefore, targeting serotonergic receptors would also intervene in AD progression.
2.2. Vascular Dementia
Vascular dementia (VaD) is predominant in 8–15% of cognitively-impaired aged patients. Neuropathological investigation of VaD shows multifocal and/or diffuse lesions in the subcortical and strategically important brain areas, including the thalamus, frontobasal, and limbic system, and hippocampal sclerosis to multi-infarct encephalopathy [32]. These pathophysiologies further impair cognition, behaviour, execution, and memory. Further, mAChR1 reduction due to cerebrovascular occlusion results in cognitive dysfunction in VaD [33]. In addition, D1R reduction in the hippocampal dentate gyrus in a VaD rat model resulted in learning and memory deficits [34]. The γ-aminobutyric acid receptor (GABAR) in the dentate gyrus regulates synaptic plasticity, learning, and memory. In a VaD rat hippocampus, both GABAR1 and GABAR2 were found to be reduced, which resulted in spatial learning and memory deficits [35].
The 5-hydroxytryptamine receptors (5-HTRs) are prevalent in the frontal and temporal cortices [36] and are key players in cognitive function and memory formation. In particular, the 5-HT1A and 5-HT2A receptors are related to the correlation and preservation of cognition [37]. Reduced 5-HT1A receptors in AD brains leads to cognitive impairments [8,38] and could also affect VaD as well. Hence, although GPCRs have not yet been studied extensively in VaD models, GPCRs could be potential therapeutic targets in VaD as well.
2.3. Frontotemporal Dementia
Frontotemporal dementia (FTD) is a special type of dementia affecting both the front and sides of the brain. FTD causes verbal, executive, and behavioural impairments. FTD is mostly diagnosed in people younger than 65 [9], with the disease progression starting at ~45 years of age. As in other types of dementia, the patient’s condition worsens with age. FTD, in some cases, also has a microtubule-associated-protein-tau-based pathology. The mutations in the tau gene lead to different forms of tauopathy [39], including progressive supranuclear palsy and corticobasal degeneration, although most FTD patients do not show apparent tau gene or protein anomalies [39]. Clinical management of FTD is quite challenging, and it is important to understand the neurobiological substrate in order to ease the process. The role of GPCRs in FTD-induced cognitive deficits makes them an interesting target in terms of halting the disease’s progression.
FTD is a combination of impairments in neuropsychiatry and social behaviour. A recent study demonstrated that the neuropeptide oxytocin impacts social cognition and behaviour in FTD patients [40]. Oxytocin and vasopressin, another neuropeptide, are important in social cognition, trust, behaviour, and facial expressions. These neuropeptides are related to the pituitary and synthesised in the hypothalamus, primarily in large magnocellular neurons located in the supraoptic and paraventricular nuclei [41]. They are released into various brain regions, such as the amygdala and anterior cingulate cortex, for the paracrine signalling of the oxytocin receptor (OXTR) and vasopressin receptor (AVPR1a), and these receptors have a pathophysiological impact on FTD [42]. Intranasal vasopressin administration improves social cognition and facial expressions in humans, especially in the eye region [42]. Oxytocin administration in a similar paradigm also improved facial expressions and social interactions [42]. The presence of oxytocin also facilitates GABAergic synapse development, which blocks fear and anxiety [8,43].
Several serotonin receptors, including 5-HT1A and 5-HT2A, decline in the anterior cingulate cortex [44], orbitofrontal and medial prefrontal cortexes [45], and the frontal and temporal cortexes [44] of FTD patients. However, in AD patients, the levels of the 5-HT1A and 5-HT2A receptors fall in the hippocampus and prefrontal cortex region, which causes cognitive deficits. In contrast, under similar conditions, the FTD patient develops neuropsychiatric symptoms [38]. Moreover, the mGluR5 and NMDA receptors are colocalized in cortical regions of the brain and are also involved in the formation of receptor-dependent synaptic plasticity [46]. A recent study showed mGluR5 decreases in the paralimbic cortex region of FTD patients and may lead to the neurodegeneration observed [47]. Finally, mGluR5 activation enhances NMDA receptor action, while inhibition of the receptor blocks NMDA receptors [48].
2.4. Parkinson’s Disease
Parkinson’s disease (PD) is another prevalent neurodegenerative disease and is the second most ubiquitous age-linked disorder. The aetiology of PD includes motor and nonmotor dysfunctions [49,50,51]. One-quarter of PD patients are reported to develop mild cognitive impairment (MCI), with declines in memory (13.3%), visuospatial (11.0%), and attention/executive function (10.1%) [52]. A 20-year follow-up study of some newly diagnosed PD patients reported the occurrence of dementia in 83% of the cases [53]. However, most PD patients are diagnosed with elevated dopamine D1 and D2 receptors in the striatum and substantia nigra, which leads to dopamine denervation supersensitivity [54]. A study of PD animals showed that a D1 agonist improved visuospatial accuracy, whereas the use of a D2 antagonist induces attention impairment [55]. Therefore, the crucial roles of D1 and D2 in visuospatial and attentional dysfunction in PD–MCI are well supported. A good number of nondopaminergic transmitters are also affected in PD patients, and these transmitters are considered to contribute to cognitive deficits [56]. A post-mortem brain study of PD reported the loss of the cholinergic system, leading to cognitive decline. The cholinergic deficit is also associated with late-onset dementia in PD, whereas Aβ plaque and Lewy bodies are associated with an earlier onset of dementia [57,58]. Several serotonergic receptors are also found to be associated with cognition PD, such as the 5-HT1B receptor, which has been found to be downregulated in the early stage of PD progression [59].
2.5. Multiple Sclerosis
Within the context of multiple sclerosis’ (MS’s) pathophysiology, around 40–60% of patients complain of cognitive dysfunction [60]. Previously, it was supposed that depression and cognition are two independent domains that persist in MS. Eventually, it was understood that at a certain point in MS-induced depression, MS patients experience severe difficulties with working memory, execution, and information-processing time [61,62,63]. When proinflammatory cytokines in the central nervous system (CNS) increase, less serotonin is released from the synapses, causing the depression and resulting in the malfunction of the noradrenergic and serotonergic circuits. In this context, antidepressant therapeutics alone might not be sufficient to improve cognitive function in MS. For example, a study conducted in 2008 with two groups of MS patients reported only a slight difference between the group being treated with antidepressants and the placebo group. In that study, 57% of MS patients treated with paroxetine (a selective serotonin reuptake inhibitor (SSRI)) received 50% lower depression scores on the Hamilton Rating Scale, while the placebo group reduced their scores by 40% under similar conditions [64].
2.6. Huntington’s Disease
Progressive motor, behavioural, and cognitive impairment are the common features of Huntington’s disease (HD). Cognitive impairment is inevitable in HD. A great deal of evidence indicates that glutamate-mediated excitotoxicity, which includes wide mGluR2 and mGluR5 expression in the neocortical layers, hippocampus, striatum, thalamus/hypothalamus, and cerebellum, is one of the major contributors to HD progression [62,65,66]. It is also evident that mGluR regulates motor function and Huntington (HTT) protein aggregation in HD. A study showed that treatment with the mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reduces hyperactivity and improves motor coordination in R6/2 HD transgenic mice [67]. Genetic deletion of mGluR5 has also been reported to improve motor function and reduce HTT protein aggregation in HdhQ111/Q111 knock-in mice [67]. On the other hand, both D1R and D2R expression declines in the early and late phase of HD [68]. The hyperactivity of dopamine in the early phase of HD, i.e., activation of D2R, increases HTT protein aggregation [69]. Thus, suppressing/inhibiting D2R with an antagonist would decrease HTT protein aggregation and reduce cell death via striatal protection, which may be beneficial in the treatment of early-stage HD and could potentially delay disease progression [70].
3. Therapeutic Targets for Neurodegenerative Disorders Based on Different GPCR Classes
Due to the selectivity in the function, specific distribution, and number of family members, GPCRs possess a potential therapeutic index and are important targets in different neurological conditions; some of the GPCRs listed in Table 1 are therapeutic targets for cognition and memory deficits. The important roles of different GPCRs are discussed below.

Table 1.
Therapeutics for different neurodegenerative disorders—new agents, Phase II or III clinical trials [13].
3.1. 5-HT Receptors
The serotonergic neurotransmitters play a critical role in cognitive and behavioural functions. These receptors are distributed in different brain regions, including the cortex, amygdala, and hippocampus, and are associated with learning and memory [71]. Their roles in cognition and memory have made them important drug targets in neurodegenerative disorders. Some evidence suggests that the use of agonists of the 5-HT subtype, such as 5-HT2A/2C or 5-HT4, can prevent memory impairment and improve learning ability. A similar effect can also be obtained by using an antagonist of 5-HT1A or 5-HT3 and the 5-HT1B receptor [72].
The 5-HT6 receptor (5-HT6R) has been shown to regulate several neurotransmitter pathways, including the serotonergic, cholinergic, glutamatergic, and GABAergic systems [73]. In addition, several studies have established that this receptor is involved in learning and memory processes. These studies also claim that 5-HT6R antagonists could improve cognitive functions; some agents are in the preclinical stage [74,75]. Many of these agents are associated with substantial improvements in different cognitive tasks and enhanced memory retention or formation in rodents [76]. To date, at least three candidates have already reached Phase II/III clinical trials as novel therapeutic agents for the treatment of AD [77]. Indeed, the serotonin receptor 5-HT6R is an attractive drug target for reversing memory loss and learning disabilities associated with NDDs [77,78,79]. A recent study has discovered a new benzimidazole-based compound that is an antagonist of 5-HT6R and improves the novel object identification task in memory-deficit mice [76].
Moreover, in a Phase I trial, PRX-07034, a selective 5-HT6R antagonist, showed high selectivity for 5-HT6Rs over other 5-HT receptors and nonserotonin receptors. This derivative at doses of 1 and 3 mg/kg enhanced short-term memory and improved cognitive flexibility in rats [80]. Although Phase II trials have not yet been concluded [81,82], this drug candidate could be useful in treating dementia in AD [83]. Another selective antagonist, AVN-322, which is a derivative of AVN-101 and AVN-211, is undergoing Phase I trials for AD and schizophrenia. This drug has been reported to reverse the detrimental cognitive effects of scopolamine and MK-80 [84]. The AVN-101 is a multitarget serotonin antagonist that blocks 5-HT7R and has a low, but potential, affinity towards 5-HT6, 5-HT2A, 5-HT2C, and adrenergic receptors. It is also a dynamic candidate that is an antagonist of both 5-HT6 and 5-HT7. Both receptors follow the same signal transduction pathway, which is important for learning, memory, and anxiety. After completing Phase I trials, the drug is poised to start Phase II trials for AD and anxiety [85]. In addition, the benefit of AVN-101 in treating AD progression is the symptomatic relief of anxiety, depression, sleep disorders, and associated mood disorders [85]. Another derivative, AVN-211, has been reported to be a well-tolerated antagonist at a dose of 20 mg/kg of body weight and is suggested in AD therapy for its positive impact on cognition [86].
Idalopirdine is an antagonist of 5-HT6R that was developed initially for schizophrenia. Later analysis revealed its positive effect on cognition, and it was recommended for use in AD-associated dementia. Idalopirdine blocks 5-HT6R and increases acetylcholine in the CNS. In particular, it increases acetylcholine by inhibiting CYP206, an enzyme that is involved in the metabolism of donepezil. Therefore, cotreatment with donepezil increases donepezil’s bioavailability [87]. During a trial, 90 mg of idalopirdine taken daily with donepezil (10 mg) improved cognitive function significantly [87]. An AChE inhibitor, donepezil, and an NMDAR inhibitor, memantine, were used in a clinical trial involving VaD patients and resulted in cognitive improvement [88]. Monotherapy with intepirdine was found to be well tolerated, but its efficacy was lower than expected in AD. Further, the results of different studies were inconsistent. For instance, one study found a significant change in global function but not cognition [89], while another report failed to find any significant improvements [90,91].
