Association between Microsatellite Instability Status and Peri-Operative Release of Circulating Tumour Cells in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Blood Samples
2.2. CTC Enrichment and Enumeration
2.3. Clinical and Histopathological Data
2.4. Statistical Analysis
3. Results
3.1. Clinical and Surgical Characteristics
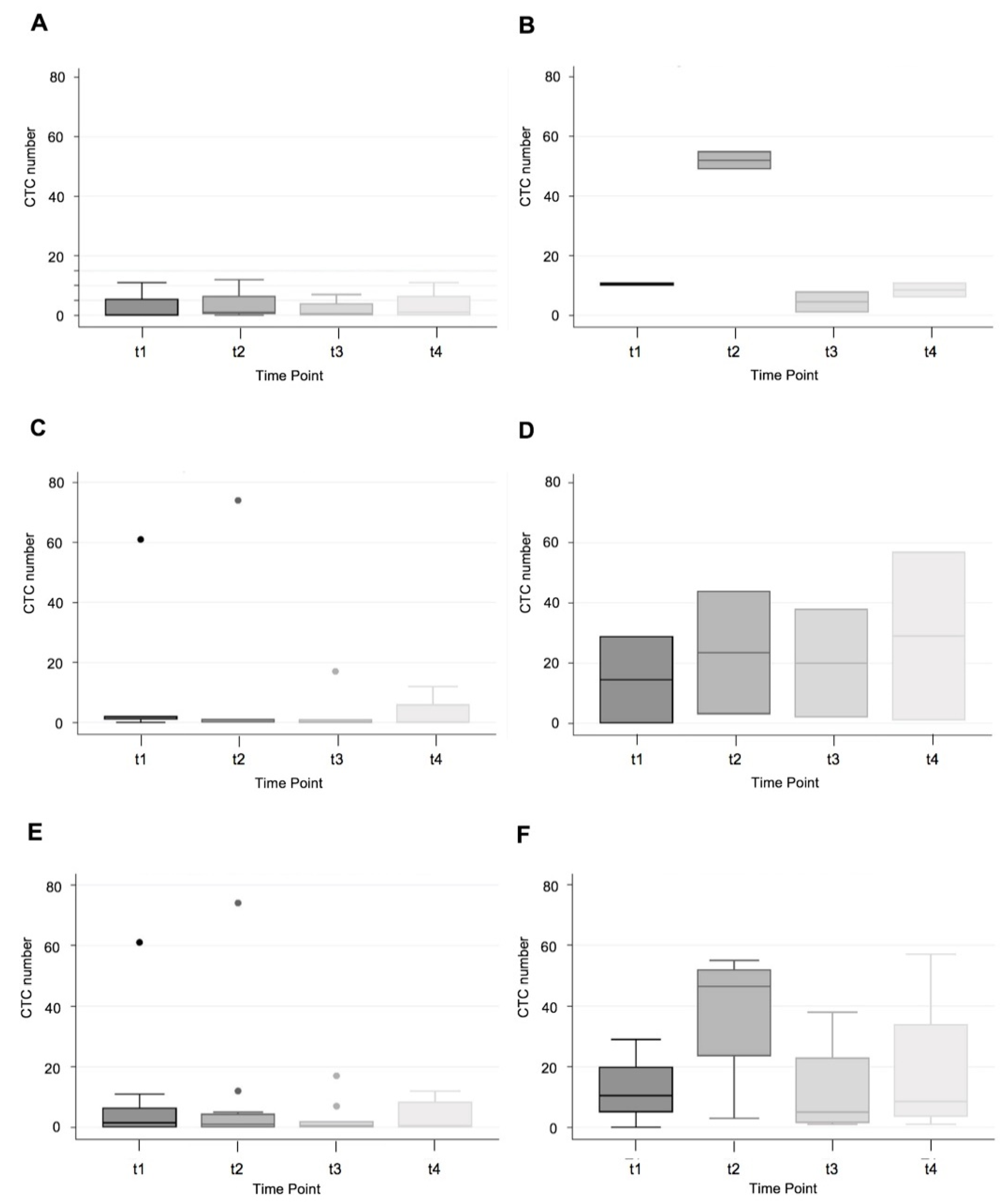
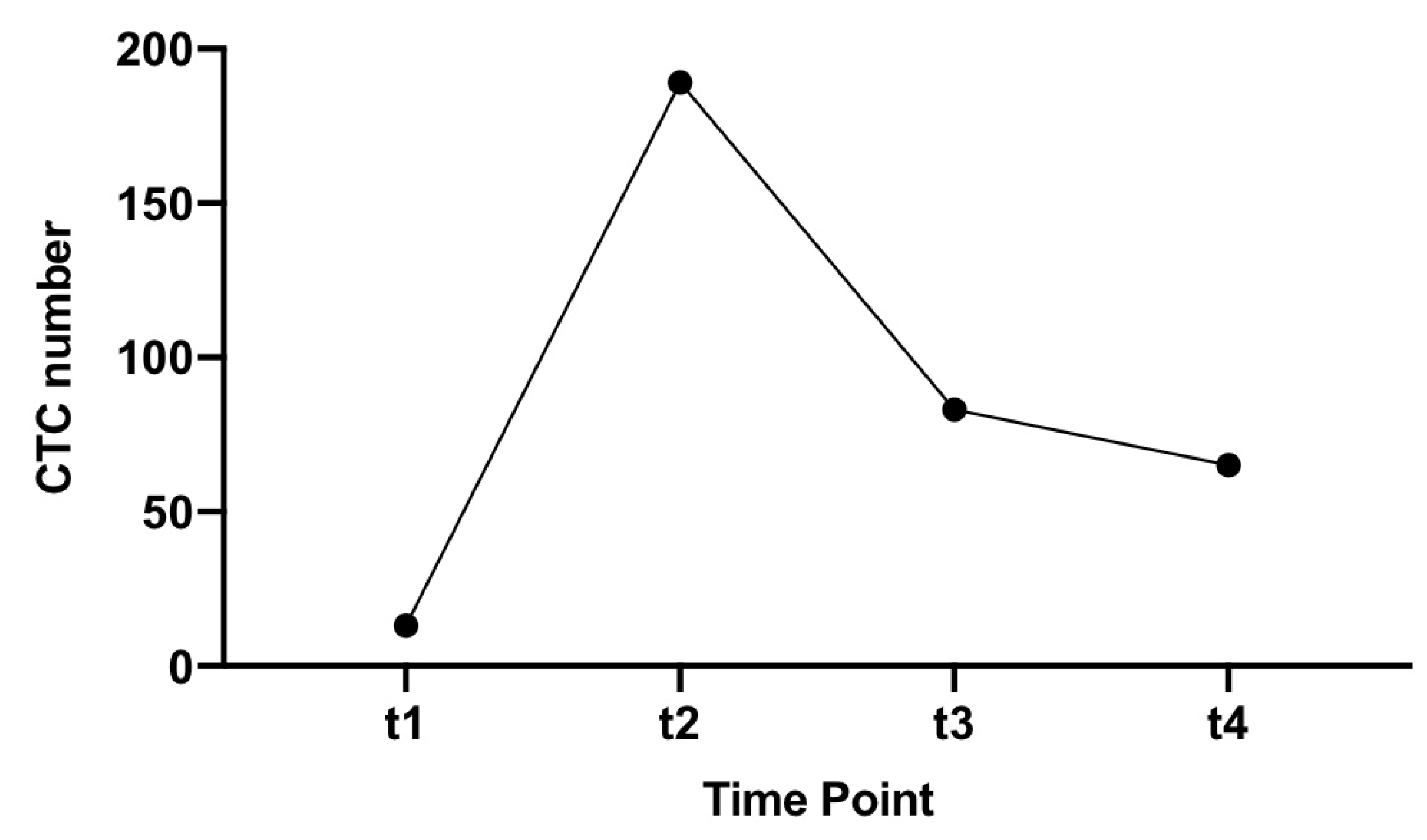
3.2. CTC Yield in All Patients
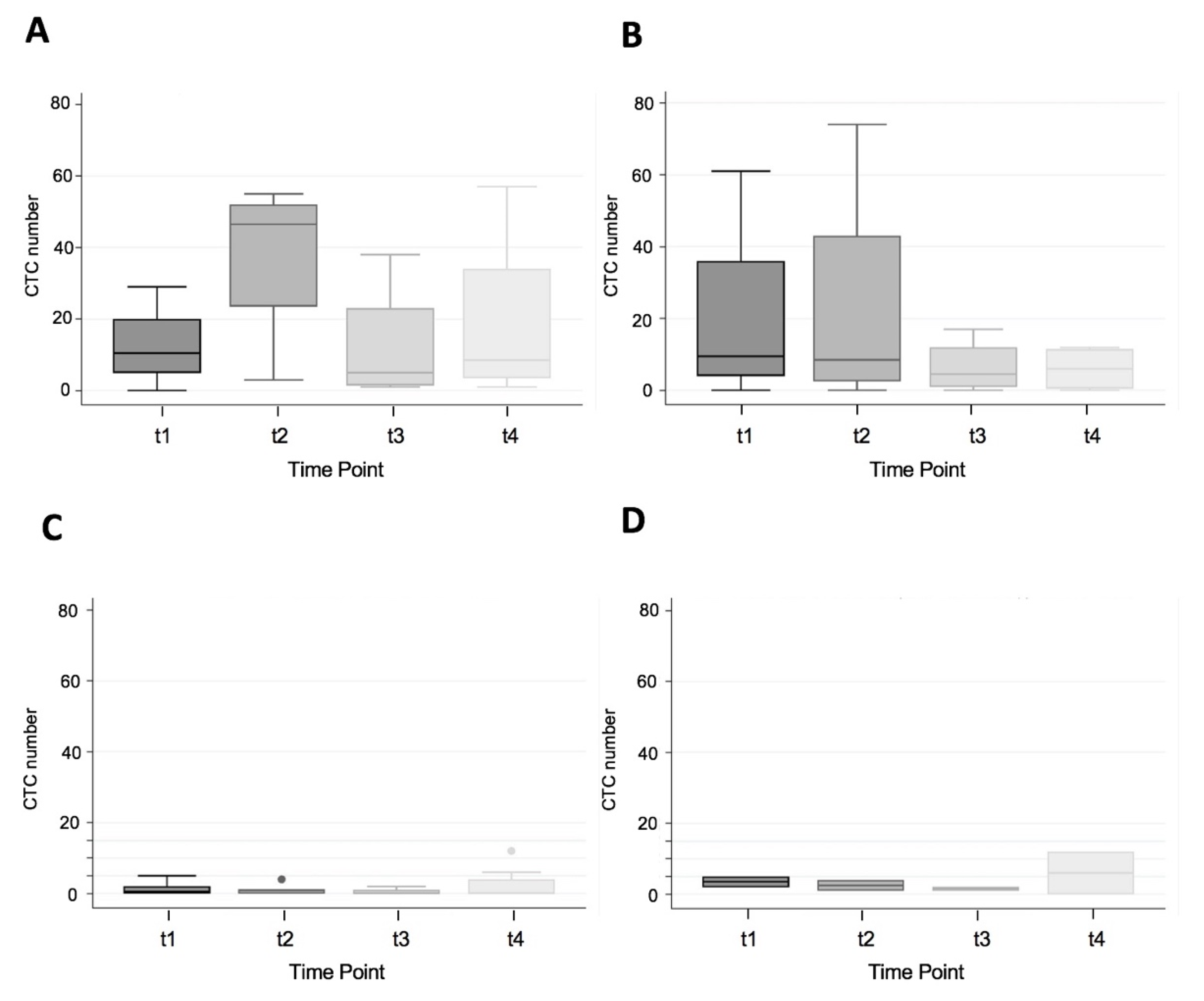
3.3. CTC Yield in Cancer Patients Only
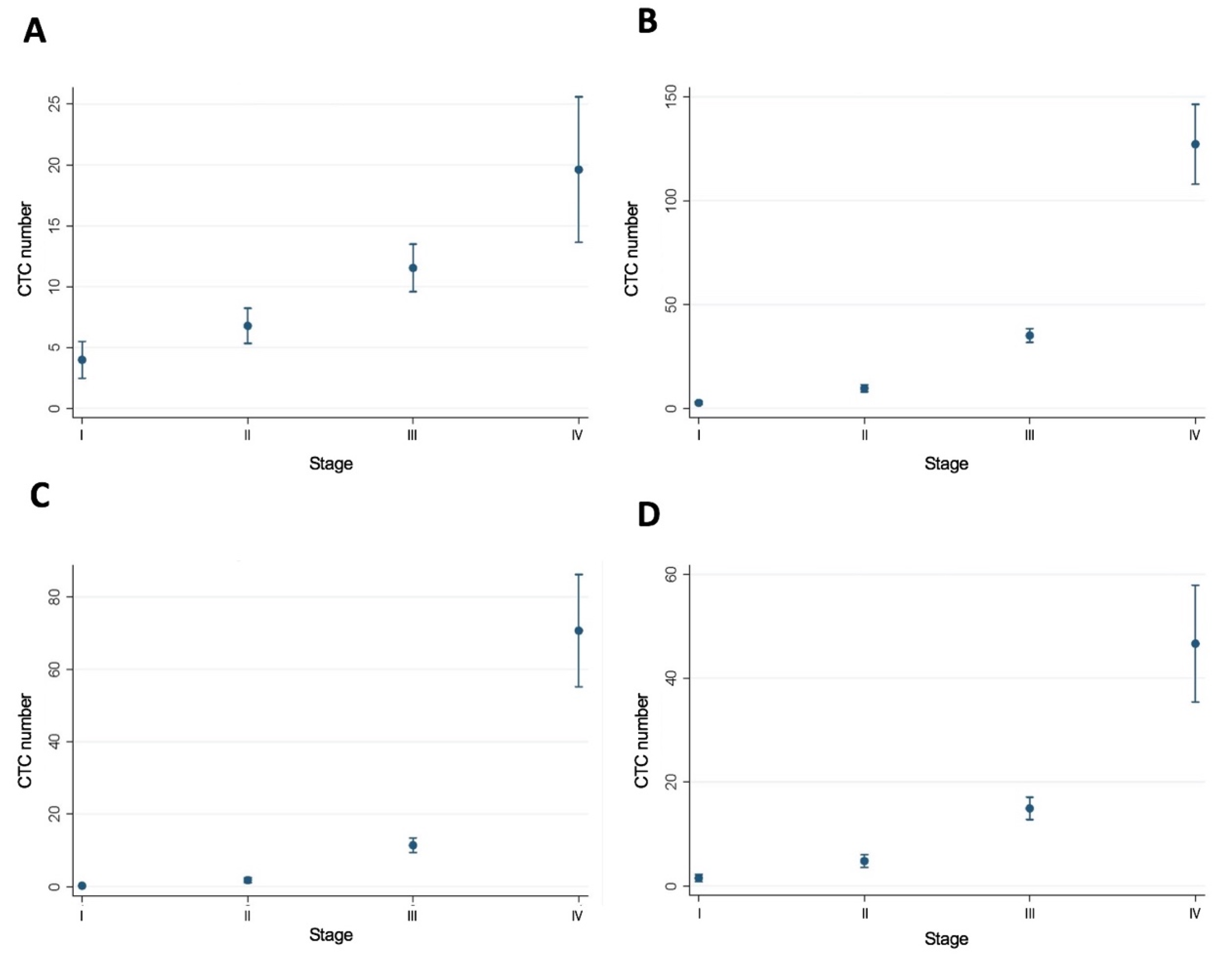
3.4. MSI Status and CTC Number by Stage of CRC
3.5. MSI Status and CTC by Side (Right vs. Left) Stage I-III Colon Cancer
3.6. Poisson Regression Model with Post-Estimation Marginal Fundamental Analysis
3.7. Non-Parametric Wilcoxon–Mann–Whitney U-test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra392. [Google Scholar] [CrossRef]
- Michael-Robinson, J.M.; Biemer-Huttmann, A.; Purdie, D.M.; Walsh, M.D.; Simms, L.A.; Biden, K.G.; Young, J.P.; Leggett, B.A.; Jass, J.R.; Radford-Smith, G.L. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut 2001, 48, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef]
- Guastadisegni, C.; Colafranceschi, M.; Ottini, L.; Dogliotti, E. Microsatellite instability as a marker of prognosis and response to therapy: A meta-analysis of colorectal cancer survival data. Eur. J. Cancer 2010, 46, 2788–2798. [Google Scholar] [CrossRef]
- Steinert, G.; Scholch, S.; Koch, M.; Weitz, J. Biology and significance of circulating and disseminated tumour cells in colorectal cancer. Langenbeck’s Arch. Surg. 2012, 397, 535–542. [Google Scholar] [CrossRef]
