Oocyte Maturation in Starfish
Abstract
1. Introduction
2. Activation of G-Protein Coupled Receptor by Maturation Inducing Hormone 1-MA
3. The Effectors of G-Protein
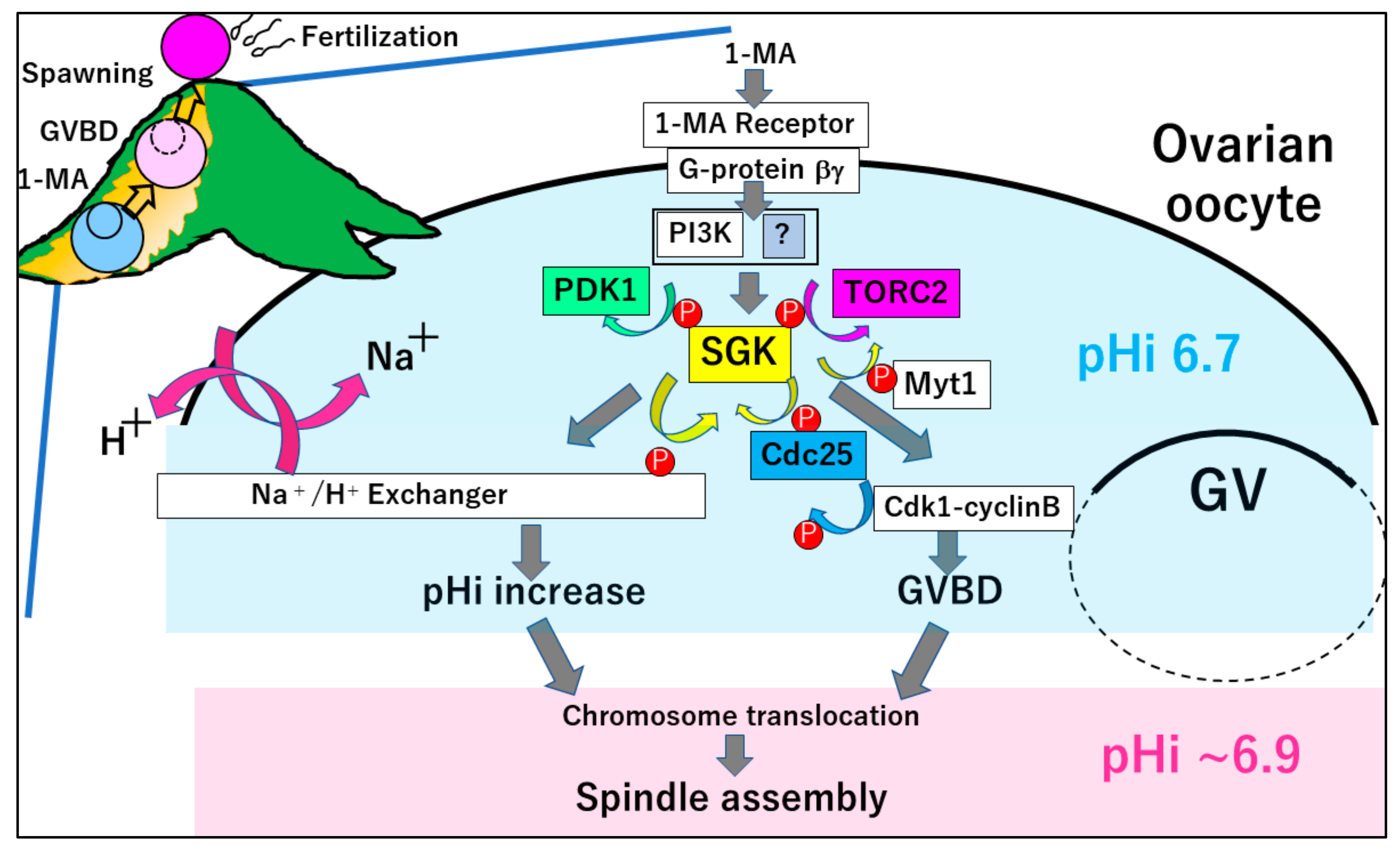
3.1. SGK-Dependent GVBD
3.2. SGK-Dependent Spindle Formation
4. MI Arrest in the Ovaries
5. Oocyte Maturation in Mammals
6. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanatani, H.; Shirai, H.; Nakanishi, K.; Kurokawa, T. Isolation and indentification on meiosis inducing substance in starfish Asterias amurensis. Nature 1969, 221, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. MPF-based meiotic cell cycle control: Half a century of lessons from starfish oocytes. Proc. Jpn. Acad. Ser. B 2018, 94, 180–203. [Google Scholar] [CrossRef]
- Harada, K.; Oita, E.; Chiba, K. Metaphase I arrest of starfish oocytes induced via the MAP kinase pathway is released by an increase of intracellular pH. Dev. Camb. Engl. 2003, 130, 4581–4586. [Google Scholar] [CrossRef] [PubMed]
- Oita, E.; Harada, K.; Chiba, K. Degradation of polyubiquitinated cyclin B is blocked by the MAPK pathway at the metaphase I arrest in starfish oocytes. J. Biol. Chem. 2004, 279, 18633–18640. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Hirohashi, N.; Chiba, K. Involvement of mitogen-activating protein kinase and intracellular pH in the duration of the metaphase I (MI) pause of starfish oocytes after spawning. Dev. Growth Differ. 2008, 50, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K. Evolution of the acquisition of fertilization competence and polyspermy blocks during meiotic maturation. Mol. Reprod. Dev. 2011, 78, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Plachot, M.; Mandelbaum, J. Oocyte maturation, fertilization and embryonic growth in vitro. Br. Med. Bull. 1990, 46, 675–694. [Google Scholar] [CrossRef]
- Voronina, E.; Wessel, G.M. The regulation of oocyte maturation. Curr. Top. Dev. Biol. 2003, 58, 53–110. [Google Scholar]
- Conti, M. Hormones and growth factors in the regulation of oocyte maturation. In Biology and Pathology of the Oocyte: Role in Fertility, Medicine and Nuclear Reprograming; Trounson, A., Gosden, R., Eichenlaub-Ritter, U., Eds.; Cambridge University Press, University Printing House: Shaftesbury Road, Cambridge, UK, 2013; pp. 109–118. [Google Scholar]
- Abbara, A.; Clarke, S.A.; Dhillo, W.S. Novel Concepts for Inducing Final Oocyte Maturation in In Vitro Fertilization Treatment. Endocr. Rev. 2018, 39, 593–628. [Google Scholar] [CrossRef]
- Mita, M. Starfish Gonadotropic Hormone: From Gamete-Shedding Substance to Relaxin-Like Gonad-Stimulating Peptide. Front. Endocrinol. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Tadenuma, H.; Takahashi, K.; Chiba, K.; Hoshi, M.; Katada, T. Properties of 1-methyladenine receptors in starfish oocyte membranes: Involvement of pertussis toxin-sensitive GTP-binding protein in the receptor-mediated signal transduction. Biochem. Biophys. Res. Commun. 1992, 186, 114–121. [Google Scholar] [CrossRef]