Latrepirdine, or Dimebon, is an antihistamine developed originally for allergic rhinitis. Later, the drug was found to have procognitive effects, which are the result of antagonising 5-HT6R. Latrepirdine was also reported to stabilise mitochondria and works as a neuroprotective drug [82]. Based on fact and figures, latrepirdine was recommended for clinical trials in both AD and HD in the 2000s [82]. After completion of a small pilot study, the drug passed its Phase I and II clinical trials. However, in the Phase III trial, use of latrepirdine was terminated due to lack of efficacy [82,92]. SUVN-502 is another selective 5-HT6R antagonist. After completing Phase I trials with no notable adverse effects and better tolerance [93], the drug is currently in Phase II clinical trials involving patients with moderate AD [82]. This drug has been reported to promote acetylcholine and glutamate in the CNS when administered in combination with donepezil [94,95]. Agomelatine, a 5-HT2C antagonist, is another drug undergoing clinical trials that promises to improve prefrontal dopaminergic tone in FTD patients [96]. One clinical trial involving agomelatine reported that it reduces depression in AD patients significantly and decreases depression and motor symptoms in PD patients [97,98]. These data suggest that the 5-HT2C receptor could also be an important target in neurodegenerative disorder therapy.
Tryptophan (TRP) is an essential amino acid and serotonin precursor, and its depletion can disrupt serotonin synthesis in the brain. That depletion could also produce a paradigm of the serotonin deficit models, which has been demonstrated in different animals, including mice [99], rats [100], primates [101], and humans [102]. Several studies have assessed the relation between TRP depletion and cognitive function. For instance, Mendelsohn et al. [103] used a diet lacking in TRP but enriched in large neutral amino acids to produce an almost 50% downregulation of 5-HT levels in the cortex, striatum, and hippocampus [103]. That study, however, did not find any significant effect of TRP depletion on spatial, episodic, or working memory and instead reported semantic memory improvement even in the depleted state [103].
Moreover, despite the modest effect of TRP depletion on sustained attention, such as vigilance, it does not significantly affect specific impaired executive functions such as planning, decision making, and responding [103,104]. To the contrary, tryptophan-rich supplementation of a regular diet can enhance serotonin synthesis, and a clinical study found that tryptophan supplementation improved reaction times, visual memory, and attention [105]. Chronic ingestion of docosahexaenoic acid phospholipids with melatonin and tryptophan for 12 weeks also improves mild cognition impairment in elderly patients [106].
Vortioxetine [13], a serotonin transporter inhibitor (SERT), also works as an antagonist of 5-HT3, 5-HT7, and 5-HT1D receptors, an agonist of 5-HT1A, and a partial agonist of 5-HT1B. A recent meta-analysis of a 6–8-week treatment with vortioxetine (5-20 mg/day) produced an incremental reduction in depression symptoms, and an increasing effect was associated with an increase in dose [107]. Vortioxetine was also shown to improve cognitive performance in patients with acute major depressive disorder [107]. Similarly, a study involving chronic citalopram use (20 mg; single dose daily) resulted in improved impulse response and contextual information processing abilities on a delayed nonmatching to sample task (DNMST) in healthy controls (n = 20). Acute administration (24 hr) of that drug showed no effect on working memory or impulsive responses. The authors of the study also suggested that DNMST makes a contribution to the activation of 5-HT1A receptors in the entorhinal cortex and hippocampus [108].
Chronic consumption of a diet high in saturated fats and low in fibre is associated with obesity and cognitive decline. In this context, dietary supplementation can prevent cognitive decline by altering serotoninergic signalling in the brain. A recent study with high-fat, low-fibre (5% dietary fibre)-induced obese models has demonstrated an association with the upregulation of 5-HT1AR and 5-HT2AR binding density in the rat brain in comparison to the low-fat diet group [109]. With the inclusion of galacto-oligosaccharides and resistant starch, receptor binding densities in the hippocampal and hypothalamic region are reduced, improving cognitive function.
3.2. Dopamine Receptors
In the late 1950s, Carlsson identified dopamine as a potential neurotransmitter in the brain. Later, it was discovered that a progressive decrease in dopamine is associated with the pathophysiology of PD [51,110,111]. This finding introduced levodopa, the metabolic precursor of dopamine, into the symptomatic treatment of PD. In the past few years, several investigations have been conducted on dopamine and cognitive function [112,113,114,115]. Although dopamine neurons are very few in number compared to the total neuronal population in the brain (< 1/100,000), they are involved in neuroendocrine regulation, mood, motivation, and psychological processes, including working memory and learning [111,112]. Although no specific mechanism has yet been confirmed, the inhibition of tyrosine hydroxylase activity and tyrosine conversion into dopamine and norepinephrine are involved in long-term memory formation [116]. For example, vasopressin is a hypothalamic neuropeptide found to improve memory and does so by interacting with dopamine in the amygdala and serotonin of the hippocampus [117].
As previously mentioned, some evidence suggests that dopamine modulates working memory, but its specific role is not yet fully defined, and its effect in memory processing has not been clarified. Haloperidol, a D2R antagonist, has recently been investigated for its effect on working memory improvement as well as for its distracting-information-ignoring capability. The study was designed with two testing sessions. In one session, participants took a placebo tablet, and in the other, they took Haloperidol 2.5 mg [118]. The study showed that the deleterious effect of haloperidol on response conflict is associated with the negative effect of the drug on ignoring. The authors also suggest that D2R protects memory content from distraction through a general process, and inhibition of D2R could result in the impairment of response conflict as well as reduced quality of recall [118]. D1R and D2R have been found to be important targets in FTD as well. Both DR antagonists (antipsychotics) and agonists (specifically, D2R) are used frequently in FTD treatment. Commonly used antipsychotics, such as olanzapine, quetiapine, and risperidone, have a high affinity for D2R and dissociate rapidly, resulting in very few side effects [119]. A lack of presynaptic dopaminergic nerve terminal and postsynaptic D2R binding in the striatum is prevalent in FTD patients, and most of them complain of rigidity and bradykinesia. Therefore, FTD patients are currently being treated with the DR agonists carbidopa and levodopa to improve behavioural and psychotic symptoms.
However, SK609, a recently designed selective-small-molecule agonist of D3R, selectively inhibits norepinephrine reuptake as well as increases dopamine by 160% at an i.p. (intraperitoneal) dose of 4 mg/kg. SK609 improved rats’ performance in an attention task. Additionally, SK609 has been reported to improve cognitive function in low-performing rats. Interestingly, the molecule did not produce any side effects mediated by DA transporter (DAT) activity [120], such as spontaneous locomotor activity.
3.3. Cannabinoid Receptors
Extensive evidence indicates that the endocannabinoid system has modulatory effects on cognitive function, and these effects have a substantial role in memory acquisition, consolidation, and extinction [121]. However, the introduction of cannabinoid drugs often induces opposite effects, when used in anxiety, cognition, and other behavioural deficiencies, depending on the stress level and the aversiveness of the context [121,122]. It has been demonstrated that the use of an endocannabinoid receptor inhibitor under different environmental conditions has a substantial influence on cognitive function without affecting locomotor or emotional behaviour [122]. Although it is difficult to define the exact role of the cannabinoid receptor, cannabinoid signalling influences memory processing. This influence has been demonstrated in several studies [121,122,123].
In particular, Δ9-tetrahydrocannabinol (THC) is a cannabinoid receptor agonist that acts at CB1R to produce a wide range of biological and behavioural activity. This ligand’s association with cognitive deficits and poor decision making has been identified. Using Wistar rats in a rat gambling task study, it has been reported that a high dose of THC reduces premature responses, while another synthetic agonist produced the opposite reaction [124]. Although acute or limited chronic use of THC does not affect subjects, long-term exposure can impair impulse control and attentional function [125]. Therefore, chronic activation of CB1R or antagonism can impair or improve task performance, which makes it an interesting target. For example, in an animal study, the CB1 antagonist rimonabant produced an improvement in decision making [124]. Meanwhile, cannabinoid receptor agonists, such as AEA (N-arachidonoyl ethanolamine) and noladin, have been reported to protect against Aβ-induced neurotoxicity in the human teratocarcinoma cell line NTERA-2/cl-D1. The effect was exerted through the CB1R- and mitogen-activated protein kinase (MAPK) pathway [126]. Treatment with the CB1R antagonist SR141716A failed to protect against Aβ-induced amnesia [127]. Another study with a triple transgenic mouse model of AD (3xTg-AD) reported that CB1R is up-regulated in the anterior thalamus at the age of 4 months, while the CB1R activity decreased gradually in the nucleus basalis of Meynert at 15 months of age [128].
Activation of CB1R and CB2R was reported to affect the upregulation of PPARγ signalling in an animal model study [129], where Aβ-induced neuroinflammation, neurodegeneration, and spatial memory impairment was attenuated. Activation of CB2R using a lower-dose agonist, JWH-015, eradicated native Aβ from human tissue and cleared a synthetic pathogenic Aβ peptide in a human macrophage cell line (THP-1). This effect was attributed to the antagonist SR144528, which is selective for CB2R, and the plaque removal effect induced by JWH-015 was reversed [130]. A selective CB2R agonist 1-((3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl) carbonyl) piperidine (MDA7) has shown a modulatory effect on cognitive impairment induced by bilateral microinjection of Aβ (1-40) fibrils into the hippocampal CA1 area of rats. Intraperitoneal treatment of MDA7 (15 mg/kg) for 14 days reduced microglial CD11b expression, promoted Aβ clearance, and restored synaptic plasticity, cognition, and memory [131].
The increased expression of CB2R has been noted with reduced CB1R in late-onset HD, mainly in glial cells [132]. The selective CB2R antagonist, SR144528, provides striatal neuron protection in HD rats; the mechanism includes glial cells [133]. Genetic deletion of CB2R also exacerbates HD, but CB2R-selective agonists can reduce striatal neurodegeneration through microglial activation [134]. SR141716 has also been reported to aggravate malonate-induced striatal pathology in HD rats [135]. However, some studies report that cannabidiol, a phytoconstituent and an allosteric modulator of CB1R [136], can rescue neuronal loss induced by THC by preventing THC-induced CB1R loss [137]. Similarly, VCE-003.2, a cannabigerol derivative, improved the antioxidant barrier in 3-nitropropionic-acid-induced HD mice brains [138]. This agent also showed neuroprotection in SOD1G93A transgenic amyotrophic lateral sclerosis (ALS) mice by targeting CB2R and inhibiting endocannabinoid inactivation [139]. Based on different reports, it is speculated that CBR antagonists may provide a better therapeutic insight into HD treatment.
3.4. Cholinergic Receptors
Cholinergic receptors are divided into two classes, including the muscarinic and nicotinic receptor families. These two families are further subclassed according to the occurrence of many ACh receptor subtypes, and their differential dendritic, somatic, axonal, and synaptic localisation contributes to the varied roles that these receptors play in the CNS [140]. Five subtypes of mAChRs (M1 to M5) have been defined and pharmacologically characterized in the CNS. Their expression has been detected at very high levels in the subcortical structures and the cerebral cortex [141]. A modest level of mAChR expression was also reported in the frontal cortex, parietal cortex, temporal cortex, entorhinal cortex, occipital cortex, and insular and cingulate cortexes, with the highest values recorded for the temporal and occipital cortexes [141]. M1 receptors are the most abundantly expressed mAChRs [141].
Nicotinic AChRs (nAChRs) constitute the second cholinergic receptor type. This type of receptor relies on ligand-gated ion channels and contains five subunits, which are assembled into homomeric or heteromeric subunit combinations. The pharmacological and biological characteristics of these receptors are determined by this combination [142]. The nAChR subunits are composed of α4, α6, α7, β2, and β3 subunits and are mostly expressed in the striatum [143,144]. They are expressed on glutamatergic and dopaminergic neuron terminals, GABAergic interneurons, and cholinergic interneurons (ChIs) but are not present in medium spiny neurons (MSNs) [145,146]. However, nAChRs can express in both pre- and post-synaptic neurons. Thus, they can depolarise and increase excitability, causing glutamate, DA, and GABA to release [147,148].