- Hiraiwa, K.; Takeuchi, H.; Hasegawa, H.; Saikawa, Y.; Suda, K.; Ando, T.; Kumagai, K.; Irino, T.; Yoshikawa, T.; Matsuda, S.; et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann. Surg. Oncol. 2008, 15, 3092–3100. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, J.; Feng, J.G.; Ju, H.X.; Zhu, Y.P.; Feng, H.Y.; Li, D.C. Detection of circulating tumor cells in peripheral blood of colorectal cancer patients without distant organ metastases. Cell. Oncol. 2013, 36, 43–53. [Google Scholar] [CrossRef]
- Gazzaniga, P.; Gianni, W.; Raimondi, C.; Gradilone, A.; Russo, G.L.; Longo, F.; Gandini, O.; Tomao, S.; Frati, L. Circulating tumor cells in high-risk nonmetastatic colorectal cancer. Tumour Biol. 2013, 34, 2507–2509. [Google Scholar] [CrossRef]
- Katsuno, H.; Zacharakis, E.; Aziz, O.; Rao, C.; Deeba, S.; Paraskeva, P.; Ziprin, P.; Athanasiou, T.; Darzi, A. Does the presence of circulating tumor cells in the venous drainage of curative colorectal cancer resections determine prognosis? A meta-analysis. Ann. Surg. Oncol. 2008, 15, 3083–3091. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Rahbari, N.N.; Buchler, M.W.; Koch, M.; Weitz, J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: A meta-analysis. Ann. Surg. Oncol. 2013, 20, 2156–2165. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Aigner, M.; Thorlund, K.; Mollberg, N.; Motschall, E.; Jensen, K.; Diener, M.K.; Büchler, M.W.; Koch, M.; Weitz, J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010, 138, 1714–1726. [Google Scholar] [CrossRef]
- Thorsteinsson, M.; Jess, P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer—a review. Eur J. Surg Oncol. 2011, 37, 459–465. [Google Scholar] [CrossRef]
- Gazzaniga, P.; Raimondi, C.; Gradilone, A.; Biondi Zoccai, G.; Nicolazzo, C.; Gandini, O.; Longo, F.; Tomao, S.; Lo Russo, G.; Seminara, P.; et al. Circulating tumor cells in metastatic colorectal cancer: Do we need an alternative cutoff? J. Cancer Res. Clin. Oncol. 2013, 139, 1411–1416. [Google Scholar] [CrossRef]