- Shilling, F.; Chiba, K.; Hoshi, M.; Kishimoto, T.; Jaffe, L.A. Pertussis toxin inhibits 1-methyladenine-induced maturation in starfish oocytes. Dev. Biol. 1989, 133, 605–608. [Google Scholar] [CrossRef]
- Chiba, K.; Tadenuma, H.; Matsumoto, M.; Takahashi, K.; Katada, T.; Hoshi, M. The primary structure of the alpha subunit of a starfish guanosine-nucleotide-binding regulatory protein involved in 1-methyladenine-induced oocyte maturation. Eur. J. Biochem. 1992, 207, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Tadenuma, H.; Chiba, K.; Takahashi, K.; Hoshi, M.; Katada, T. Purification and characterization of a GTP-binding protein serving as pertussis toxin substrate in starfish oocytes. Arch. Biochem. Biophys. 1991, 290, 411–417. [Google Scholar] [CrossRef]
- Chiba, K.; Kontani, K.; Tadenuma, H.; Katada, T.; Hoshi, M. Induction of starfish oocyte maturation by the beta gamma subunit of starfish G protein and possible existence of the subsequent effector in cytoplasm. Mol. Biol. Cell 1993, 4, 1027–1034. [Google Scholar] [CrossRef]
- Jaffe, L.A.; Gallo, C.J.; Lee, R.H.; Ho, Y.K.; Jones, T.L. Oocyte maturation in starfish is mediated by the beta gamma-subunit complex of a G-protein. J. Cell Biol. 1993, 121, 775–783. [Google Scholar] [CrossRef]
- Hiraoka, D.; Aono, R.; Hanada, S.-I.; Okumura, E.; Kishimoto, T. Two new competing pathways establish the threshold for cyclin-B-Cdk1 activation at the meiotic G2/M transition. J. Cell Sci. 2016, 129, 3153–3166. [Google Scholar] [CrossRef]
- Bruhn, M.A.; Pearson, R.B.; Hannan, R.D.; Sheppard, K.E. AKT-independent PI3-K signaling in cancer - emerging role for SGK3. Cancer Manag. Res. 2013, 5, 281–292. [Google Scholar]
- Nakano, T.; Kontani, K.; Kurosu, H.; Katada, T.; Hoshi, M.; Chiba, K. G-protein betagamma subunit-dependent phosphorylation of 62-kDa protein in the early signaling pathway of starfish oocyte maturation induced by 1-methyladenine. Dev. Biol. 1999, 209, 200–209. [Google Scholar] [CrossRef]
- Sadler, K.C.; Ruderman, J.V. Components of the signaling pathway linking the 1-methyladenine receptor to MPF activation and maturation in starfish oocytes. Dev. Biol. 1998, 197, 25–38. [Google Scholar] [CrossRef]
- Hosoda, E.; Hiraoka, D.; Hirohashi, N.; Omi, S.; Kishimoto, T.; Chiba, K. SGK regulates pH increase and cyclin B–Cdk1 activation to resume meiosis in starfish ovarian oocytes. J. Cell Biol. 2019, 218, 3612–3629. [Google Scholar] [CrossRef]
- Hiraoka, D.; Hosoda, E.; Chiba, K.; Kishimoto, T. SGK phosphorylates Cdc25 and Myt1 to trigger cyclin B-Cdk1 activation at the meiotic G2/M transition. J. Cell Biol. 2019, 218, 3597–3611. [Google Scholar] [CrossRef]