The nAChRs form good targets in the treatment of neurodegeneration. In recent years, several agonists and partial agonists of the α7 subunit have been evaluated in the treatment of NDDs. Daily nicotine administration in a PD animal model produced improvements in motor coordination. That treatment also showed a beneficial effect on neuronal survival, as well as on microglial and astrocytic activation, providing neuroprotection against MPTP/MPP+ toxicity [149]. DMXBA (GTS-21) and ABT-107, both α7nAChR agonists, have also produced beneficial effects by attenuating nigrostriatal damage in 6-hydroxydopamine (6-OHDA)-induced rats [51]. Another agonist, PHA 543613, attenuates early-stage HD induced by striatal quinolinic acid lesions. PHA 543613 reduced microglial activation to protect neurons [150]. In another study, α7nAChR-agonist-treated 3xTg-AD mice showed improved cognitive function [151]. However, PNU-282987 has also produced improved motor activity, anxiety, and learning and memory development in the B6C3-Tg mice model for AD [152]. Similarly, two other agonists, AR-R17779 and ABBF (N-[(3R)-1-azabicyclo [2.2.2]oct-3-yl]-7-[2-(methoxy) phenyl]-1-benzofuran-2-carboxamide), are also reported to improve learning and memory [153,154].
ANAVEX2-73, a σ1R (sigma 1 receptor) agonist and muscarinic receptor ligand, has been studied on the Aβ25-35-injected AD mice model [155]. Because Aβ25-35 injection modulates mitochondrial respiration in the hippocampus, ANAVEX2-73 (0.01-1 mg/kg IP) restored respiration to normal and prevented Aβ25-35-induced increases in lipid peroxidation levels, Bax/Bcl-2 ratio, and cytochrome c release into the cytosol [155].
3.5. Metabotropic Glutamate Receptors
Metabotropic glutamate receptors (mGluRs) are members of the C GPCR family, and they consist of eight subtypes (mGlu1 to mGlu8) that are further subdivided into three groups depending on their amino acid sequences, G-protein coupling, and pharmacological characteristics. Group I consists of mGlu1 and mGlu5 receptors, which are coupled to Gq/G11 [156]. The Group II (mGlu2, mGlu3) and Group III (mGlu4, mGlu6, mGlu7, mGlu8) subtypes are coupled to Gi/Go. Both of these groups regulate adenylate cyclase negatively and can also activate the MAP kinase and PI-3-kinase pathways [156,157]. However, overexpression of mGlu5 has been reported in different neurodegenerative disorders [157]. Specifically, Aβ plaques have been found in the surroundings of astrocytes, spinal cord lesions, and MS lesions, ALS, PD, and in the hippocampal astrocytes of Down syndrome patients [158,159].
Several mGluRs agonists, antagonists, and positive and negative allosteric modulators have been studied using different neurodegenerative animal models. Collectively, these data on mGluRs provide an insight into the development of therapeutics for treating NDDs. However, LY341495 (an antagonist to Group I/II mGluR) was reported as blocking Aβ-enhanced long-term depression and improving synaptic plasticity [160]. The same study also reported that pretreatment with an mGluR1/5 agonist, 3,5-dihydroxyphenylglycine (DHPG), decreased Aβ-enhanced long-term depression [160]. SIB1757, a noncompetitive antagonist of mGluR5, prevented Aβ oligomer-induced synaptic N-Methyl-D-aspartic acid receptor NMDAR reduction [161]. A comparison study targeting Group II mGluRs showed that an mGlu2R positive allosteric modulator, N-4′-cyano-biphenyl-3-yl)-N-(3-pyridinylmethyl)-ethanesulfonamide hydrochloride (LY566332), amplified Aβ-induced neurodegeneration [162]. Treatment with the antagonist (2S,1′S,2′S)-2-(9-xanthylmethyl)-2-(2′-carboxycyclopropyl) glycine (LY341495) of mGlu2/3R prevented this effect. Similarly, the dual mGlu2/3 receptor agonist (−)-2-oxa-4-aminobicyclo [3.1.0] exhane-4,6-dicarboxylic acid (LY379268) exhibited neuroprotection via a paracrine mechanism mediated by transforming growth factor-β1 [162]. Therefore, dual activation of mGlu2R and mGlu3R may be a prime target for providing neuroprotection against Aβ-induced toxicity, and negative modulation of mGlu5R would also be a good target for PD and AD treatment.
3.6. Orphan GPCRs
More than 140 GPCRs remain mysterious and are referred to as ‘orphans’ (oGPCRs); most of them do not have any known ligands. Although very little is known about their endogenous or exogenous ligands, these so-called oGPCRS have gained considerable attention as drug targets [163]. Several findings have postulated the critical role of oGPCRs in the cognitive deficits in disorders such as AD and schizophrenia [164,165,166,167]. For example, an expression map of the mouse brain has listed 78 oGPCRs and showed that many of them are relevant to cognition, motivation, and emotional processing [168]. That study reported that oGPCRs, such as GPR17, GPR27, GPR37, GPR39, GPR63, GPR85, GPR88, GPR123 (Adgra1), GPR125 (Adgra3), GPR153, GPR176, and GPRc5c, are highly expressed in the prefrontal cortex (PFC) region of the mouse brain, which is involved in cognition and learning. Both mouse and human brain analyses have shown that four oGPCRs are highly expressed, including GPR88, GPR123, GPR149, and GPR151, but information about these oGPCRs is minimal, with the exception of GPR88 [168].
However, GPR3 has exhibited stable and enhanced expression throughout the ageing process in ten different regions of the healthy human brain, which has been linked to AD pathogenesis in multiple cohort studies [166]. Additionally, GPR3, independent of G-protein coupling, recruits β-arrestin 2 and promotes γ-secretase activity, which increases Aβ precursor protein (APP) cleavage and accelerates Aβ peptide production and accumulation [169]. Thus, deletion of GPR3 in the AD mouse alleviated cognitive impairment and restored memory [166]. Although GPR158 improves memory through transducing osteocalcin and regulating IP3 and BDNF in the CA3 neurons [167], GPR158 overexpression in PFC has a potential role in depressive behaviour, which is reversed by its depletion [170].
GPR52 is a nonodorant GPCR and is colocalized with both D1 and D2 receptors in the basal ganglia neurons. Histological experiments suggest that GPR52 promotes and regulates the Cre-lox system and may also modulate cognition and emotion, as it has involvement with both dopaminergic and glutaminergic neurotransmission [171]. Thus, the incorporation of the GPR52 antagonist may potentiate cognitive improvement and exert anxiolytic activity in psychiatric disorders [171]. GPR3 knockout also produced anxiety and depressive behaviour, with no noticeable locomotor impairment under stressful conditions. However, the lack of GPR3 has no preventive action in the learning involved in fear memory in a similar stressful condition in mice [172]. GPR3 also regulates serotonin (5-HT) and dopamine (DA) synthesis and reuptake, which makes it a primary target as well. A study has reported the possibility that serotonin reduction in the frontal cortex and hippocampus causes aggressive behaviours in GPR3 knockout mice [172]. This finding indicates that GPR3 modulates the serotonergic and dopaminergic system, which makes it a potential target in the therapy of AD or schizophrenia.
GPR55 is highly expressed in the pyramidal cells in the hippocampal CA1 and CA3 layers and modulates the synaptic plasticity of pyramidal cells [173]. However, GPR85 is highly expressed in the dentate gyrus region of the hippocampus [174,175] and prominently expresses in the phases of neuronal differentiation in the developing cerebral cortex [176]. This expression suggests a possible role of GPR85 in cognition, and this receptor could become a potential drug target as well.
4. An Emerging Paradigm in the Development of Therapeutics for Neurodegenerative Disorders
4.1. Allosteric Modulators of GPCRs in the Treatment of Neurodegeneration
Allosteric ligands bind to GPCRs at their endogenous ligand-binding sites. This binding is distinct from the conventional regulation of the downstream GPCR effect due to the interaction between agonists and ligand-binding pockets (Figure 2) [177]. Allosteric ligands provide an opportunity to manipulate the GPCR functions for potential therapeutic benefit. However, their complex actions are challenging for new drug screening and development. Several studies focusing on areas such as biased signalling by allosteric ligands have exploited the interaction mechanisms between allosteric ligands and GPCRs, and learning how these interactions modulate the effects would be beneficial for drug discovery.
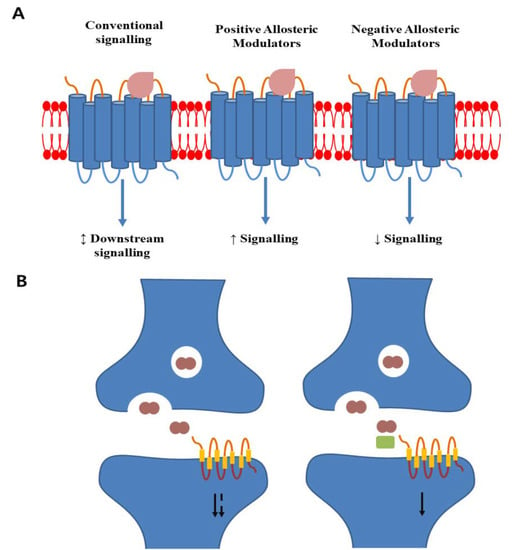
Figure 2.
Schematic display of allosteric modulator action on GPCRs. (A) Conventional agonist binding makes conformational changes and activates downstream signalling. Positive allosteric modulators bind to a distinct site and enhance conventional ligand-induced signalling. Negative allosteric modulators binding decreases conventional agonist efficacy and reduces downstream signalling. (B) In normal physiology, neurotransmitters are released into the synaptic cleft, binding to postsynaptic GPCRs, and activating downstream signalling. The duration of signalling can be degraded by metabolizing enzymes. A positive allosteric modulator (green rectangle) cobinding with the metabolites can extend the duration of receptor activation and enhance signalling (based on [177]).
Allosteric modulators include ions, ligands, small and large molecules, and protein complexes. They could become favourable pharmaceutical products if developed into low-molecular-weight, nonpeptidic molecules able to cross the blood–brain barrier readily. Allosteric modulators are divided into two major categories based on receptor signalling, i.e., positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) (Figure 2). They neither activate nor inhibit the receptors, unlike the ligand. They do not bind to the conventional binding site but instead bind to a site that is distinct and highly diverse from the active site. Therefore, PAMs and NAMs could reduce side effects, maintain natural rhythm, and control the potency and efficacy of the drug response [178]. Furthermore, recent progress in neurodegenerative disorder research, including PD, AD, and cerebellar ataxia research, has turned up a potential disease-modifying treatment via allosterism [179,180]. The mAChRs subclasses M1 and M4 are major targets for schizophrenia, AD, and PD [181,182]. Although the M1/M4 agonist xanomeline showed improved cognitive functions in a Phase III clinical trial for schizophrenia, xanomeline is associated with gastrointestinal side effects, and a PAM could possibly be a potential and safe alternative. Several pharmaceutical studies have identified active M1 PAMs in lower animal models, but the safety margin needs to be confirmed [183,184]. M1 PAM MK-7622 was terminated after a Phase IIa/IIb clinical trial. However, several selective M4 PAMs, including LY2033298, VU0152100, VU0152099, and VU0467485, have been evaluated in preclinical models of schizophrenia [185]. A recent crystallization approach to the M1 and M4 receptors provided a structural basis for understanding PAMs [186], which could be a potential target to develop drugs for AD. However, allosterism in the treatment of neurodegenerative disorders depends on the availability of the allosteric modulators. Many potential targets need to be proven or disproven in the neurodegeneration treatment options provided by the GPCR family. PAMs or NAMs of these members could efficiently reduce neurodegenerative progression and reduce therapeutic side effects. For example, allosteric modulators of several members of mGluR showed symptomatic therapeutic potential in a preclinical study of AD. Development of these modulators for clinical study in humans could change the therapeutic strategy for AD [178].