- Peach, G.; Kim, C.; Zacharakis, E.; Purkayastha, S.; Ziprin, P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: A systematic review. Br. J. Cancer 2010, 102, 1327–1334. [Google Scholar] [CrossRef]
- Yalcin, S.; Kilickap, S.; Portakal, O.; Arslan, C.; Hascelik, G.; Kutluk, T. Determination of circulating tumor cells for detection of colorectal cancer progression or recurrence. Hepato-gastroenterology 2010, 57, 1395–1398. [Google Scholar]
- Steinert, G.; Scholch, S.; Niemietz, T.; Iwata, N.; García, S.A.; Behrens, B.; Voigt, A.; Kloor, M.; Benner, A.; Bork, U. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014, 74, 1694–1704. [Google Scholar] [CrossRef]
- Schwitalle, Y.; Kloor, M.; Eiermann, S.; Linnebacher, M.; Kienle, P.; Knaebel, H.P.; Tariverdian, M.; Benner, A.; Doeberitz, M.v.K. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 2008, 134, 988–997. [Google Scholar] [CrossRef]
- Buckowitz, A.; Knaebel, H.P.; Benner, A.; Bläker, H.; Gebert, J.; Kienle, P.; Doeberitz, M.v.K.; Kloor, M. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br. J. Cancer 2005, 92, 1746–1753. [Google Scholar] [CrossRef]
- Kazama, Y.; Watanabe, T.; Kanazawa, T.; Tanaka, J.; Tanaka, T.; Nagawa, H. Microsatellite instability in poorly differentiated adenocarcinomas of the colon and rectum: Relationship to clinicopathological features. J. Clin. Pathol. 2007, 60, 701–704. [Google Scholar] [CrossRef]
- Toh, J.; Chapuis, P.H.; Bokey, L.; Chan, C.; Spring, K.J.; Dent, O.F. Competing risks analysis of microsatellite instability as a prognostic factor in colorectal cancer. Br. J. Surg. 2017, 104, 1250–1259. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, G.H. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J. Gastroenterol. WJG 2014, 20, 4230–4243. [Google Scholar] [CrossRef] [PubMed]