- Okumura, E.; Fukuhara, T.; Yoshida, H.; Hanada Si, S.; Kozutsumi, R.; Mori, M.; Tachibana, K.; Kishimoto, T. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat. Cell Biol. 2002, 4, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Nakagawa, T.; Nakaya, F.; Hirohashi, N.; Chiba, K. Arrest at metaphase of meiosis I in starfish oocytes in the ovary is maintained by high CO2 and low O2 concentrations in extracellular fluid. Zoolog. Sci. 2013, 30, 975–984. [Google Scholar] [CrossRef]
- Putney, L.K.; Barber, D.L. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J. Biol. Chem. 2003, 278, 44645–44649. [Google Scholar] [CrossRef]
- Lénárt, P.; Bacher, C.P.; Daigle, N.; Hand, A.R.; Eils, R.; Terasaki, M.; Ellenberg, J. A contractile nuclear actin network drives chromosome congression in oocytes. Nature 2005, 436, 812–818. [Google Scholar] [CrossRef]
- Mori, M.; Somogyi, K.; Kondo, H.; Monnier, N.; Falk, H.J.; Machado, P.; Bathe, M.; Nédélec, F.; Lénárt, P. An Arp2/3 nucleated F-actin shell fragments nuclear membranes at nuclear envelope breakdown in starfish oocytes. Curr. Biol. CB 2014, 24, 1421–1428. [Google Scholar] [CrossRef]
- Mori, M.; Monnier, N.; Daigle, N.; Bathe, M.; Ellenberg, J.; Lénárt, P. Intracellular transport by an anchored homogeneously contracting F-actin meshwork. Curr. Biol. CB 2011, 21, 606–611. [Google Scholar] [CrossRef]
- Bun, P.; Dmitrieff, S.; Belmonte, J.M.; Nédélec, F.J.; Lénárt, P. A disassembly-driven mechanism explains F-actin-mediated chromosome transport in starfish oocytes. eLife 2018, 7, e31469. [Google Scholar] [CrossRef]
- Mehlmann, L.M.; Kalinowski, R.R.; Ross, L.F.; Parlow, A.F.; Hewlett, E.L.; Jaffe, L.A. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev. Biol. 2006, 299, 345–355. [Google Scholar] [CrossRef]
- Mehlmann, L.M.; Saeki, Y.; Tanaka, S.; Brennan, T.J.; Evsikov, A.V.; Pendola, F.L.; Knowles, B.B.; Eppig, J.J.; Jaffe, L.A. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 2004, 306, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.E.; Lane, S.I.R.; Jones, K.T. The control of meiotic maturation in mammalian oocytes. Curr. Top. Dev. Biol. 2013, 102, 207–226. [Google Scholar] [PubMed]
- Jaffe, L.A.; Egbert, J.R. Regulation of Mammalian Oocyte Meiosis by Intercellular Communication Within the Ovarian Follicle. Annu. Rev. Physiol. 2017, 79, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, Y.-Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, S.; Weeks, J.L.; Hsieh, M.; Menniti, F.S.; Conti, M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol. Reprod. 2009, 81, 595–604. [Google Scholar] [CrossRef]