4.2. Neuropeptides as a Target to Treat Neurodegenerations
Neuropeptides are small amino acid molecules that can regulate neuronal activity. They possess the ability to modulate different functions such as thermoregulation, reproductive behaviour, food and water intake, and circadian rhythms. However, neuropeptides also have a role in inflammatory responses and pain sensitivity. Several neuropeptides have been identified as being related to neuroinflammation, including the proopiomelanocortin (POMC) gene-derived peptides, neuropeptide Y (NPY), vasoactive intestinal peptide (VIP), somatostatin (SST), calcitonin gene-related peptide (CGRP), and cortistatin (CST). Moreover, a mammalian homolog of the nicotinamide adenine dinucleotide (NAD)-dependent deacetylase sirtuin family member, silent information regulator 1 (SIRT1), is related to AD progression. Studies have demonstrated that SIRT1 decreases ROCK1, a serine/threonine Rho kinase, and regulates β-amyloid metabolism by promoting α-secretase enzyme activity. Therefore, SIRT1 promoters could prevent the formation of β-amyloid oligomers and senile plaques by inducing APP processing in the nonamyloidogenic pathway [187]. Natural derivatives, such as resveratrol, oxyresveratrol, and 2,3,4′,5-tetrahydroxystilbene-2-O-b-D-glucoside (TSG), have been reported to enhance or activate SIRT1 and downregulate neurodegeneration [188]. It has also been observed that SIRT1 overexpression decreases the amyloid plaque burden and improves behavioural phenotypes through the deacetylation of retinoic acid receptor β (RARβ), a transcriptional activator of disintegrin- and metalloproteinase-domain-containing protein 10 (ADAM10), that processes APP through the nonamyloidogenic pathway [189]. SIRT1 expression may deacetylate tau proteins directly, diminish the formation of neurofibrillary tangles, and suppress tau-induced pathology [190].
NPY is present in the hippocampus and produced by γ-aminobutyric acid (GABA)-ergic interneurons. NPY is also distributed in the cerebral cortex, hypothalamus, thalamus, brainstem, and cerebellum and plays a major role in the regulation of learning, memory, feeding, and endocrine secretions. Successive reports indicate that NYP expression is reduced with the progression of AD, PD, and HD [191,192]. NYP could protect neuronal cell death from excessive GluRs activation by reducing the Ca2+ influx in the presynaptic nerve terminal through the protein kinase A and p38K pathways [193]. NPY also plays an important role in adult neurogenesis regulation. Spencer et al. (2016) developed a technique to evaluate NYP’s role in neurogenesis by fusing NYP vectors to the brain transport peptide (apolipoprotein B) of an APP-tg mouse model. That study showed that the proliferation of neural precursor cells in the subgranular zone of the hippocampus increased significantly without further differentiation into neurons [194]. NPY-regulated neurogenesis can be observed in the dentate gyrus, caudal subventricular zone (cSVZ), and subcallosal zone via the proliferative effect of Y1 receptors on neuroblasts [195]. Moreover, NYP administration protected neurons from microglia-caused inflammation in the striatum and substantia nigra of 6-OHDA-lesioned rats as a PD model [196]. Thus, NPY could be a potential therapeutic target for preventing neurodegeneration.
Finally, ghrelin, a 28-amino acid peptide that possesses the ability to stimulate growth hormone (GH) release from the pituitary, is highly expressed in the hypothalamus. The ghrelin receptor (GHSR) forms a heterodimer with D1R and D2R and can alter G-protein coupling as well as ligand binding potency [197]. Some evidence suggests that ghrelin exerts neuroprotection against NDDs [197]. Leptin, a cytokine-like 167-amino acid peptide, regulates the production of POMC and NYP at the hypothalamus and can modulate neurogenesis, synaptogenesis, neuronal excitability, and neuroprotection in extrahypothalamic sites [198].
5. Conclusions
NDDs get worse day by day. Unfortunately, their therapeutics are disappointing and limited to symptomatic treatment. However, targeting the underlying pathobiology of the NDDs would disrupt disease progression and improve the conditions [199]. Herein, several GPCRs and their agonists or antagonists under screening or approved for AD, PD, MS, or HD, have been discussed. In addition, allosteric modulation of GPCRs or the targeting of neuropeptides would intervene in neurodegeneration and improve associated cognitive deficits. Interestingly, the number of GPCRs found to be involved in the pathophysiology of NDDs is increasing and widening GPCR-based targets. For example, CB2R expression increases, along with a noticeable reduction in CB1R, in late-onset HD. Thus, CB2R would be a potential target for HD therapy. Furthermore, α7nAChR is also a potential target, and several potential agonists have been assessed. Some of them have moved to clinical trials. Several oGPCRs have been found to be associated with the pathophysiological conditions involved in neurodegeneration. Several studies have concluded and consider oGPCRs to be novel targets for solving neuropsychiatric disorders, including anxiety, depression, and cognition in AD, PD, HD, and schizophrenia.
Neuropeptides, such as NYP, GHSR, POMC, CGRP, and SIRT1, would also be interesting targets for improving cognitive functions. Their association with neurodegenerative disease progression has been well established in the last few years. NYP has a potential therapeutic role in the most prevalent neurodegenerative and neuroimmune diseases and counteracts depressive symptoms in NDDs. Stimulating these neuropeptides causes neuronal survival and neuroproliferation by attenuating neuroinflammation and excitotoxicity and inducing autophagy. Similarly, SIRT1 and GHSR protect against neurodegeneration and improve behavioural phenotypes. However, the roles of different neuropeptides in NDDs and cognitive improvement are more complex and not yet fully uncovered. On the other hand, several potential AMs of GPCRs have been mentioned lately for the treatment of neurodegenerative disorders. Although many potential candidates exist, there is still a need to discover and develop potential compounds for treatment. For example, AMs of mGluRs have shown potential in symptomatic relief. In this case, selective PAMs or NAMs of mGluR7, mGluR3, and mGluR8 would be potential targets. The mGluR5 NAM has also exhibited disease-modifying effects in an AD animal model study. Thus, the development of GPCR-allosteric modulators would be an alternative therapeutic option for neurodegeneration and cognitive deficits.
A combinatorial therapeutic approach based on disease progression and variety would also be beneficial. Multiple GPCRs would be targeted to either slow down or stop disease progression. For example, inhibition of both A2AR (adenosine 2A receptor) and the corticotrophin-releasing hormone receptor 1 (CRHR1) by inhibitors may improve cognitive function and reduce depression in AD patients. At the same time, combination therapy could overcome monotherapy-associated side effects. Indeed, an in-depth mechanistic study is required to clarify the interactions between different neurochemical pathways in combinatorial therapy. As such, the combination of L-DOPA and the A2AR antagonist istradefylline targets two neurochemical pathways. Istradefylline reduces the relevant side effects of L-DOPA monotherapy in PD patients. GPR3 regulates both serotonergic and dopaminergic synthesis; thus, targeting this receptor with an agonist would maintain 5-HT and dopamine at normal levels and improve depression and aggressive behaviour. In several neuroinflammation cases, it has been observed that abnormal protein accumulation regulates the pathological changes in the brain and causes mitochondrial dysfunction or oxidative stress. Therefore, neuropeptides, such as vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide, which have been shown to inhibit mitochondrial apoptosis and act on GPCRs, could be an alternative therapeutic option for targeting the GPCRs involved in neuroprotection, thus providing neuronal protection against oxidative stress and inflammation and intervention against NDDs. Collectively, recent and past evidence indicates that targeting either single or multiple GPCRs could potentially intervene against NDDs either by preventing or halting disease progression. Recently discussed neuropeptides and allosteric targets that are directly involved in several aspects of neurodegeneration, such as adhesion GPCRs, could become the next frontier in the development of alternative neurodegenerative treatments [178].
In conclusion, natural active compounds show promising neuroprotective effects in several disease models. The potential of these compounds in terms of acting on GPCRs needs to be studied [5,6,200]. Several recent studies reported that nuclear receptor subfamily 4 group A member 2 [199], NACHT, LRR, and PYD domains containing protein 3 [201], and glutamate receptors [202] have great potential in neuroprotective therapy. The correlation between GPCRs and these target molecules would be a fascinating area of research.
Author Contributions
S.A., D.-K.C., M.J., and I.-S.K. conceptualised and designed the study; D.-K.C. and I.-S.K. also supervised and corresponded. S.A. and M.E.H. reviewed the literature, wrote the manuscript, and compiled the tables. M.E.H., M.J., and S.-H.J. helped in revising the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Basic Science Research Program supported this research through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (Grant No. NRF-2018R1C1B6005129 and 2020R1A2B5B02002032).
Acknowledgments
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2018R1C1B6005129 and 2020R1A2B5B02002032).
Conflicts of Interest
All authors declare no conflicts of interest.
Abbreviations
| 5-HT1AR | 5-hydroxy tryptamine receptor 1A |
| 5-HT2AR | 5-hydroxy tryptamine receptor 2A |
| A2AR | adenosine 2a receptor |
| CB1R | cannabinoid type 1 receptor |
| CB2R | cannabinoid type 2 receptor |
| CRHR1 | corticotrophin-releasing hormone receptor 1 |
| D1R | dopamine D1 receptor |
| D2R | dopamine D2 receptor |
| GABA | γ-aminobutyric acid |
| GPR3 | G-protein-coupled receptor 3 |
| GPR88 | G-protein-coupled receptor 88 |
| GPR55 | G-protein-coupled receptor 55 |
| GPR52 | G-protein-coupled receptor 52 |
| 6-OHDA | 6-hydroxydopamine |
| mGluR | metabotropic glutamate receptors |
| nAChRs | nicotinic acetylcholin receptors |
| mAChR | muscharinic acetylcholin receptors |
| THC | Δ9-tetrahydrocannabinol |
| TRP | tryptophan |
References