- Greenson, J.K.; Bonner, J.D.; Ben-Yzhak, O.; Cohen, H.I.; Miselevich, I.; Resnick, M.B.; Trougouboff, P.; Tomsho, L.; Kim, E.; Low, M.; et al. Phenotype of microsatellite unstable colorectal carcinomas: Well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am. J. Surg. Pathol. 2003, 27, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Smyrk, T.C.; Watson, P.; Kaul, K.; Lynch, H.T. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 2001, 91, 2417–2422. [Google Scholar] [CrossRef]
- Tougeron, D.; Fauquembergue, E.; Rouquette, A.; Pessot, F.L.; Sesboüé, R.; Laurent, M.; Berthet, P.; Mauillon, J.; Fiore, F.D.; Sabourin, J.C. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod. Pathol. 2009, 22, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Pages, F.; Berger, A.; Camus, M.; Sanchez-Cabo, F.; Costes, A.; Molidor, R.; Mlecnik, B.; Kirilovsky, A.; Nilsson, M.; Damotte, D.; et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New Engl. J. Med. 2005, 353, 2654–2666. [Google Scholar] [CrossRef]
- Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Berger, A.; Bindea, G.; Meatchi, T.; Bruneval, P.; Trajanoski, Z.; Fridman, W.H.; Page’s, F.; et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 2011, 29, 610–618. [Google Scholar] [CrossRef]
- Ling, A.; Edin, S.; Wikberg, M.L.; Oberg, A.; Palmqvist, R. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br. J. Cancer 2014, 110, 2551–2559. [Google Scholar] [CrossRef]
- Svennevig, J.L.; Lunde, O.C.; Holter, J.; Bjorgsvik, D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br. J. Cancer 1984, 49, 375–377. [Google Scholar] [CrossRef][Green Version]
- Jass, J.R. Lymphocytic infiltration and survival in rectal cancer. J. Clin. Pathol. 1986, 39, 585–589. [Google Scholar] [CrossRef]
