RNA-Based Strategies for Cardiac Reprogramming of Human Mesenchymal Stromal Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Fluorescence-Activated Cell Sorting
2.3. Cardiac Reprogramming
2.4. IF Staining and Calcium Imaging
2.5. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
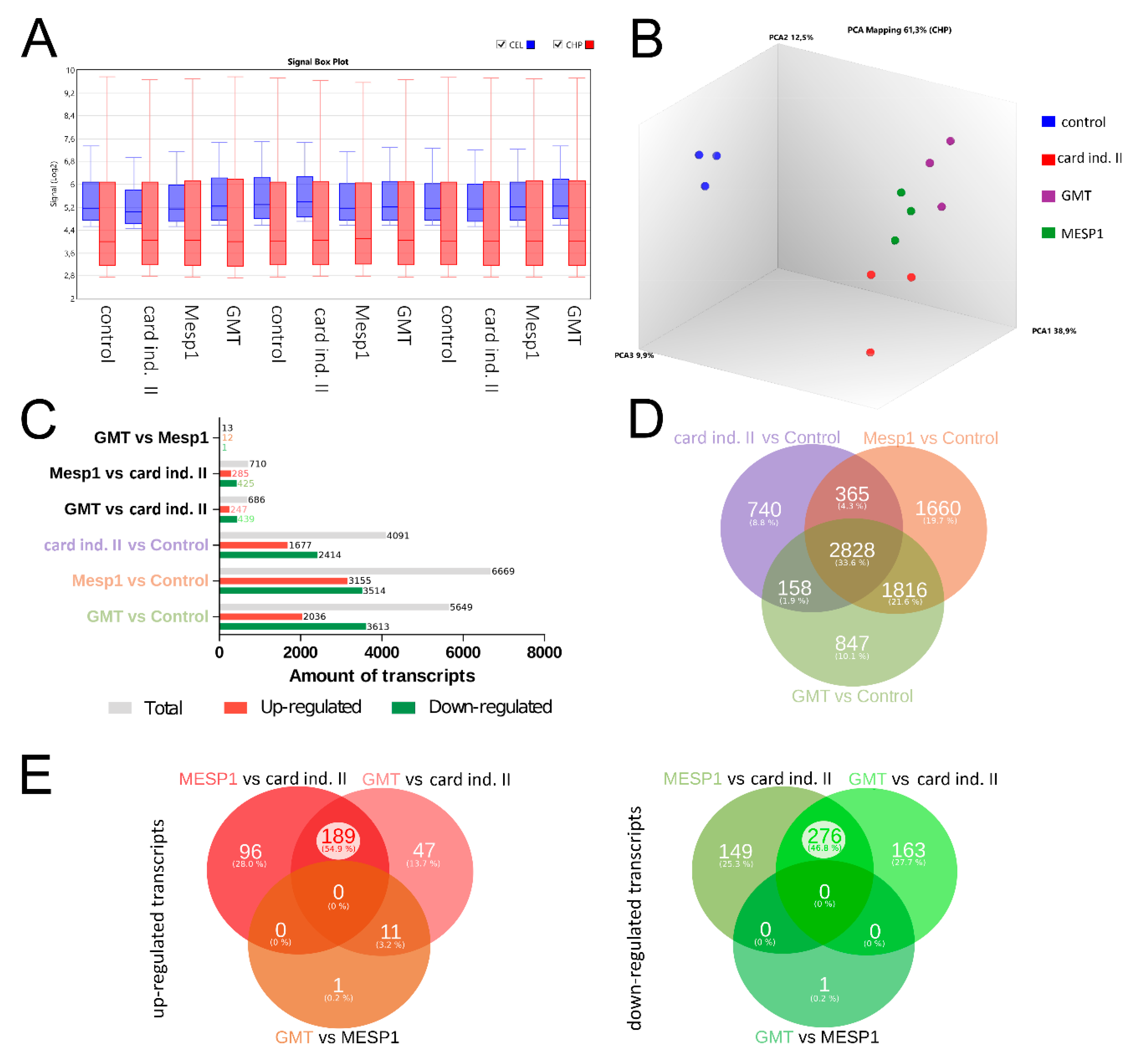
2.6. Microarray Analysis
2.7. Statistical Analysis
3. Results
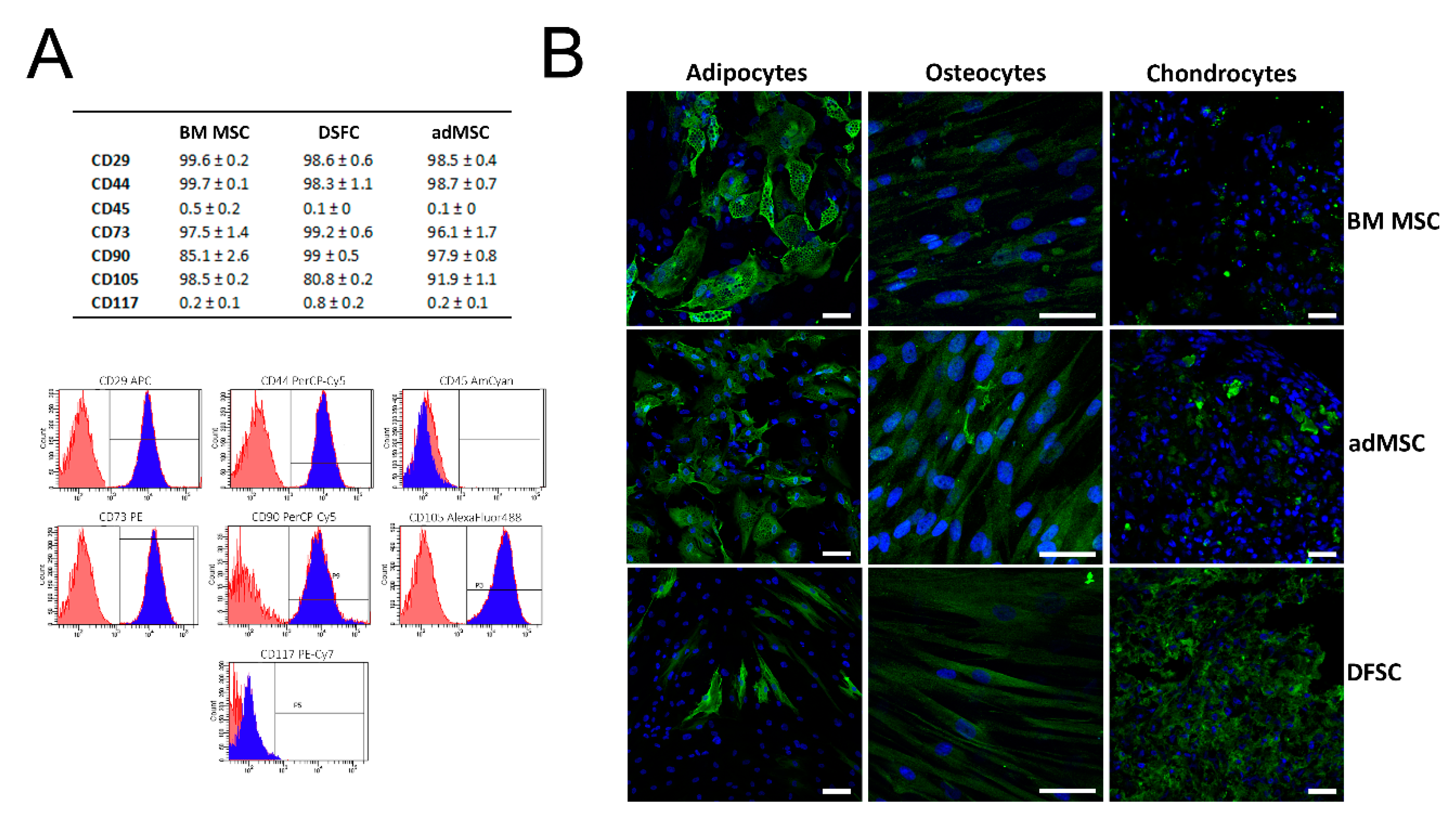
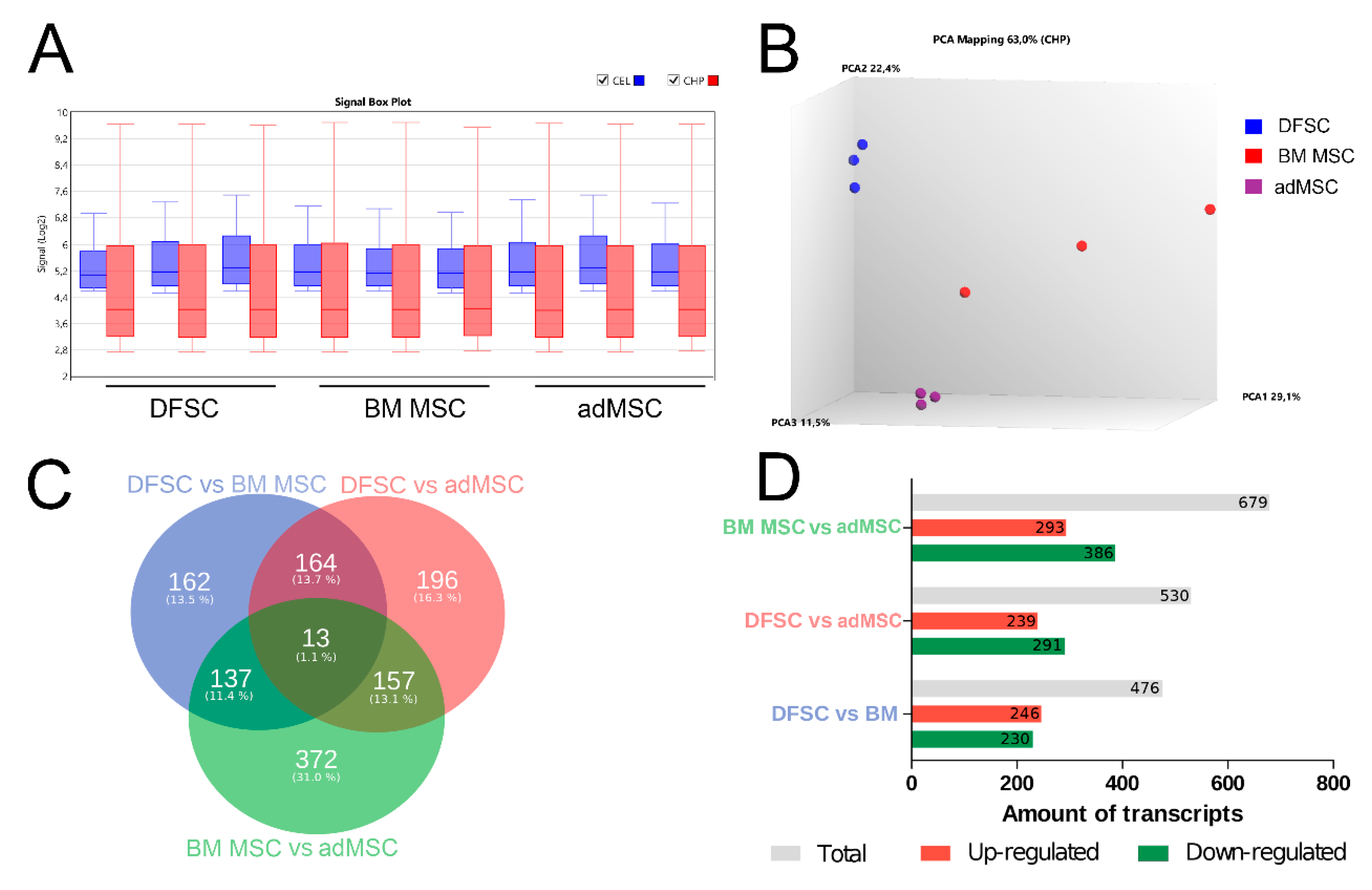
3.1. Characterization of Isolated MSC
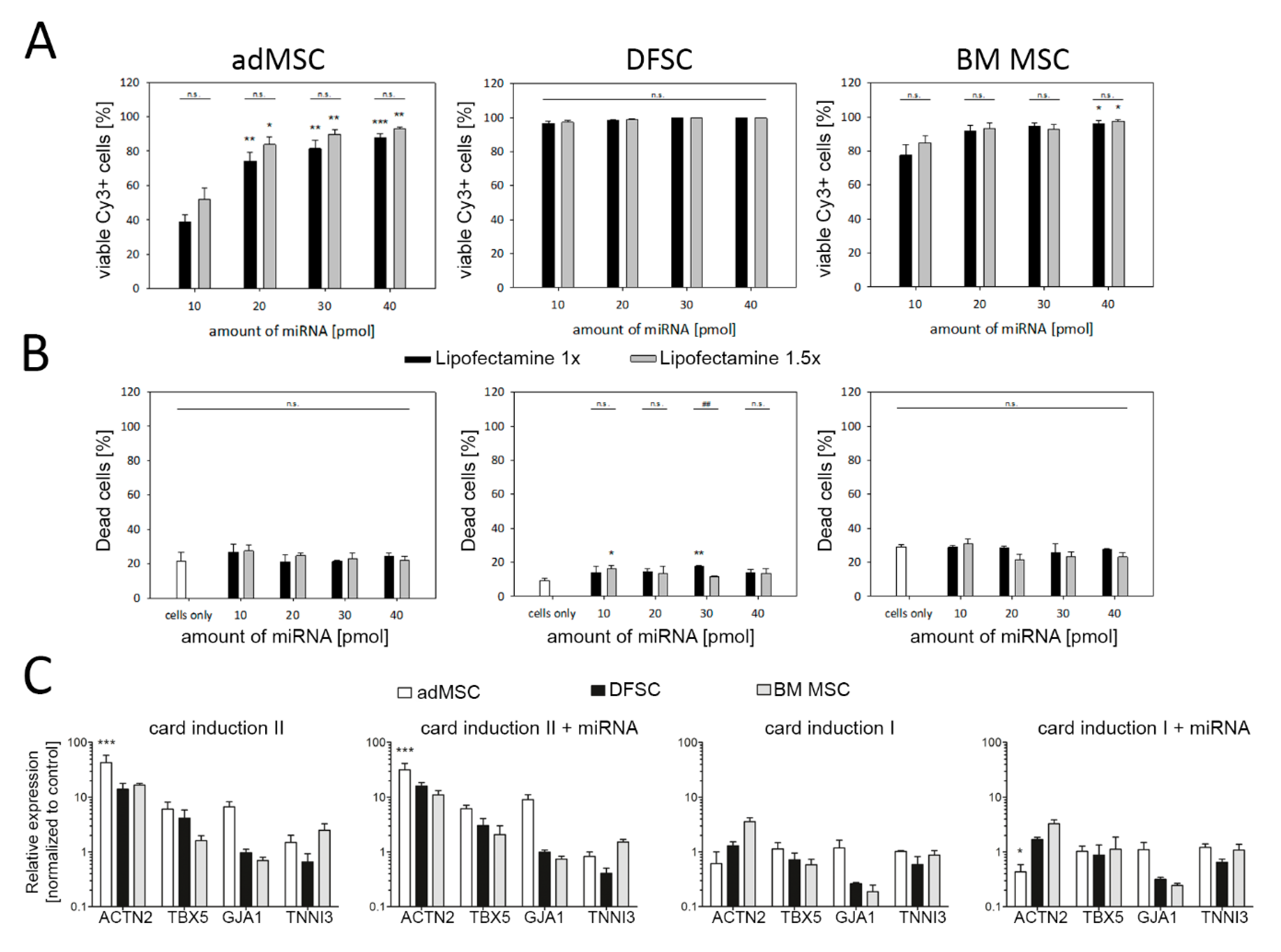
3.2. Reprogramming of MSC Using miRNA and Cardiac Induction Cell Culture Conditions
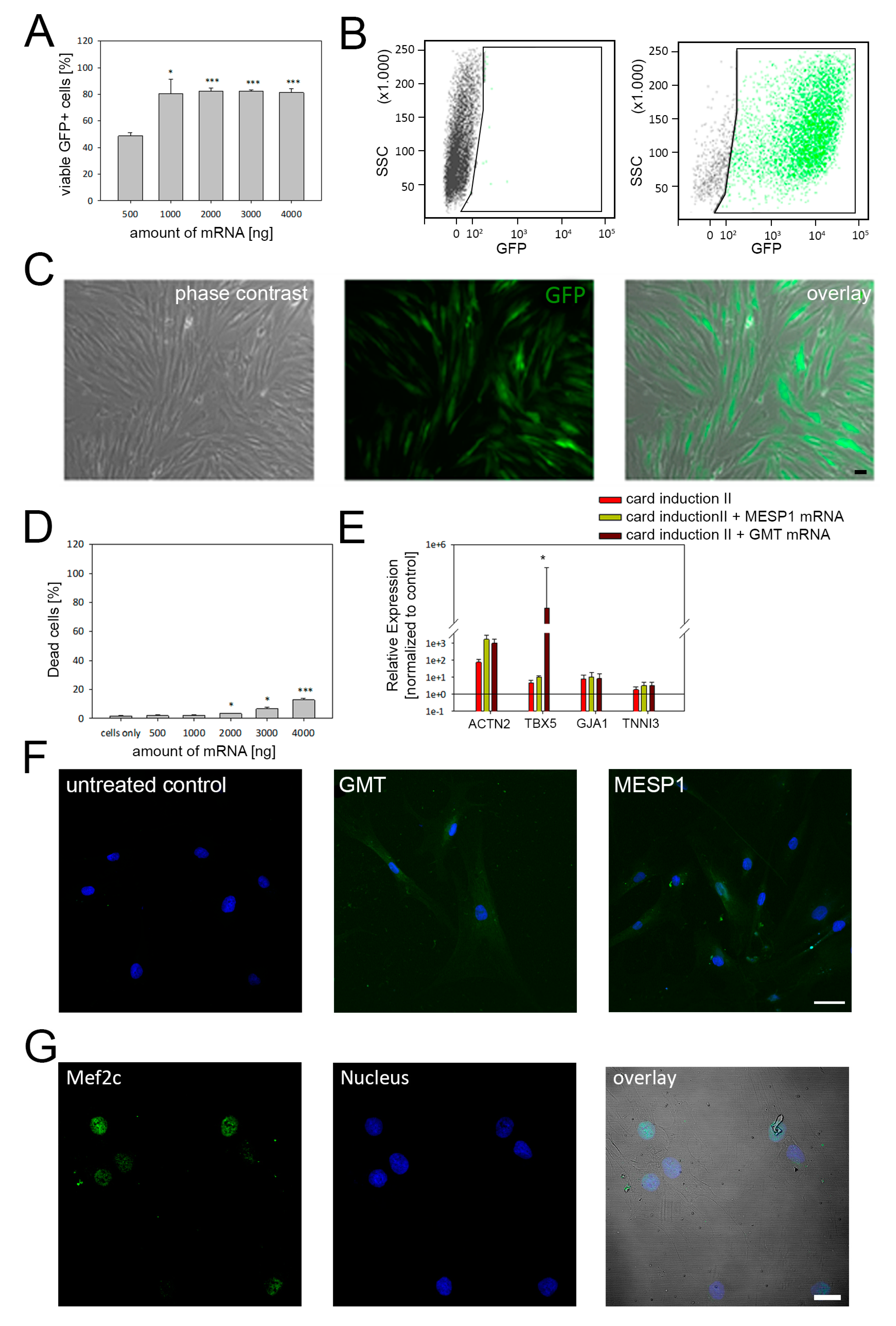
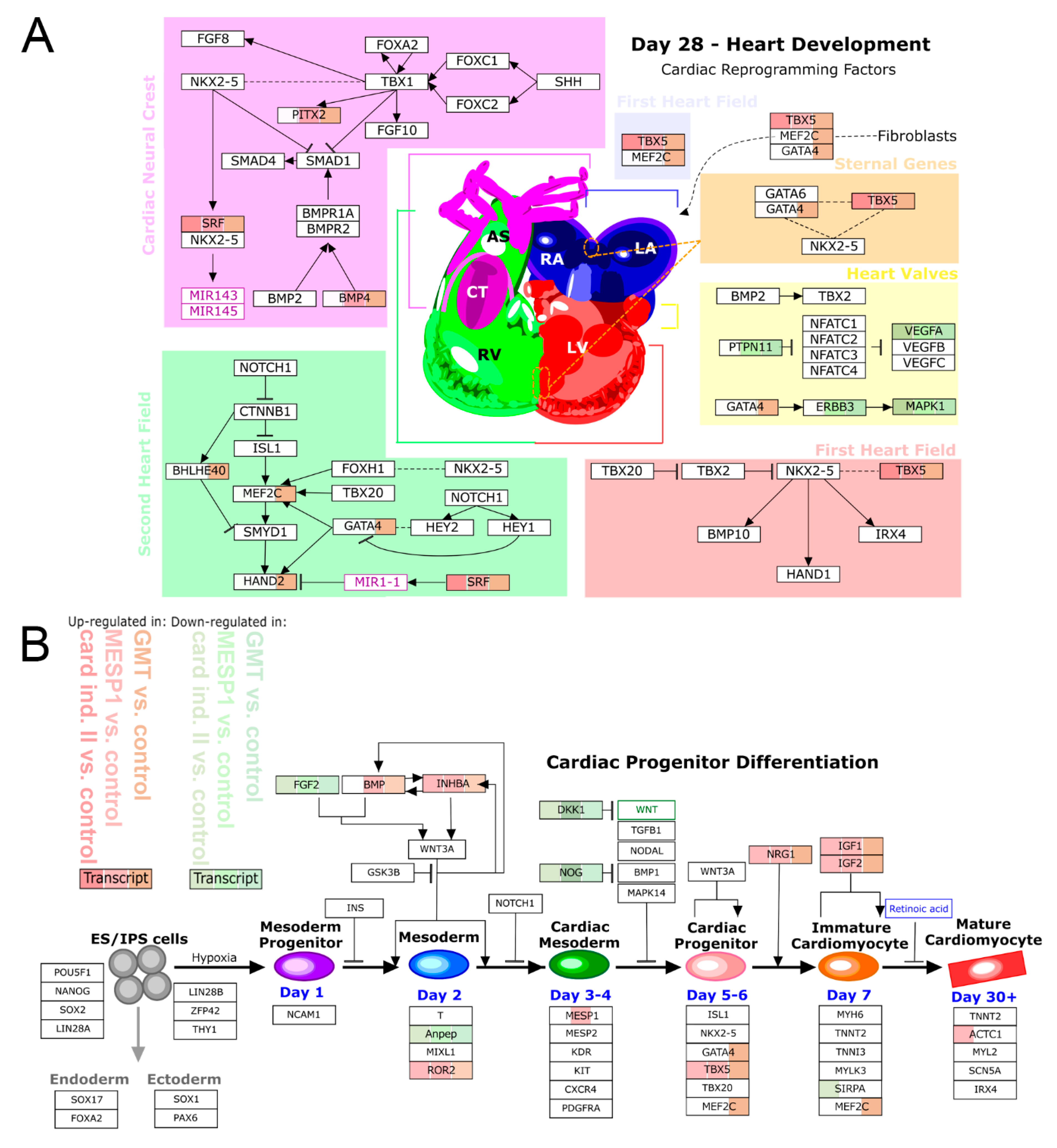
3.3. mRNA-Based Reprogramming of adMSC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rajabzadeh, N.; Fathi, E.; Farahzadi, R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Collichia, M.; Jones, D.A.; Beirne, A.-M.; Hussain, M.; Weeraman, D.; Rathod, K.; Veerapen, J.; Lowdell, M.; Mathur, A. Umbilical cord-derived mesenchymal stromal cells in cardiovascular disease: review of preclinical and clinical data. Cytotherapy 2019, 21, 1007–1018. [Google Scholar] [CrossRef]
- Guerrouahen, B.S.; Sidahmed, H.; Al Sulaiti, A.; Al Khulaifi, M.; Cugno, C. Enhancing Mesenchymal Stromal Cell Immunomodulation for Treating Conditions Influenced by the Immune System. Stem Cells Int. 2019, 2019, 7219297. [Google Scholar] [CrossRef]
- Aguilera-Castrejon, A.; Pasantes-Morales, H.; Montesinos, J.J.; Cortés-Medina, L.V.; Castro-Manrreza, M.E.; Mayani, H.; Ramos-Mandujano, G. Improved Proliferative Capacity of NP-Like Cells Derived from Human Mesenchymal Stromal Cells and Neuronal Transdifferentiation by Small Molecules. Neurochem. Res. 2017, 42, 415–427. [Google Scholar] [CrossRef]
- Tsai, W.-L.; Yeh, P.-H.; Tsai, C.-Y.; Ting, C.-T.; Chiu, Y.-H.; Tao, M.-H.; Li, W.-C.; Hung, S.-C. Efficient programming of human mesenchymal stem cell-derived hepatocytes by epigenetic regulations. J. Gastroenterol. Hepatol. 2017, 32, 261–269. [Google Scholar] [CrossRef]
- Papadimou, E.; Morigi, M.; Iatropoulos, P.; Xinaris, C.; Tomasoni, S.; Benedetti, V.; Longaretti, L.; Rota, C.; Todeschini, M.; Rizzo, P.; et al. Direct Reprogramming of Human Bone Marrow Stromal Cells into Functional Renal Cells Using Cell-free Extracts. Stem Cell Reports 2015, 4, 685–698. [Google Scholar] [CrossRef]