- Sachdev, P.; Kalaria, R.; O’Brien, J.; Skoog, I.; Alladi, S.; Black, S.E.; Blacker, D.; Blazer, D.G.; Chen, C.; Chui, H.; et al. Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis. Assoc. Disord. 2014, 28, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Neurodegeneration: What is it and where are we? J. Clin. Investig. 2003, 111, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Azam, S.; Cho, D.-Y.; Haque, M.E.; Kim, I.-S.; Choi, D.-K. The Methanol Extract of Allium cepa L. Protects Inflammatory Markers in LPS-Induced BV-2 Microglial Cells and Upregulates the Antiapoptotic Gene and Antioxidant Enzymes in N27-A Cells. Antioxidants 2019, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Azam, S.; Jo, S.-H.; Kim, I.-S.; Dash, R.; Choi, D.-K. Potential Therapeutic Targets of Quercetin and Its Derivatives: Its Role in the Therapy of Cognitive Impairment. J. Clin. Med. 2019, 8, 1789. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Kim, J.; Karthivashan, G.; Park, S.-Y.; Ganesan, P.; Choi, D.-K. Emerging signals modulating potential of ginseng and its active compounds focusing on neurodegenerative diseases. J. Ginseng Res. 2019, 43, 163–171. [Google Scholar] [CrossRef]
- Jakaria, M.; Haque, M.E.; Kim, J.; Cho, D.Y.; Kim, I.S.; Choi, D.K. Active ginseng components in cognitive impairment: Therapeutic potential and prospects for delivery and clinical study. Oncotarget 2018, 9, 33601–33620. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.-H.; Uddin, M.S.; Kim, I.-S.; Choi, D.-K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Huang, Y.; Todd, N.; Thathiah, A. The role of GPCRs in neurodegenerative diseases: Avenues for therapeutic intervention. Curr. Opin. Pharmacol. 2017, 32, 96–110. [Google Scholar] [CrossRef]
- Warren, J.D.; Rohrer, J.D.; Rossor, M.N. Clinical review. Frontotemporal dementia. BMJ Clin. Res. Ed. 2013, 347, f4827. [Google Scholar] [CrossRef]
- Jicha, G.A.; Nelson, P.T. Management of frontotemporal dementia: Targeting symptom management in such a heterogeneous disease requires a wide range of therapeutic options. Neurodegener. Dis. Manag. 2011, 1, 141–156. [Google Scholar] [CrossRef]
- Gabryelewicz, T.; Masellis, M.; Berdynski, M.; Bilbao, J.M.; Rogaeva, E.; St George-Hyslop, P.; Barczak, A.; Czyzewski, K.; Barcikowska, M.; Wszolek, Z.; et al. Intra-familial clinical heterogeneity due to FTLD-U with TDP-43 proteinopathy caused by a novel deletion in progranulin gene (PGRN). J. Alzheimers Dis. JAD 2010, 22, 11–1133. [Google Scholar] [CrossRef] [PubMed]
- Guerram, M.; Zhang, L.Y.; Jiang, Z.Z. G-protein coupled receptors as therapeutic targets for neurodegenerative and cerebrovascular diseases. Neurochem. Int. 2016, 101, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.G.; Ramos, M.L.S.; Amaro, A.J.; Dias, R.A.; Vieira, S.I. Gi/o-Protein Coupled Receptors in the Aging Brain. Front. Aging Neurosci. 2019, 11. [Google Scholar] [CrossRef]
- Mombaerts, P.J. Genes and ligands for odorant, vomeronasal and taste receptors. Nat. Rev. Neurosci. 2004, 5, 263–278. [Google Scholar] [CrossRef]
- Heng, B.C.; Aubel, D.; Fussenegger, M. An overview of the diverse roles of G-protein coupled receptors (GPCRs) in the pathophysiology of various human diseases. Biotechnol. Adv. 2013, 31, 1676–1694. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A.J. G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Masuram, S.; Schiöth, H.B. The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 9–26. [Google Scholar] [CrossRef]
- Laschet, C.; Dupuis, N.; Hanson, J.J. The G protein-coupled receptors deorphanization landscape. Biochem. Pharmacol. 2018, 153, 62–74. [Google Scholar] [CrossRef]
- Barthet, G.; Framery, B.; Gaven, F.; Pellissier, L.; Reiter, E.; Claeysen, S.; Bockaert, J.; Dumuis, A. 5-hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on Src activation but not on G protein or beta-arrestin signaling. Mol. Biol. Cell 2007, 18, 1979–1991. [Google Scholar] [CrossRef]
- Maletínská, L.; Popelová, A.; Železná, B.; Bencze, M.; Kuneš, J. The impact of anorexigenic peptides in experimental models of Alzheimer’s disease pathology. J. Endocrinol. 2019, 240, R47. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The therapeutics of Alzheimer’s disease: Where we stand and where we are heading. Ann. Neurol. 2013, 74, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Farlow, M.R.; Graham, S.M.; Alva, G. Memantine for the treatment of Alzheimer’s disease: Tolerability and safety data from clinical trials. Drug Saf. 2008, 31, 577–585. [Google Scholar] [CrossRef]
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef]
- Hamilton, A.; Esseltine, J.L.; DeVries, R.A.; Cregan, S.P.; Ferguson, S.S. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer’s disease. Mol. Brain 2014, 7, 40. [Google Scholar] [CrossRef]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef]
- Lo, A.C.; De Maeyer, J.H.; Vermaercke, B.; Callaerts-Vegh, Z.; Schuurkes, J.A.; D’Hooge, R. SSP-002392, a new 5-HT4 receptor agonist, dose-dependently reverses scopolamine-induced learning and memory impairments in C57Bl/6 mice. Neuropharmacology 2014, 85, 178–189. [Google Scholar] [CrossRef]
- Zhang, G.; Ásgeirsdóttir, H.N.; Cohen, S.J.; Munchow, A.H.; Barrera, M.P.; Stackman, R.W., Jr. Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology 2013, 64, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. The pathology of “vascular dementia”: A critical update. J. Alzheimers Dis. JAD 2008, 14, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.-I.; Ogawa, N.; Asanuma, M.; Kondo, Y.; Nomura, M.J. Relationship between cholinergic dysfunction and discrimination learning disabilities in Wistar rats following chronic cerebral hypoperfusion. Brain Res. 1996, 729, 55–65. [Google Scholar] [CrossRef]
- Wan, P.; Wang, S.; Zhang, Y.; Lv, J.; Jin, Q.J. Involvement of dopamine D1 receptors of the hippocampal dentate gyrus in spatial learning and memory deficits in a rat model of vascular dementia. Die Pharm. Int. J. Pharm. Sci. 2014, 69, 709–710. [Google Scholar]
- Li, C.-J.; Lu, Y.; Zhou, M.; Zong, X.-G.; Li, C.; Xu, X.-L.; Guo, L.-J.; Lu, Q.J. Activation of GABA B receptors ameliorates cognitive impairment via restoring the balance of HCN1/HCN2 surface expression in the hippocampal CA1 area in rats with chronic cerebral hypoperfusion. Mol. Neurobiol. 2014, 50, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Glikmann-Johnston, Y.; Saling, M.M.; Reutens, D.C.; Stout, J.C.J. Hippocampal 5-HT1A receptor and spatial learning and memory. Front. Pharmacol. 2015, 6, 289. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.S.; Ballard, C.G.; Kalaria, R.N.; Perry, R.; Hortobagyi, T.; Francis, P.T. Increased binding to 5-HT1A and 5-HT2A receptors is associated with large vessel infarction and relative preservation of cognition. Brain 2009, 132, 1858–1865. [Google Scholar] [CrossRef]
- King, M.V.; Marsden, C.A.; Fone, K.C.J. A role for the 5-HT1A, 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol. Sci. 2008, 29, 482–492. [Google Scholar] [CrossRef]
- Neary, D.; Snowden, J.; Mann, D. Frontotemporal dementia. Lancet Neurol. 2005, 4, 771–780. [Google Scholar] [CrossRef]
- Jesso, S.; Morlog, D.; Ross, S.; Pell, M.D.; Pasternak, S.H.; Mitchell, D.G.; Kertesz, A.; Finger, E.C. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain J. Neurol. 2011, 134, 2493–2501. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, Z.R.; Young, L.J. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Theodosis, D.T.; Koksma, J.-J.; Trailin, A.; Langle, S.L.; Piet, R.; Lodder, J.C.; Timmerman, J.; Mansvelder, H.; Poulain, D.A.; Oliet, S.H.R.; et al. Oxytocin and estrogen promote rapid formation of functional GABA synapses in the adult supraoptic nucleus. Mol. Cell. Neurosci. 2006, 31, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, M.; Anchisi, D.; Pelati, O.; Zuffi, M.; Matarrese, M.; Moresco, R.M.; Fazio, F.; Perani, D. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann. Neurol. 2005, 57, 216–225. [Google Scholar] [CrossRef]
- Bowen, D.; Procter, A.; Mann, D.; Snowden, J.; Esiri, M.; Neary, D.; Francis, P. Imbalance of a serotonergic system in frontotemporal dementia: Implication for pharmacotherapy. Psychopharmacology 2008, 196, 603–610. [Google Scholar] [CrossRef]
- Alagarsamy, S.; Rouse, S.T.; Junge, C.; Hubert, G.; Gutman, D.; Smith, Y.; Conn, P.J. NMDA-induced phosphorylation and regulation of mGluR5. Pharmacol. Biochem. Behav. 2002, 73, 299–306. [Google Scholar] [CrossRef]
- Leuzy, A.; Zimmer, E.R.; Dubois, J.; Pruessner, J.; Cooperman, C.; Soucy, J.-P.; Kostikov, A.; Schirmaccher, E.; Désautels, R.; Gauthier, S.J.B.S.; et al. In vivo characterization of metabotropic glutamate receptor type 5 abnormalities in behavioral variant FTD. Brain Struct. Funct. 2016, 221, 1387–1402. [Google Scholar] [CrossRef]
- Homayoun, H.; Moghaddam, B.J. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: Rate-dependent influence and interaction with NMDA receptors. Cereb. Cortex 2005, 16, 93–105. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 13, 318–324. [Google Scholar] [CrossRef]
- More, S.V.; Choi, D.-K. Emerging preclinical pharmacological targets for Parkinson’s disease. Oncotarget 2016, 7, 29835–29863. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Hisahara, S.; Shimohama, S. Dopamine Receptors and Parkinson’s Disease. Int. J. Med. Chem. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Pezze, M.-A.; Dalley, J.W.; Robbins, T.W. Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2007, 32, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 634–650. [Google Scholar] [CrossRef]
- Ballard, C.; Ziabreva, I.; Perry, R.; Larsen, J.P.; O’Brien, J.; McKeith, I.; Perry, E.; Aarsland, D. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 2006, 67, 1931–1934. [Google Scholar] [CrossRef]
- Shimada, H.; Hirano, S.; Shinotoh, H.; Aotsuka, A.; Sato, K.; Tanaka, N.; Ota, T.; Asahina, M.; Fukushi, K.; Kuwabara, S.; et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 2009, 73, 273–278. [Google Scholar] [CrossRef]
- Varrone, A.; Svenningsson, P.; Marklund, P.; Fatouros-Bergman, H.; Forsberg, A.; Halldin, C.; Nilsson, L.G.; Farde, L. 5-HT1B receptor imaging and cognition: A positron emission tomography study in control subjects and Parkinson’s disease patients. Synapse 2015, 69, 365–374. [Google Scholar] [CrossRef]
- Benedict, R.H.; Cookfair, D.; Gavett, R.; Gunther, M.; Munschauer, F.; Garg, N.; Weinstock-Guttman, B. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J. Int. Neuropsychol. Soc. JINS 2006, 12, 549–558. [Google Scholar] [CrossRef]
- Lubrini, G.; Perianez, J.A.; Rios-Lago, M.; Frank, A. Processing speed in relapsing-remitting multiple sclerosis: The role played by the depressive symptoms. Rev. Neurol. 2012, 55, 585–592. [Google Scholar] [PubMed]
- Arnett, P.A.; Higginson, C.I.; Voss, W.D.; Bender, W.I.; Wurst, J.M.; Tippin, J.M. Depression in multiple sclerosis: Relationship to working memory capacity. Neuropsychology 1999, 13, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.; Magalhaes, S.; Richard, J.F.; Audet, B.; Moore, C. The link between multiple sclerosis and depression. Nat. Rev. Neurol. 2014, 10, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ehde, D.M.; Kraft, G.H.; Chwastiak, L.; Sullivan, M.D.; Gibbons, L.E.; Bombardier, C.H.; Wadhwani, R. Efficacy of paroxetine in treating major depressive disorder in persons with multiple sclerosis. Gen. Hosp. Psychiatry 2008, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F.; Ferrante, R.J.; Swartz, K.J.; Kowall, N.W. Chronic quinolinic acid lesions in rats closely resemble Huntington’s disease. J. Neurosci. Off. J. Soc. Neurosci. 1991, 11, 1649–1659. [Google Scholar] [CrossRef]