- Shunyakov, L.; Ryan, C.K.; Sahasrabudhe, D.M.; Khorana, A.A. The influence of host response on colorectal cancer prognosis. Clin. Colorectal Cancer 2004, 4, 38–45. [Google Scholar] [CrossRef]
- Dahlin, A.M.; Henriksson, M.L.; Van Guelpen, B.; Stenling, R.; Öberg, A.; Rutegård, J.; Palmqvist, R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod. Pathol. 2011, 24, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Ohtani, H.; Mizoi, T.; Naito, Y.; Nagura, H.; Ohuchi, A.; Ohuchi, K.; Shiiba, K.; Kurokawa, Y.; Satomi, S. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: Possible association with suppression of micrometastasis. Br. J. Cancer 2004, 91, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Lugli, A.; Baker, K.; Roth, S.; Minoo, P.; Hayashi, S.; Terracciano, L.; Jass, J.R. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J. Pathol. 2007, 212, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Prall, F.; Duhrkop, T.; Weirich, V.; Ostwald, C.; Lenz, P.; Nizze, H.; Barten, M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum. Pathol. 2004, 35, 808–816. [Google Scholar] [CrossRef]
- Koch, M.; Beckhove, P.; Op den Winkel, J.; Autenrieth, D.; Wagner, P.; Nummer, D.; Specht, S.; Antolovic, D.; Galindo, L. Schmitz-Winnenthal, F.H. Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann. Surg. 2006, 244, 986–992. [Google Scholar] [CrossRef]
- Lamberti, C.; Lundin, S.; Bogdanow, M.; Pagenstecher, C.; Friedrichs, N.; Büttner, R.; Sauerbruch, T. Microsatellite instability did not predict individual survival of unselected patients with colorectal cancer. Int. J. Colorectal Dis. 2007, 22, 145–152. [Google Scholar] [CrossRef]