- Cai, B.; Li, J.; Wang, J.; Luo, X.; Ai, J.; Liu, Y.; Wang, N.; Liang, H.; Zhang, M.; Chen, N.; et al. microRNA-124 Regulates Cardiomyocyte Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells Via Targeting STAT3 Signaling. Stem Cells 2012, 30, 1746–1755. [Google Scholar] [CrossRef]
- Li, J.; Zhu, K.; Wang, Y.; Zheng, J.; Guo, C.; Lai, H.; Wang, C. Combination of IGF-1 gene manipulation and 5-AZA treatment promotes differentiation of mesenchymal stem cells into cardiomyocyte-like cells. Mol. Med. Rep. 2015, 11, 815–820. [Google Scholar] [CrossRef]
- Loo, Z.X.; Kunasekaran, W.; Govindasamy, V.; Musa, S.; Abu Kasim, N.H. Comparative analysis of cardiovascular development related genes in stem cells isolated from deciduous pulp and adipose tissue. Sci. World J. 2014, 2014, 186508. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Szaraz, P.; Gratch, Y.S.; Iqbal, F.; Librach, C.L. In Vitro Differentiation of Human Mesenchymal Stem Cells into Functional Cardiomyocyte-like Cells. J. Vis. Exp. 2017, 9, 55757. [Google Scholar] [CrossRef] [PubMed]
- Markmee, R.; Aungsuchawan, S.; Narakornsak, S.; Tancharoen, W.; Bumrungkit, K.; Pangchaidee, N.; Pothacharoen, P.; Puaninta, C. Differentiation of mesenchymal stem cells from human amniotic fluid to cardiomyocyte-like cells. Mol. Med. Rep. 2017, 16, 6068–6076. [Google Scholar] [CrossRef]
- Shen, X.; Pan, B.; Zhou, H.; Liu, L.; Lv, T.; Zhu, J.; Huang, X.; Tian, J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J. Biomed. Sci. 2017, 24, 29. [Google Scholar] [CrossRef]
- O’Connor, K.C. Molecular Profiles of Cell-to-Cell Variation in the Regenerative Potential of Mesenchymal Stromal Cells. Stem Cells Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Elahi, K.C.; Klein, G.; Avci-Adali, M.; Sievert, K.D.; MacNeil, S.; Aicher, W.K. Human Mesenchymal Stromal Cells from Different Sources Diverge in Their Expression of Cell Surface Proteins and Display Distinct Differentiation Patterns. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- D’Alimonte, I.; Mastrangelo, F.; Giuliani, P.; Pierdomenico, L.; Marchisio, M.; Zuccarini, M.; Di Iorio, P.; Quaresima, R.; Caciagli, F.; Ciccarelli, R. Osteogenic Differentiation of Mesenchymal Stromal Cells: A Comparative Analysis Between Human Subcutaneous Adipose Tissue and Dental Pulp. Stem Cells Dev. 2017, 26, 843–855. [Google Scholar] [CrossRef]
- Kwon, A.; Kim, Y.; Kim, M.; Kim, J.; Choi, H.; Jekarl, D.W.; Lee, S.; Kim, J.M.; Shin, J.-C.; Park, I.Y. Tissue-specific Differentiation Potency of Mesenchymal Stromal Cells from Perinatal Tissues. Sci. Rep. 2016, 6, 23544. [Google Scholar] [CrossRef]