- Bruno, V.; Ksiazek, I.; Battaglia, G.; Lukic, S.; Leonhardt, T.; Sauer, D.; Gasparini, F.; Kuhn, R.; Nicoletti, F.; Flor, P.J. Selective blockade of metabotropic glutamate receptor subtype 5 is neuroprotective. Neuropharmacology 2000, 39, 2223–2230. [Google Scholar] [CrossRef]
- Schiefer, J.; Sprunken, A.; Puls, C.; Luesse, H.G.; Milkereit, A.; Milkereit, E.; Johann, V.; Kosinski, C.M. The metabotropic glutamate receptor 5 antagonist MPEP and the mGluR2 agonist LY379268 modify disease progression in a transgenic mouse model of Huntington’s disease. Brain Res. 2004, 1019, 246–254. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, E.A.; Cepeda, C.; Levine, M.S. Dopamine imbalance in Huntington’s disease: A mechanism for the lack of behavioral flexibility. Front. Neurosci. 2013, 7, 114. [Google Scholar] [CrossRef]
- Charvin, D.; Vanhoutte, P.; Pages, C.; Borrelli, E.; Caboche, J. Unraveling a role for dopamine in Huntington’s disease: The dual role of reactive oxygen species and D2 receptor stimulation. Proc. Natl. Acad. Sci. USA 2005, 102, 12218–12223. [Google Scholar] [CrossRef]
- Charvin, D.; Roze, E.; Perrin, V.; Deyts, C.; Betuing, S.; Pages, C.; Regulier, E.; Luthi-Carter, R.; Brouillet, E.; Deglon, N.; et al. Haloperidol protects striatal neurons from dysfunction induced by mutated huntingtin in vivo. Neurobiol. Dis. 2008, 29, 22–29. [Google Scholar] [CrossRef]
- Meneses, A. 5-HT system and cognition. Neurosci. Biobehav. Rev. 1999, 23, 1111–1125. [Google Scholar] [CrossRef]
- Buhot, M.C.; Martin, S.; Segu, L. Role of serotonin in memory impairment. Ann. Med. 2000, 32, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Codony, X.; Vela, J.M.; Ramírez, M.J. 5-HT6 receptor and cognition. Curr. Opin. Pharmacol. 2011, 11, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Fone, K.C. An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function. Neuropharmacology 2008, 55, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Meneses, A.; Pérez-García, G.; Ponce-Lopez, T.; Castillo, C. 5-HT6 receptor memory and amnesia: Behavioral pharmacology–learning and memory processes. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 96, pp. 27–47. [Google Scholar]
- González-Vera, J.A.; Medina, R.A.; Martín-Fontecha, M.; Gonzalez, A.; de la Fuente, T.; Vázquez-Villa, H.; García-Cárceles, J.; Botta, J.; McCormick, P.J.; Benhamú, B.; et al. A new serotonin 5-HT6 receptor antagonist with procognitive activity—Importance of a halogen bond interaction to stabilize the binding. Sci. Rep. 2017, 7, 41293. [Google Scholar] [CrossRef] [PubMed]
- Benhamú, B.; Martin-Fontecha, M.; Vázquez-Villa, H.; Pardo, L.; Lopez-Rodriguez, M.L.J. Serotonin 5-HT6 receptor antagonists for the treatment of cognitive deficiency in Alzheimer’s disease. J. Med. Chem. 2014, 57, 7160–7181. [Google Scholar] [CrossRef]
- Ramírez, M.J. 5-HT 6 receptors and Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 15. [Google Scholar]
- Marazziti, D.; Rutigliano, G.; Catena-Dell’Osso, M.; Baroni, S.; Dell’Osso, L. The 5-HT6 receptor antagonism approach in Alzheimer’s disease. Drugs Future 2014, 39, 133–140. [Google Scholar] [CrossRef]
- Mohler, E.G.; Baker, P.M.; Gannon, K.S.; Jones, S.S.; Shacham, S.; Sweeney, J.A.; Ragozzino, M.E. The effects of PRX-07034, a novel 5-HT6 antagonist, on cognitive flexibility and working memory in rats. Psychopharmacology 2012, 220, 687–696. [Google Scholar] [CrossRef]
- Ferrero, H.; Solas, M.; Francis, P.T.; Ramirez, M.J. Serotonin 5-HT6 Receptor Antagonists in Alzheimer’s Disease: Therapeutic Rationale and Current Development Status. CNS Drugs 2017, 31, 19–32. [Google Scholar] [CrossRef]
- Andrews, M.; Tousi, B.; Sabbagh, M.N. 5HT6 Antagonists in the Treatment of Alzheimer’s Dementia: Current Progress. Neurol. Ther. 2018, 7, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Halford, J.C.; Harrold, J.A.; Boyland, E.J.; Lawton, C.L.; Blundell, J.E. Serotonergic drugs: Effects on appetite expression and use for the treatment of obesity. Drugs 2007, 67, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Ivachtchenko, A.V.; Ivanenkov, Y.A.; Veselov, M.S.; Okun, I.M. AVN-322 is a Safe Orally Bio-Available Potent and Highly Selective Antagonist of 5-HT6R with Demonstrated Ability to Improve Impaired Memory in Animal Models. Curr. Alzheimer Res. 2017, 14, 268–294. [Google Scholar] [CrossRef] [PubMed]
- Ivachtchenko, A.V.; Lavrovsky, Y.; Okun, I. AVN-101: A Multi-Target Drug Candidate for the Treatment of CNS Disorders. J. Alzheimers Dis. JAD 2016, 53, 583–620. [Google Scholar] [CrossRef]
- Ivachtchenko, A.V.; Lavrovsky, Y.; Ivanenkov, Y.A. AVN-211, Novel and Highly Selective 5-HT6 Receptor Small Molecule Antagonist, for the Treatment of Alzheimer’s Disease. Mol. Pharm. 2016, 13, 945–963. [Google Scholar] [CrossRef]
- Schneider, L.S. Idalopirdine for Alzheimer’s disease: Written in the stars. Lancet Neurol. 2014, 13, 1063–1065. [Google Scholar] [CrossRef]
- Roman, G.C.; Wilkinson, D.G.; Doody, R.S.; Black, S.E.; Salloway, S.P.; Schindler, R.J. Donepezil in vascular dementia: Combined analysis of two large-scale clinical trials. Dement. Geriatr. Cogn. Disord. 2005, 20, 338–344. [Google Scholar] [CrossRef]
- Maher-Edwards, G.; Zvartau-Hind, M.; Hunter, A.J.; Gold, M.; Hopton, G.; Jacobs, G.; Davy, M.; Williams, P. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer’s disease. Curr. Alzheimer Res. 2010, 7, 374–385. [Google Scholar] [CrossRef]
- Maher-Edwards, G.; Dixon, R.; Hunter, J.; Gold, M.; Hopton, G.; Jacobs, G.; Hunter, J.; Williams, P. SB-742457 and donepezil in Alzheimer disease: A randomized, placebo-controlled study. Int. J. Geriatr. Psychiatry 2011, 26, 536–544. [Google Scholar] [CrossRef]
- Maher-Edwards, G.; Watson, C.; Ascher, J.; Barnett, C.; Boswell, D.; Davies, J.; Fernandez, M.; Kurz, A.; Zanetti, O.; Safirstein, B.; et al. Two randomized controlled trials of SB742457 in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2015, 1, 23–36. [Google Scholar] [CrossRef]
- Doody, R.S.; Gavrilova, S.I.; Sano, M.; Thomas, R.G.; Aisen, P.S.; Bachurin, S.O.; Seely, L.; Hung, D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: A randomised, double-blind, placebo-controlled study. Lancet 2008, 372, 207–215. [Google Scholar] [CrossRef]
- Nirogi, R.; Kandikere, V.; Mudigonda, K.; Bhyrapuneni, G.; Jasti, V. Safety, tolerability and pharmacokinetics of SUVN-502—A 5-HT6 receptor antagonist, first in human phase-1 Single Ascending Dose (SAD) study. Alzheimers Dement. J. Alzheimers Assoc. 2009, 5, P250. [Google Scholar] [CrossRef]
- Nirogi, R.; Ajjala, D.R.; Aleti, R.; Rayapati, L.; Pantangi, H.R.; Boggavarapu, R.K.; Padala, N.S.P. Development and validation of sensitive LC-MS/MS method for the quantification of SUVN-502 and its metabolite and its application for first in human pharmacokinetic study. J. Pharm. Biomed. Anal. 2017, 145, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Shinde, A.; Kambhampati, R.S.; Mohammed, A.R.; Saraf, S.K.; Badange, R.K.; Bandyala, T.R.; Bhatta, V.; Bojja, K.; Reballi, V.J. Discovery and Development of 1-[(2-Bromophenyl) sulfonyl]-5-methoxy-3-[(4-methyl-1-piperazinyl) methyl]-1 H-indole Dimesylate Monohydrate (SUVN-502): A Novel, Potent, Selective and Orally Active Serotonin 6 (5-HT6) Receptor Antagonist for Potential Treatment of Alzheimer’s Disease. J. Med. Chem. 2017, 60, 1843–1859. [Google Scholar] [PubMed]
- Callegari, I.; Mattei, C.; Benassi, F.; Krueger, F.; Grafman, J.; Yaldizli, Ö.; Sassos, D.; Massucco, D.; Scialò, C.; Nobili, F. Agomelatine improves apathy in frontotemporal dementia. Neurodegener. Dis. 2016, 16, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, D.; Pappa, D.; Katirtzoglou, E.; Siarkos, K.; Tzavellas, E.; Papadimitriou, G.; Politis, A. EPA-1197–Agomelatine for treating apathy in alzheimer’s disease. Eur. Psychiatry 2014, 29, 1. [Google Scholar] [CrossRef]
- Avila, A.; Cardona, X.; Martin-Baranera, M.; Leon, L.; Caballol, N.; Millet, P.; Bello, J.J. Agomelatine for depression in Parkinson disease: Additional effect on sleep and motor dysfunction. J. Clin. Psychopharmacol. 2015, 35, 719–723. [Google Scholar] [CrossRef]
- Biskup, C.S.; Sanchez, C.L.; Arrant, A.; Van Swearingen, A.E.; Kuhn, C.; Zepf, F.D. Effects of acute tryptophan depletion on brain serotonin function and concentrations of dopamine and norepinephrine in C57BL/6J and BALB/cJ mice. PLoS ONE 2012, 7, e35916. [Google Scholar] [CrossRef]
- Jans, L.A.; Korte-Bouws, G.A.; Korte, S.M.; Blokland, A. The effects of acute tryptophan depletion on affective behaviour and cognition in Brown Norway and Sprague Dawley rats. J. Psychopharmacol. 2010, 24, 605–614. [Google Scholar] [CrossRef]
- Young, S.N.; Ervin, F.R.; Pihl, R.O.; Finn, P. Biochemical aspects of tryptophan depletion in primates. Psychopharmacology 1989, 98, 508–511. [Google Scholar] [CrossRef]
- Riedel, W.J.; Klaassen, T.; Deutz, N.E.; van Someren, A.; van Praag, H.M. Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology 1999, 141, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, D.; Riedel, W.J.; Sambeth, A. Effects of acute tryptophan depletion on memory, attention and executive functions: A systematic review. Neurosci. Biobehav. Rev. 2009, 33, 926–952. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.L.; Silliman, R.A.; Guadagnoli, E.; Mor, V. The effect of less than definitive care on breast carcinoma recurrence and mortality. Cancer 2000, 89, 1739–1747. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Wittwer, J.; Vargas, K.; Hogan, E.; Holmes, A.; Rogers, P.J.; Goralczyk, R.; Gibson, E.L. Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br. J. Nutr. 2015, 113, 350–365. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Faliva, M.; Mozzoni, M.; Antoniello, N.; Cazzola, R.; Savare, R.; Cerutti, R.; Grossi, E.; Cestaro, B. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr. Neurosci. 2012, 15, 46–54. [Google Scholar] [CrossRef]
- Pae, C.-U.; Wang, S.-M.; Han, C.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Serretti, A. Vortioxetine: A meta-analysis of 12 short-term, randomized, placebo-controlled clinical trials for the treatment of major depressive disorder. J. Psychiatry Neurosci. 2015, 40, 174–186. [Google Scholar] [CrossRef]
- Almeida, S.; Glahn, D.C.; Argyropoulos, S.V.; Frangou, S. Acute citalopram administration may disrupt contextual information processing in healthy males. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2010, 25, 87–91. [Google Scholar] [CrossRef]
- Yu, Y.; Patch, C.; Weston-Green, K.; Zhou, Y.; Zheng, K.; Huang, X.F. Dietary Galacto-Oligosaccharides and Resistant Starch Protect Against Altered CB1 and 5-HT1A and 2A Receptor Densities in Rat Brain: Implications for Preventing Cognitive and Appetite Dysfunction During a High-Fat Diet. Mol. Nutr. Food Res. 2018, 62, e1800422. [Google Scholar] [CrossRef]