- Xiao, H.; Yoon, Y.S.; Hong, S.M.; Ae Roh, S.; Cho, D.H.; Yu, C.S.; Kim, J.C. Poorly differentiated colorectal cancers: Correlation of microsatellite instability with clinicopathologic features and survival. Am. J. Clin. Pathol. 2013, 140, 341–347. [Google Scholar] [CrossRef]
- Thibodeau, S.N.; Bren, G.; Schaid, D. Microsatellite instability in cancer of the proximal colon. Science 1993, 260, 816–819. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Kim, J.; Hong, S.M.; Lee, J.L.; Kim, C.W.; Park, I.J.; Lim, S.B.; Yu, C.S.; Kim, J.C. Clinical implications of mucinous components correlated with microsatellite instability in patients with colorectal cancer. Colorectal Dis. 2015, 17, O161–O167. [Google Scholar] [CrossRef]
- Karahan, B.; Argon, A.; Yildirim, M.; Vardar, E. Relationship between MLH-1, MSH-2, PMS-2,MSH-6 expression and clinicopathological features in colorectal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 4044–4053. [Google Scholar]
- Maby, P.; Tougeron, D.; Hamieh, M.; Mlecnik, B.; Kora, H.; Bindea, G.; Angell, H.K.; Fredriksen, T.; Elie, N.; Fauquembergue, E.; et al. Correlation between density of CD8+ T cell infiltrates in microsatellite unstable colorectal cancers and frameshift mutations: A rationale for personalized immunotherapy. Cancer Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Saeterdal, I.; Bjorheim, J.; Lislerud, K.; Gjertsen, M.K.; Bukholm, I.K.; Olsen, O.C.; Nesland, J.M.; Eriksen, J.A.; Møller, M.; Lindblom, A.; et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 13255–13260. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yu, X.; Wang, H.; Zhang, S.; Zhao, Z.; Xu, R. Clinical significance of mismatch repair gene expression in sporadic colorectal cancer. Exp. Ther. Med. 2014, 8, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
| Patient Characteristics | Microsatellite Status | |
|---|---|---|
| MSI-H | MSS | |
| Patient number | 4 | 13 |