- Norris, R.P.; Ratzan, W.J.; Freudzon, M.; Mehlmann, L.M.; Krall, J.; Movsesian, M.A.; Wang, H.; Ke, H.; Nikolaev, V.O.; Jaffe, L.A. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Dev. Camb. Engl. 2009, 136, 1869–1878. [Google Scholar] [CrossRef]
- Robinson, J.W.; Zhang, M.; Shuhaibar, L.C.; Norris, R.P.; Geerts, A.; Wunder, F.; Eppig, J.J.; Potter, L.R.; Jaffe, L.A. Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev. Biol. 2012, 366, 308–316. [Google Scholar] [CrossRef]
- Egbert, J.R.; Shuhaibar, L.C.; Edmund, A.B.; Van Helden, D.A.; Robinson, J.W.; Uliasz, T.F.; Baena, V.; Geerts, A.; Wunder, F.; Potter, L.R.; et al. Dephosphorylation and inactivation of NPR2 guanylyl cyclase in granulosa cells contributes to the LH-induced decrease in cGMP that causes resumption of meiosis in rat oocytes. Dev. Camb. Engl. 2014, 141, 3594–3604. [Google Scholar] [CrossRef]
- Iwamatsu, T.; Chang, M.C. Factors involved in the fertilization of mouse eggs in vitro. J. Reprod. Fertil. 1971, 26, 197–208. [Google Scholar] [CrossRef]
- Das, D.; Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar] [CrossRef]
- Chi, M.N.; Guo, S.T.; Wilmott, J.S.; Guo, X.Y.; Yan, X.G.; Wang, C.Y.; Liu, X.Y.; Jin, L.; Tseng, H.-Y.; Liu, T.; et al. INPP4B is upregulated and functions as an oncogenic driver through SGK3 in a subset of melanomas. Oncotarget 2015, 6, 39891–39907. [Google Scholar] [CrossRef] [PubMed]
- Gasser, J.A.; Inuzuka, H.; Lau, A.W.; Wei, W.; Beroukhim, R.; Toker, A. SGK3 Mediates INPP4B-Dependent PI3K Signaling in Breast Cancer. Mol. Cell 2014, 56, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Macartney, T.; Hornberger, A.; Anderson, K.E.; Tovell, H.; Prescott, A.R.; Alessi, D.R. Mechanism of activation of SGK3 by growth factors via the Class 1 and Class 3 PI3Ks. Biochem. J. 2018, 475, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Bago, R.; Malik, N.; Munson, M.J.; Prescott, A.R.; Davies, P.; Sommer, E.; Shpiro, N.; Ward, R.; Cross, D.; Ganley, I.G.; et al. Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem. J. 2014, 463, 413–427. [Google Scholar] [CrossRef]
- He, P.; Lee, S.-J.; Lin, S.; Seidler, U.; Lang, F.; Fejes-Toth, G.; Naray-Fejes-Toth, A.; Yun, C.C. Serum- and glucocorticoid-induced kinase 3 in recycling endosomes mediates acute activation of Na+/H+ exchanger NHE3 by glucocorticoids. Mol. Biol. Cell 2011, 22, 3812–3825. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiba, K. Oocyte Maturation in Starfish. Cells 2020, 9, 476. https://doi.org/10.3390/cells9020476
Chiba K. Oocyte Maturation in Starfish. Cells. 2020; 9(2):476. https://doi.org/10.3390/cells9020476
Chicago/Turabian StyleChiba, Kazuyoshi. 2020. "Oocyte Maturation in Starfish" Cells 9, no. 2: 476. https://doi.org/10.3390/cells9020476
APA StyleChiba, K. (2020). Oocyte Maturation in Starfish. Cells, 9(2), 476. https://doi.org/10.3390/cells9020476