- Carlsson, A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol. Rev. 1959, 11, 490–493. [Google Scholar]
- Girault, J.-A.; Greengard, P. The Neurobiology of Dopamine Signaling. Arch. Neurol. 2004, 61, 641–644. [Google Scholar] [CrossRef]
- Westbrook, A.; Braver, T.S. Dopamine Does Double Duty in Motivating Cognitive Effort. Neuron 2016, 89, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Wang, M.; Paspalas, C.D. Dopamine’s Actions in Primate Prefrontal Cortex: Challenges for Treating Cognitive Disorders. Pharmacol. Rev. 2015, 67, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Badgaiyan, R.D.; Dunston, G.M.; Baron, D.; Modestino, E.J.; McLaughlin, T.; Steinberg, B.; Gold, M.S.; Gondre-Lewis, M.C. The DRD2 Taq1A A1 Allele May Magnify the Risk of Alzheimer’s in Aging African-Americans. Mol. Neurobiol. 2018, 55, 5526–5536. [Google Scholar] [CrossRef]
- Karthivashan, G.; Ganesan, P.; Park, S.-Y.; Lee, H.-W.; Choi, D.-K. Lipid-based nanodelivery approaches for dopamine-replacement therapies in Parkinson’s disease: From preclinical to translational studies. Biomaterials 2020, 232, 119704. [Google Scholar] [CrossRef]
- van Wimersma Greidanus, T.B.; Jolles, J.; De Wied, D. Hypothalamic neuropeptides and memory. Acta Neurochir. 1985, 75, 99–105. [Google Scholar] [CrossRef]
- Fallon, S.J.; Muhammed, K.; Drew, D.S.; Ang, Y.-S.; Manohar, S.G.; Husain, M. Dopamine guides competition for cognitive control: Common effects of haloperidol on working memory and response conflict. Cortex 2019, 113, 156–168. [Google Scholar] [CrossRef]
- Kapur, S.; Seeman, P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? A new hypothesis. Am. J. Psychiatry 2001, 158, 360–369. [Google Scholar] [CrossRef]
- Marshall, C.A.; Brodnik, Z.D.; Mortensen, O.V.; Reith, M.E.A.; Shumsky, J.S.; Waterhouse, B.D.; España, R.A.; Kortagere, S. Selective activation of Dopamine D3 receptors and norepinephrine transporter blockade enhances sustained attention. Neuropharmacology 2019, 148, 178–188. [Google Scholar] [CrossRef]
- Morena, M.; Campolongo, P. The endocannabinoid system: An emotional buffer in the modulation of memory function. Neurobiol. Learn. Mem. 2014, 112, 30–43. [Google Scholar] [CrossRef]
- Campolongo, P.; Ratano, P.; Manduca, A.; Scattoni, M.L.; Palmery, M.; Trezza, V.; Cuomo, V. The endocannabinoid transport inhibitor AM404 differentially modulates recognition memory in rats depending on environmental aversiveness. Front. Behav. Neurosci. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, P.; Roozendaal, B.; Trezza, V.; Hauer, D.; Schelling, G.; McGaugh, J.L.; Cuomo, V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc. Natl. Acad. Sci. USA 2009, 106, 4888–4893. [Google Scholar] [CrossRef] [PubMed]
- Ferland, J.-M.N.; Carr, M.R.; Lee, A.M.; Hoogeland, M.E.; Winstanley, C.A.; Pattij, T. Examination of the effects of cannabinoid ligands on decision making in a rat gambling task. Pharmacol. Biochem. Behav. 2018, 170, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Irimia, C.; Polis, I.Y.; Stouffer, D.; Parsons, L.H. Persistent effects of chronic Δ9-THC exposure on motor impulsivity in rats. Psychopharmacology 2015, 232, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Milton, N.G. Anandamide and noladin ether prevent neurotoxicity of the human amyloid-beta peptide. Neurosci. Lett. 2002, 332, 127–130. [Google Scholar] [CrossRef]
- Mazzola, C.; Micale, V.; Drago, F. Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur. J. Pharmacol. 2003, 477, 219–225. [Google Scholar] [CrossRef]
- Manuel, I.; Lombardero, L.; LaFerla, F.M.; Gimenez-Llort, L.; Rodriguez-Puertas, R. Activity of muscarinic, galanin and cannabinoid receptors in the prodromal and advanced stages in the triple transgenic mice model of Alzheimer’s disease. Neuroscience 2016, 329, 284–293. [Google Scholar] [CrossRef]
- Fakhfouri, G.; Ahmadiani, A.; Rahimian, R.; Grolla, A.A.; Moradi, F.; Haeri, A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-gamma pathway. Neuropharmacology 2012, 63, 653–666. [Google Scholar] [CrossRef]
- Tolon, R.M.; Nunez, E.; Pazos, M.R.; Benito, C.; Castillo, A.I.; Martinez-Orgado, J.A.; Romero, J. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Res. 2009, 1283, 148–154. [Google Scholar] [CrossRef]
- Wu, J.; Bie, B.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol. Aging 2013, 34, 791–804. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.; Romero, J.; Velasco, G.; Tolon, R.M.; Ramos, J.A.; Guzman, M. Cannabinoid CB2 receptor: A new target for controlling neural cell survival? Trends Pharmacol. Sci. 2007, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, O.; Gonzalez, S.; Aroyo, I.; Pazos, M.R.; Benito, C.; Lastres-Becker, I.; Romero, J.P.; Tolon, R.M.; Mechoulam, R.; Brouillet, E.; et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: Relevance for Huntington’s disease. Glia 2009, 57, 1154–1167. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Aguado, T.; Pazos, M.R.; Julien, B.; Carrasco, C.; Resel, E.; Sagredo, O.; Benito, C.; Romero, J.; Azcoitia, I.; et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain J. Neurol. 2009, 132, 3152–3164. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Bizat, N.; Boyer, F.; Hantraye, P.; Brouillet, E.; Fernandez-Ruiz, J. Effects of cannabinoids in the rat model of Huntington’s disease generated by an intrastriatal injection of malonate. Neuroreport 2003, 14, 813–816. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Biased Type 1 Cannabinoid Receptor Signaling Influences Neuronal Viability in a Cell Culture Model of Huntington Disease. Mol. Pharmacol. 2016, 89, 364–375. [Google Scholar] [CrossRef]
- Diaz-Alonso, J.; Paraiso-Luna, J.; Navarrete, C.; Del Rio, C.; Cantarero, I.; Palomares, B.; Aguareles, J.; Fernandez-Ruiz, J.; Bellido, M.L.; Pollastro, F.; et al. VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Sci. Rep. 2016, 6, 29789. [Google Scholar] [CrossRef]
- Rodriguez-Cueto, C.; Santos-Garcia, I.; Garcia-Toscano, L.; Espejo-Porras, F.; Bellido, M.; Fernandez-Ruiz, J.; Munoz, E.; de Lago, E. Neuroprotective effects of the cannabigerol quinone derivative VCE-003.2 in SOD1(G93A) transgenic mice, an experimental model of amyotrophic lateral sclerosis. Biochem. Pharmacol. 2018, 157, 217–226. [Google Scholar] [CrossRef]
- Colangelo, C.; Shichkova, P.; Keller, D.; Markram, H.; Ramaswamy, S. Cellular, Synaptic and Network Effects of Acetylcholine in the Neocortex. Front. Neural Circuits 2019, 13. [Google Scholar] [CrossRef]
- Wevers, A. Localisation of pre- and postsynaptic cholinergic markers in the human brain. Behav. Brain Res. 2011, 221, 341–355. [Google Scholar] [CrossRef]
- Dani, J.A. Overview of nicotinic receptors and their roles in the central nervous system. Biol. Psychiatry 2001, 49, 166–174. [Google Scholar] [CrossRef]
- Quik, M.; Bordia, T.; O’Leary, K. Nicotinic receptors as CNS targets for Parkinson’s disease. Biochem. Pharmacol. 2007, 74, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Wonnacott, S. α6β2* and α4β2* Nicotinic Acetylcholine Receptors as Drug Targets for Parkinson’s Disease. J. Pharmacol. Rev. 2011, 63, 938–966. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.B.; Bussert, T.G.; Kreitzer, A.C.; Seal, R.P. Striatal Cholinergic Neurotransmission Requires VGLUT3. J. Neurosci. 2014, 34, 8772–8777. [Google Scholar] [CrossRef] [PubMed]
- English, D.F.; Ibanez-Sandoval, O.; Stark, E.; Tecuapetla, F.; Buzsáki, G.; Deisseroth, K.; Tepper, J.M.; Koos, T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat. Neurosci. 2011, 15, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Patel, J.C.; Cragg, S.J. Dopamine release in the basal ganglia. Neuroscience 2011, 198, 112–137. [Google Scholar] [CrossRef]
- Zhou, F.-M.; Liang, Y.; Dani, J.A. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 2001, 4, 1224–1229. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wu, J.; Zhu, C.; Hui, Y.; Han, Y.; Huang, Z.; Ellsworth, K.; Fan, W. α7 nicotinic acetylcholine receptor-mediated neuroprotection against dopaminergic neuron loss in an MPTP mouse model via inhibition of astrocyte activation. J. Neuroinflammation 2012, 9, 98. [Google Scholar] [CrossRef]
- Foucault-Fruchard, L.; Doméné, A.; Page, G.; Windsor, M.; Emond, P.; Rodrigues, N.; Dollé, F.; Damont, A.; Buron, F.; Routier, S.; et al. Neuroprotective effect of the alpha 7 nicotinic receptor agonist PHA 543613 in an in vivo excitotoxic adult rat model. Neuroscience 2017, 356, 52–63. [Google Scholar] [CrossRef]
- Medeiros, R.; Castello, N.A.; Cheng, D.; Kitazawa, M.; Baglietto-Vargas, D.; Green, K.N.; Esbenshade, T.A.; Bitner, R.S.; Decker, M.W.; LaFerla, F.M. alpha7 Nicotinic receptor agonist enhances cognition in aged 3xTg-AD mice with robust plaques and tangles. Am. J. Pathol. 2014, 184, 520–529. [Google Scholar] [CrossRef]
- Vicens, P.; Heredia, L.; Torrente, M.; Domingo, J.L. Behavioural effects of PNU-282987 and stress in an animal model of Alzheimer’s disease. Psychogeriatr. Off. J. Jpn. Psychogeriatr. Soc. 2017, 17, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, M.; Selbach, K.; Schneider, R.; Schiegel, E.; Boess, F.; Schreiber, R. AR-R 17779 improves social recognition in rats by activation of nicotinic alpha7 receptors. Psychopharmacology 2004, 172, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Boess, F.G.; De Vry, J.; Erb, C.; Flessner, T.; Hendrix, M.; Luithle, J.; Methfessel, C.; Riedl, B.; Schnizler, K.; van der Staay, F.J.; et al. The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxa mide improves working and recognition memory in rodents. J. Pharmacol. Exp. Ther. 2007, 321, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Lahmy, V.; Long, R.; Morin, D.; Villard, V.; Maurice, T. Mitochondrial protection by the mixed muscarinic/sigma1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Abeta25-35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front. Cell. Neurosci. 2014, 8, 463. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Iyer, A.M.; van Scheppingen, J.; Milenkovic, I.; Anink, J.J.; Lim, D.; Genazzani, A.A.; Adle-Biassette, H.; Kovacs, G.G.; Aronica, E. Metabotropic glutamate receptor 5 in Down’s syndrome hippocampus during development: Increased expression in astrocytes. Curr. Alzheimer Res. 2014, 11, 694–705. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Copani, A.; Nicoletti, F.; Sortino, M.A.; Caraci, F. Metabotropic Glutamate Receptors in Glial Cells: A New Potential Target for Neuroprotection? Front. Mol. Neurosci. 2018, 11, 414. [Google Scholar] [CrossRef]
- Chen, X.; Lin, R.; Chang, L.; Xu, S.; Wei, X.; Zhang, J.; Wang, C.; Anwyl, R.; Wang, Q. Enhancement of long-term depression by soluble amyloid β protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3. Neuroscience 2013, 253, 435–443. [Google Scholar] [CrossRef]
- Renner, M.; Lacor, P.N.; Velasco, P.T.; Xu, J.; Contractor, A.; Klein, W.L.; Triller, A. Deleterious Effects of Amyloid β Oligomers Acting as an Extracellular Scaffold for mGluR5. Neuron 2010, 66, 739–754. [Google Scholar] [CrossRef]