| Age | 85.5 (54–86) | 66 (44–86) |
| Female:male | 3:1 | 6:7 |
| Right colon | 4 (100%) | 5 (38.5%) |
| Left colon | 0 | 3 (23.1%) |
| Rectal/rectosigmoid | 0 | 5 (38.5%) |
| Grade | ||
| High | 3 (75%) | 1 (8.3%) |
| Moderate | 1 (25%) | 10 (83.3%) |
| Low | 0 | 1 (8.3%) |
| BRAF mutant:wild-type | 3:1 | N/A |
| Stage | ||
| I | 0 | 3 (23.1%) |
| II | 2 (50%) | 4 (30.8%) |
| III | 2 (50%) | 5 (38.5%) |
| IV | 0 | 1 (7.7%) |
| CTC number | ||
| t1 | 10.5 (0–29) | 1 (0–61) |
| t2 | 52 (44–189) | 1 (0–74) |
| t3 | 23 (1–83) | 1 (0–17) |
| t4 | 34 (6–65) | 1 (0–12) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toh, J.W.T.; Lim, S.H.; MacKenzie, S.; de Souza, P.; Bokey, L.; Chapuis, P.; Spring, K.J. Association between Microsatellite Instability Status and Peri-Operative Release of Circulating Tumour Cells in Colorectal Cancer. Cells 2020, 9, 425. https://doi.org/10.3390/cells9020425
Toh JWT, Lim SH, MacKenzie S, de Souza P, Bokey L, Chapuis P, Spring KJ. Association between Microsatellite Instability Status and Peri-Operative Release of Circulating Tumour Cells in Colorectal Cancer. Cells. 2020; 9(2):425. https://doi.org/10.3390/cells9020425
Chicago/Turabian StyleToh, James W. T., Stephanie H. Lim, Scott MacKenzie, Paul de Souza, Les Bokey, Pierre Chapuis, and Kevin J. Spring. 2020. "Association between Microsatellite Instability Status and Peri-Operative Release of Circulating Tumour Cells in Colorectal Cancer" Cells 9, no. 2: 425. https://doi.org/10.3390/cells9020425
APA StyleToh, J. W. T., Lim, S. H., MacKenzie, S., de Souza, P., Bokey, L., Chapuis, P., & Spring, K. J. (2020). Association between Microsatellite Instability Status and Peri-Operative Release of Circulating Tumour Cells in Colorectal Cancer. Cells, 9(2), 425. https://doi.org/10.3390/cells9020425