- Leijten, J.; Georgi, N.; Moreira Teixeira, L.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc. Natl. Acad. Sci. USA 2014, 111, 13954–13959. [Google Scholar] [CrossRef]
- Occhetta, P.; Pigeot, S.; Rasponi, M.; Dasen, B.; Mehrkens, A.; Ullrich, T.; Kramer, I.; Guth-Gundel, S.; Barbero, A.; Martin, I. Developmentally inspired programming of adult human mesenchymal stromal cells toward stable chondrogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Yannarelli, G.; Pacienza, N.; Montanari, S.; Santa-Cruz, D.; Viswanathan, S.; Keating, A. OCT4 expression mediates partial cardiomyocyte reprogramming of mesenchymal stromal cells. PLoS ONE 2017, 12, e0189131. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Gaebel, R.; Skorska, A.; Voronina, N.; Lux, C.A.; Petters, J.; Sasse, S.; Zarniko, N.; Steinhoff, G.; David, R. Mechanisms of stem cell based cardiac repair-gap junctional signaling promotes the cardiac lineage specification of mesenchymal stem cells. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xia, Y. Study of adipose tissue-derived mesenchymal stem cells transplantation for rats with dilated cardiomyopathy. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 398–406. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Yang, B.; Ma, L.-N.; Dong, Y.-H. MicroRNA-1 effectively induces differentiation of myocardial cells from mouse bone marrow mesenchymal stem cells. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1665–1670. [Google Scholar] [CrossRef]
- Dai, F.; Du, P.; Chang, Y.; Ji, E.; Xu, Y.; Wei, C.; Li, J. Downregulation of MiR-199b-5p inducing differentiation of bone-marrow mesenchymal stem cells (BMSCs) toward cardiomyocyte-like cells via HSF1/HSP70 pathway. Med. Sci. Monit. 2018, 24, 2700–2710. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defned generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef]
- Jiang, Y.; Park, P.; Hong, S.M.; Ban, K. Maturation of cardiomyocytes derived from human pluripotent stem cells: Current strategies and limitations. Mol. Cells 2018, 41, 613–621. [Google Scholar]
- Chen, R.; He, J.; Wang, Y.; Guo, Y.; Zhang, J.; Peng, L.; Wang, D.; Lin, Q.; Zhang, J.; Guo, Z.; et al. Qualitative transcriptional signatures for evaluating the maturity degree of pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2019, 10, 113. [Google Scholar] [CrossRef]