- Caraci, F.; Molinaro, G.; Battaglia, G.; Giuffrida, M.L.; Riozzi, B.; Traficante, A.; Bruno, V.; Cannella, M.; Merlo, S.; Wang, X.; et al. Targeting Group II Metabotropic Glutamate (mGlu) Receptors for the Treatment of Psychosis Associated with Alzheimer’s Disease: Selective Activation of mGlu2 Receptors Amplifies β-Amyloid Toxicity in Cultured Neurons, Whereas Dual Activation of mGlu2 and mGlu3 Receptors Is Neuroprotective. Mol. Pharmacol. 2011, 79, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Wang, Y.; Li, D.-L.; Luo, J.; Liu, M.-Y. Orphan G protein-coupled receptors (GPCRs): Biological functions and potential drug targets. Acta Pharmacol. Sin. 2012, 33, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Grottick, A.; Schuelert, N.; Rosenbrock, H.; von Heimendahl, M.; Arban, R.; Hobson, S.J.B.P. 669. GPR52 Agonists Represent a Novel Approach to Treat Cognitive Deficits Associated with Schizophrenia. Boil. Psychiatry 2017, 81, S271. [Google Scholar] [CrossRef]
- Haque, M.E.; Kim, I.-S.; Jakaria, M.; Akther, M.; Choi, D.-K. Importance of GPCR-Mediated Microglial Activation in Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Skwarek-Maruszewska, A.; Horré, K.; Vandewyer, E.; Wolfs, L.; Snellinx, A.; Saito, T.; Radaelli, E.; Corthout, N.; Colombelli, J. Loss of GPR3 reduces the amyloid plaque burden and improves memory in Alzheimer’s disease mouse models. Sci. Transl. Med. 2015, 7, 309ra164. [Google Scholar] [CrossRef] [PubMed]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.-S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.J. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.T.; Maroteaux, G.; Robe, A.; Venteo, L.; Nasseef, M.T.; van Kempen, L.C.; Mechawar, N.; Turecki, G.; Darcq, E.; Kieffer, B.L.J.C.b. Expression map of 78 brain-expressed mouse orphan GPCRs provides a translational resource for neuropsychiatric research. Commun. Biol. 2018, 1, 102. [Google Scholar] [CrossRef]
- Thathiah, A.; Spittaels, K.; Hoffmann, M.; Staes, M.; Cohen, A.; Horre, K.; Vanbrabant, M.; Coun, F.; Baekelandt, V.; Delacourte, A.; et al. The orphan G protein-coupled receptor 3 modulates amyloid-beta peptide generation in neurons. Science 2009, 323, 946–951. [Google Scholar] [CrossRef]
- Sutton, L.P.; Orlandi, C.; Song, C.; Oh, W.C.; Muntean, B.S.; Xie, K.; Filippini, A.; Xie, X.; Satterfield, R.; Yaeger, J.D. Orphan receptor GPR158 controls stress-induced depression. eLife 2018, 7, e33273. [Google Scholar] [CrossRef]
- Komatsu, H.; Maruyama, M.; Yao, S.; Shinohara, T.; Sakuma, K.; Imaichi, S.; Chikatsu, T.; Kuniyeda, K.; Siu, F.K.; Peng, L.S. Anatomical transcriptome of G protein-coupled receptors leads to the identification of a novel therapeutic candidate GPR52 for psychiatric disorders. PLoS ONE 2014, 9, e90134. [Google Scholar] [CrossRef]
- Valverde, O.; Celerier, E.; Baranyi, M.; Vanderhaeghen, P.; Maldonado, R.; Sperlagh, B.; Vassart, G.; Ledent, C. GPR3 receptor, a novel actor in the emotional-like responses. PLoS ONE 2009, 4, e4704. [Google Scholar] [CrossRef] [PubMed]
- Hurst, K.; Badgley, C.; Ellsworth, T.; Bell, S.; Friend, L.; Prince, B.; Welch, J.; Cowan, Z.; Williamson, R.; Lyon, C. A putative lysophosphatidylinositol receptor GPR55 modulates hippocampal synaptic plasticity. Hippocampus 2017, 27, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Saito, T.; Takasaki, J.; Kamohara, M.; Sugimoto, T.; Kobayashi, M.; Tadokoro, M.; Matsumoto, S.-I.; Ohishi, T.; Furuichi, K.J.B.; et al. An evolutionarily conserved G-protein coupled receptor family, SREB, expressed in the central nervous system. Biochem. Biophys. Res. Commun. 2000, 272, 576–582. [Google Scholar] [CrossRef]
- Yanai, T.; Kurosawa, A.; Nikaido, Y.; Nakajima, N.; Saito, T.; Osada, H.; Konno, A.; Hirai, H.; Takeda, S. Identification and molecular docking studies for novel inverse agonists of SREB, super conserved receptor expressed in brain. Genes Cells 2016, 21, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Hellebrand, S.; Wittenberger, T.; Schaller, H.C.; Hermans-Borgmeyer, I. Gpr85, a novel member of the G-protein coupled receptor family, prominently expressed in the developing mouse cerebral cortex. Gene Expr. Patterns 2001, 1, 13–16. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Sexton, P.M. Emerging paradigms in GPCR allostery: Implications for drug discovery. Nat. Rev. Drug Discov. 2013, 12, 630–644. [Google Scholar] [CrossRef]
- Lutjens, R.; Rocher, J.P. Recent advances in drug discovery of GPCR allosteric modulators for neurodegenerative disorders. Curr. Opin. Pharmacol. 2017, 32, 91–95. [Google Scholar] [CrossRef]
- Notartomaso, S.; Zappulla, C.; Biagioni, F.; Cannella, M.; Bucci, D.; Mascio, G.; Scarselli, P.; Fazio, F.; Weisz, F.; Lionetto, L. Pharmacological enhancement of mGlu1 metabotropic glutamate receptors causes a prolonged symptomatic benefit in a mouse model of spinocerebellar ataxia type 1. Mol. Brain 2013, 6, 48. [Google Scholar] [CrossRef]
- Tison, F.; Keywood, C.; Wakefield, M.; Durif, F.; Corvol, J.C.; Eggert, K.; Lew, M.; Isaacson, S.; Bezard, E.; Poli, S.M.J.M.D. A phase 2a trial of the novel mglur5-negative allosteric modulator dipraglurant for levodopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2016, 31, 1373–1380. [Google Scholar] [CrossRef]
- Foster, D.J.; Choi, D.L.; Conn, P.J.; Rook, J.M. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer’s disease and schizophrenia. Neuropsychiatr. Dis. Treat. 2014, 10, 183–191. [Google Scholar]
- Jakubík, J.; El-Fakahany, E.E. Allosteric modulation of muscarinic acetylcholine receptors. Pharmacological 2010, 3, 2838–2860. [Google Scholar] [CrossRef] [PubMed]
- Davoren, J.E.; Lee, C.-W.; Garnsey, M.; Brodney, M.A.; Cordes, J.; Dlugolenski, K.; Edgerton, J.R.; Harris, A.R.; Helal, C.J.; Jenkinson, S.; et al. Discovery of the Potent and Selective M1 PAM-Agonist N-[(3R,4S)-3-Hydroxytetrahydro-2H-pyran-4-yl]-5-methyl-4-[4-(1,3-thiazol-4-yl)benzyl]pyridine-2-carboxamide (PF-06767832): Evaluation of Efficacy and Cholinergic Side Effects. J. Med. Chem. 2016, 59, 6313–6328. [Google Scholar] [CrossRef] [PubMed]
- Melancon, B.J.; Tarr, J.C.; Panarese, J.D.; Wood, M.R.; Lindsley, C.W. Allosteric modulation of the M1 muscarinic acetylcholine receptor: Improving cognition and a potential treatment for schizophrenia and Alzheimer’s disease. Drug Discov. Today 2013, 18, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.R.; Noetzel, M.J.; Melancon, B.J.; Poslusney, M.S.; Nance, K.D.; Hurtado, M.A.; Luscombe, V.B.; Weiner, R.L.; Rodriguez, A.L.; Lamsal, A. Discovery of VU0467485/AZ13713945: An M4 PAM evaluated as a preclinical candidate for the treatment of schizophrenia. ACS Med. Chem. Lett. 2016, 8, 233–238. [Google Scholar] [CrossRef]
- Thal, D.M.; Sun, B.; Feng, D.; Nawaratne, V.; Leach, K.; Felder, C.C.; Bures, M.G.; Evans, D.A.; Weis, W.I.; Bachhawat, P.J.N. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 2016, 531, 335–340. [Google Scholar] [CrossRef]
- Rizzi, L.; Roriz-Cruz, M. Sirtuin 1 and Alzheimer’s disease: An up-to-date review. Neuropeptides 2018, 71, 54–60. [Google Scholar] [CrossRef]
- Azam, S.; Jakaria, M.; Kim, I.S.; Kim, J.; Haque, M.E.; Choi, D.K. Regulation of Toll-Like Receptor (TLR) Signaling Pathway by Polyphenols in the Treatment of Age-Linked Neurodegenerative Diseases: Focus on TLR4 Signaling. Front. Immunol. 2019, 10, 1000. [Google Scholar] [CrossRef]
- Lee, H.R.; Shin, H.K.; Park, S.Y.; Kim, H.Y.; Lee, W.S.; Rhim, B.Y.; Hong, K.W.; Kim, C.D. Cilostazol suppresses beta-amyloid production by activating a disintegrin and metalloproteinase 10 via the upregulation of SIRT1-coupled retinoic acid receptor-beta. J. Neurosci. Res. 2014, 92, 1581–1590. [Google Scholar] [CrossRef]
- Min, S.-W.; Sohn, P.D.; Li, Y.; Devidze, N.; Johnson, J.R.; Krogan, N.J.; Masliah, E.; Mok, S.-A.; Gestwicki, J.E.; Gan, L. SIRT1 deacetylates tau and reduces pathogenic tau spread in a mouse model of tauopathy. J. Neurosci. 2018, 38, 3680–3688. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.-X.; Zheng, L.-P.; Wang, L.; Liu, Y.-F.; Yin, W.-Y.; Chen, Y.-Y.; Wang, X.-S.; Hou, S.-T.; Chen, J.-F. The adenosine A2A receptor antagonist SCH58261 reduces macrophage/microglia activation and protects against experimental autoimmune encephalomyelitis in mice. Neurochem. Int. 2019, 129, 104490. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Liu, S.; Zhao, Y.; Zhu, J.; Liu, K. Roles of Neuropeptide Y in Neurodegenerative and Neuroimmune Diseases. Front. Mol. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Santos-Carvalho, A.; Elvas, F.; Álvaro, A.R.; Ambrósio, A.F.; Cavadas, C. Neuropeptide Y receptors activation protects rat retinal neural cells against necrotic and apoptotic cell death induced by glutamate. Cell Death Dis. 2013, 4, e636. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.; Potkar, R.; Metcalf, J.; Thrin, I.; Adame, A.; Rockenstein, E.; Masliah, E.J. Systemic central nervous system (CNS)-targeted delivery of neuropeptide Y (NPY) reduces neurodegeneration and increases neural precursor cell proliferation in a mouse model of Alzheimer disease. J. Biol. Chem. 2016, 291, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Thiriet, N.; Agasse, F.; Nicoleau, C.; Guégan, C.; Vallette, F.; Cadet, J.L.; Jaber, M.; Malva, J.O.; Coronas, V.J. NPY promotes chemokinesis and neurogenesis in the rat subventricular zone. J. Neurochem. 2011, 116, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Pain, S.; Vergote, J.; Gulhan, Z.; Bodard, S.; Chalon, S.; Gaillard, A. Inflammatory process in Parkinson disease: Neuroprotection by Neuropeptide Y. Fundam. Clin. Pharmacol. 2019, 33, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Carniglia, L.; Ramírez, D.; Durand, D.; Saba, J.; Turati, J.; Caruso, C.; Scimonelli, T.N.; Lasaga, M. Neuropeptides and Microglial Activation in Inflammation, Pain, and Neurodegenerative Diseases. J. Mediat. Inflamm. 2017, 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G. Neurodevelopmental actions of leptin. Brain Res. 2010, 1350, 2–9. [Google Scholar] [CrossRef]
- Jakaria, M.; Haque, M.E.; Cho, D.Y.; Azam, S.; Kim, I.S.; Choi, D.K. Molecular Insights into NR4A2(Nurr1): An Emerging Target for Neuroprotective Therapy Against Neuroinflammation and Neuronal Cell Death. Mol. Neurobiol. 2019, 56, 5799–5814. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Jakaria, M.; Mathew, B.; Barreto, G.E.; Ashraf, G.M. Nootropic and anti-Alzheimer’s actions of medicinal plants: Molecular insight into therapeutic potential to alleviate Alzheimer’s neuropathology. Mol. Neurobiol. 2019, 56, 4925–4944. [Google Scholar] [CrossRef]
- Haque, M.E.; Akther, M.; Jakaria, M.; Kim, I.S.; Azam, S.; Choi, D.K. Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Mov. Disord. 2019. [Google Scholar] [CrossRef]
- Jakaria, M.; Park, S.Y.; Haque, M.E.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 307. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).