- D’Antonio-Chronowska, A.; Donovan, M.K.R.; Young Greenwald, W.W.; Nguyen, J.P.; Fujita, K.; Hashem, S.; Matsui, H.; Soncin, F.; Parast, M.; Ward, M.C.; et al. Association of Human iPSC Gene Signatures and X Chromosome Dosage with Two Distinct Cardiac Differentiation Trajectories. Stem Cell Rep. 2019, 13, 924–938. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Salamon, A.; Herzmann, N.; Adam, S.; Kleine, H.-D.; Matthiesen, I.; Ueberreiter, K.; Peters, K. Isolation and Differentiation Potential of Human Mesenchymal Stem Cells From Adipose Tissue Harvested by Water Jet-Assisted Liposuction. Aesthetic Surg. J. 2015, 35, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Ekat, K.; Brosemann, A.; Köntges, A.; David, R.; Lang, H. Isolation, characterization and microRNA-based genetic modification of human dental follicle stem cells. J. Vis. Exp. 2018, 2018, e58089. [Google Scholar]
- Thiele, F.; Voelkner, C.; Krebs, V.; Müller, P.; Jung, J.J.; Rimmbach, C.; Steinhoff, G.; Noack, T.; David, R.; Lemcke, H. Nkx2.5 Based Ventricular Programming of Murine ESC-Derived Cardiomyocytes. Cell. Physiol. Biochem. 2019, 53, 337–354. [Google Scholar]
- Koczan, D.; Fitzner, B.; Zettl, U.K.; Hecker, M. Microarray data of transcriptome shifts in blood cell subsets during S1P receptor modulator therapy. Sci. Data 2018, 5, 180145. [Google Scholar] [CrossRef]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Payne, J.A.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef]
- Sala, L.; Gnecchi, M.; Schwartz, P.J. Long QT Syndrome Modelling with Cardiomyocytes Derived from Human-induced Pluripotent Stem Cells. Arrhythmia Electrophysiol. Rev. 2019, 8, 105. [Google Scholar] [CrossRef]
- Brodehl, A.; Ebbinghaus, H.; Deutsch, M.-A.; Gummert, J.; Gärtner, A.; Ratnavadivel, S.; Milting, H. Human Induced Pluripotent Stem-Cell-Derived Cardiomyocytes as Models for Genetic Cardiomyopathies. Int. J. Mol. Sci. 2019, 20, 4381. [Google Scholar] [CrossRef]
- Protze, S.I.; Lee, J.H.; Keller, G.M. Human Pluripotent Stem Cell-Derived Cardiovascular Cells: From Developmental Biology to Therapeutic Applications. Cell Stem Cell 2019, 25, 311–327. [Google Scholar] [CrossRef]
- Rikhtegar, R.; Pezeshkian, M.; Dolati, S.; Safaie, N.; Afrasiabi Rad, A.; Mahdipour, M.; Nouri, M.; Jodati, A.R.; Yousefi, M. Stem cells as therapy for heart disease: iPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed. Pharmacother. 2019, 109, 304–313. [Google Scholar] [CrossRef]
- Jackson, A.O.; Tang, H.; Yin, K. HiPS-Cardiac Trilineage Cell Generation and Transplantation: a Novel Therapy for Myocardial Infarction. J. Cardiovasc. Transl. Res. 2019, 13, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.; Choi, J.R.; Dolbashid, A.S.; Wan Safwani, W.K.Z. Biosafety and bioefficacy assessment of human mesenchymal stem cells: What do we know so far? Regen. Med. 2018, 13, 219–232. [Google Scholar] [CrossRef]
- Duinsbergen, D.; Salvatori, D.; Eriksson, M.; Mikkers, H. Tumors Originating from Induced Pluripotent Stem Cells and Methods for Their Prevention. Ann. N. Y. Acad. Sci. 2009, 1176, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Seminatore, C.; Polentes, J.; Ellman, D.; Kozubenko, N.; Itier, V.; Tine, S.; Tritschler, L.; Brenot, M.; Guidou, E.; Blondeau, J.; et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke 2010, 41, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell–Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, S.; Miki, K.; Takaki, T.; Okubo, C.; Hatani, T.; Chonabayashi, K.; Nishikawa, M.; Takei, I.; Oishi, A.; Narita, M.; et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef]
- Ito, E.; Miyagawa, S.; Takeda, M.; Kawamura, A.; Harada, A.; Iseoka, H.; Yajima, S.; Sougawa, N.; Mochizuki-Oda, N.; Yasuda, S.; et al. Tumorigenicity assay essential for facilitating safety studies of hiPSC-derived cardiomyocytes for clinical application. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Oikonomopoulos, A.; Kitani, T.; Wu, J.C. Pluripotent Stem Cell-Derived Cardiomyocytes as a Platform for Cell Therapy Applications: Progress and Hurdles for Clinical Translation. Mol. Ther. 2018, 26, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Ye, L. Maturation of Pluripotent Stem Cell-Derived Cardiomyocytes: A Critical Step for Drug Development and Cell Therapy. J. Cardiovasc. Transl. Res. 2018, 11, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.J.; Butcher, J. Naturally Engineered Maturation of Cardiomyocytes. Front. Cell Dev. Biol. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.A.; Jiang, H.; Wang, X.; Helke, S.; Tsoporis, J.N.; Gong, N.; Keating, S.C.J.; Parker, T.G.; Backx, P.H.; Keating, A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells 2008, 26, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.N.; Jiang, S.; Wong, P.; Tan, J.; Chua, Y.L.; Seng Tan, Y.; Sin, Y.K.; Lim, C.H.; Chua, T.; Teh, M.; et al. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem. Biophys. Res. Commun. 2004, 324, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rendon, E.; Sweeney, D.; Lu, F.; Girdlestone, J.; Navarrete, C.; Watt, S.M. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008, 95, 137–148. [Google Scholar] [CrossRef]
- Ramkisoensing, A.A.; Pijnappels, D.A.; Askar, S.F.A.; Passier, R.; Swildens, J.; Goumans, M.J.; Schutte, C.I.; de Vries, A.A.F.; Scherjon, S.; Mummery, C.L.; et al. Human embryonic and fetal Mesenchymal stem cells differentiate toward three different cardiac lineages in contrast to their adult counterparts. PLoS ONE 2011, 6, e24164. [Google Scholar] [CrossRef]
- Shi, S.; Wu, X.; Wang, X.; Hao, W.; Miao, H.; Zhen, L.; Nie, S. Differentiation of Bone Marrow Mesenchymal Stem Cells to Cardiomyocyte-Like Cells Is Regulated by the Combined Low Dose Treatment of Transforming Growth Factor-? 1 and 5-Azacytidine. Stem Cells Int. 2016, 2016, 11. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Li, J.; Xu, S.; Zhao, Y.; Yu, S.; Ge, L.; Xu, B.; Yu, S.; Yu, S.; Ge, L.; Ge, L.; et al. Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol. Med. Rep. 2018, 18, 4969–4977. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Kakkar, A.; Nandy, S.B.; Gupta, S.; Bharagava, B.; Airan, B.; Mohanty, S. Adipose tissue derived mesenchymal stem cells are better respondents to TGFβ1 for in vitro generation of cardiomyocyte-like cells. Mol. Cell. Biochem. 2019, 460, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Bajek, A.; Olkowska, J.; Walentowicz-Sadłecka, M.; Sadłecki, P.; Grabiec, M.; Porowińska, D.; Drewa, T.; Roszkowski, K. Human adipose-derived and amniotic fluid-derived stem cells: A preliminary in vitro study comparing myogenic differentiation capability. Med. Sci. Monit. 2018, 24, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Bai, Y.; Zhang, L.; Zhang, B.; Zagidullin, N.; Carvalho, K.; Du, Z.; Cai, B. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications. Stem Cell Res. Ther. 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Neshati, V.; Mollazadeh, S.; Fazly Bazzaz, B.S.; de Vries, A.A.F.; Mojarrad, M.; Naderi-Meshkin, H.; Neshati, Z.; Mirahmadi, M.; Kerachian, M.A. MicroRNA-499a-5p Promotes Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells to Cardiomyocytes. Appl. Biochem. Biotechnol. 2018, 186, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xu, L.; Wang, M.; Guo, T.; Luo, F.; Su, N.; Yi, S.; Chen, T. MiR-149 promotes the myocardial differentiation of mouse bone marrow stem cells by targeting Dab2. Mol. Med. Rep. 2018, 17, 8502–8509. [Google Scholar] [CrossRef] [PubMed]
- Fujita, J.; Tohyama, S.; Kishino, Y.; Okada, M.; Morita, Y. Concise Review: Genetic and Epigenetic Regulation of Cardiac Differentiation from Human Pluripotent Stem Cells. Stem Cells 2019, 37, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Fu, J.-D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef]
- Chen, J.X.; Krane, M.; Deutsch, M.A.; Wang, L.; Rav-Acha, M.; Gregoire, S.; Engels, M.C.; Rajarajan, K.; Karra, R.; Abel, E.D.; et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ. Res. 2012, 111, 50–55. [Google Scholar] [CrossRef]
- Fu, J.D.; Stone, N.R.; Liu, L.; Spencer, C.I.; Qian, L.; Hayashi, Y.; Delgado-Olguin, P.; Ding, S.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports 2013, 1, 235–247. [Google Scholar] [CrossRef]
- David, R.; Brenner, C.; Stieber, J.; Schwarz, F.; Brunner, S.; Vollmer, M.; Mentele, E.; Müller-Höcker, J.; Kitajima, S.; Lickert, H.; et al. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat. Cell Biol. 2008, 10, 338–345. [Google Scholar] [CrossRef]
- Wystrychowski, W.; Patlolla, B.; Zhuge, Y.; Neofytou, E.; Robbins, R.C.; Beygui, R.E. Multipotency and cardiomyogenic potential of human adipose-derived stem cells from epicardium, pericardium, and omentum. Stem Cell Res. Ther. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Neshati, V.; Mollazadeh, S.; Fazly Bazzaz, B.S.; de Vries, A.A.; Mojarrad, M.; Naderi-Meshkin, H.; Neshati, Z.; Kerachian, M.A. Cardiomyogenic differentiation of human adipose-derived mesenchymal stem cells transduced with Tbx20-encoding lentiviral vectors. J. Cell. Biochem. 2018, 119, 6146–6153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Coller, J. A Universal Code for mRNA Stability? Trends Genet. 2016, 32, 687–688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warren, L.; Lin, C. mRNA-Based Genetic Reprogramming. Mol. Ther. 2019, 27, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Weidgang, C.E.; Russell, R.; Tata, P.R.; Kühl, S.J.; Illing, A.; Müller, M.; Lin, Q.; Brunner, C.; Boeckers, T.M.; Bauer, K.; et al. TBX3 directs cell-fate decision toward mesendoderm. Stem Cell Reports 2013, 1, 248–265. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.K.; Shi, X.; Toyama, A.; Arpke, R.W.; Dandapat, A.; Iacovino, M.; Kang, J.; Le, G.; Hagen, H.R.; Garry, D.J.; et al. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell 2013, 12, 587–601. [Google Scholar] [CrossRef]
- Lv, Y.; Gao, C.-W.; Liu, B.; Wang, H.-Y.; Wang, H.-P. BMP-2 combined with salvianolic acid B promotes cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells. Kaohsiung J. Med. Sci. 2017, 33, 477–485. [Google Scholar] [CrossRef]
- Bhuvanalakshmi, G.; Arfuso, F.; Kumar, A.P.; Dharmarajan, A.; Warrier, S. Epigenetic reprogramming converts human Wharton’s jelly mesenchymal stem cells into functional cardiomyocytes by differential regulation of Wnt mediators. Stem Cell Res. Ther. 2017, 8, 185. [Google Scholar] [CrossRef]
- Ibarra-Ibarra, B.R.; Franco, M.; Paez, A.; López, E.V.; Massó, F. Improved efficiency of cardiomyocyte-like cell differentiation from rat adipose tissue-derived mesenchymal stem cells with a directed differentiation protocol. Stem Cells Int. 2019, 2019, 8940365. [Google Scholar] [CrossRef]
- Steinle, H.; Weber, M.; Behring, A.; Mau-Holzmann, U.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Generation of iPSCs by Nonintegrative RNA-Based Reprogramming Techniques: Benefits of Self-Replicating RNA versus Synthetic mRNA. Stem Cells Int. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Yin, C.; Asfour, H.; Chen, O.; Li, Y.; Bursac, N.; Liu, J.; Qian, L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ. Res. 2015, 116, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Qayed, M.; Copland, I.; Galipeau, J. Allogeneic Versus Autologous Mesenchymal Stromal Cells and Donor-to-Donor Variability. In Mesenchymal Stromal Cells; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–120. [Google Scholar]
- McLeod, C.M.; Mauck, R.L. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur. Cell. Mater. 2017, 34, 217–231. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, P.; Wolfien, M.; Ekat, K.; Lang, C.I.; Koczan, D.; Wolkenhauer, O.; Hahn, O.; Peters, K.; Lang, H.; David, R.; et al. RNA-Based Strategies for Cardiac Reprogramming of Human Mesenchymal Stromal Cells. Cells 2020, 9, 504. https://doi.org/10.3390/cells9020504
Mueller P, Wolfien M, Ekat K, Lang CI, Koczan D, Wolkenhauer O, Hahn O, Peters K, Lang H, David R, et al. RNA-Based Strategies for Cardiac Reprogramming of Human Mesenchymal Stromal Cells. Cells. 2020; 9(2):504. https://doi.org/10.3390/cells9020504
Chicago/Turabian StyleMueller, Paula, Markus Wolfien, Katharina Ekat, Cajetan Immanuel Lang, Dirk Koczan, Olaf Wolkenhauer, Olga Hahn, Kirsten Peters, Hermann Lang, Robert David, and et al. 2020. "RNA-Based Strategies for Cardiac Reprogramming of Human Mesenchymal Stromal Cells" Cells 9, no. 2: 504. https://doi.org/10.3390/cells9020504
APA StyleMueller, P., Wolfien, M., Ekat, K., Lang, C. I., Koczan, D., Wolkenhauer, O., Hahn, O., Peters, K., Lang, H., David, R., & Lemcke, H. (2020). RNA-Based Strategies for Cardiac Reprogramming of Human Mesenchymal Stromal Cells. Cells, 9(2), 504. https://doi.org/10.3390/cells9020504





