Long-Term Survival of Transplanted Autologous Canine Liver Organoids in a COMMD1-Deficient Dog Model of Metabolic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
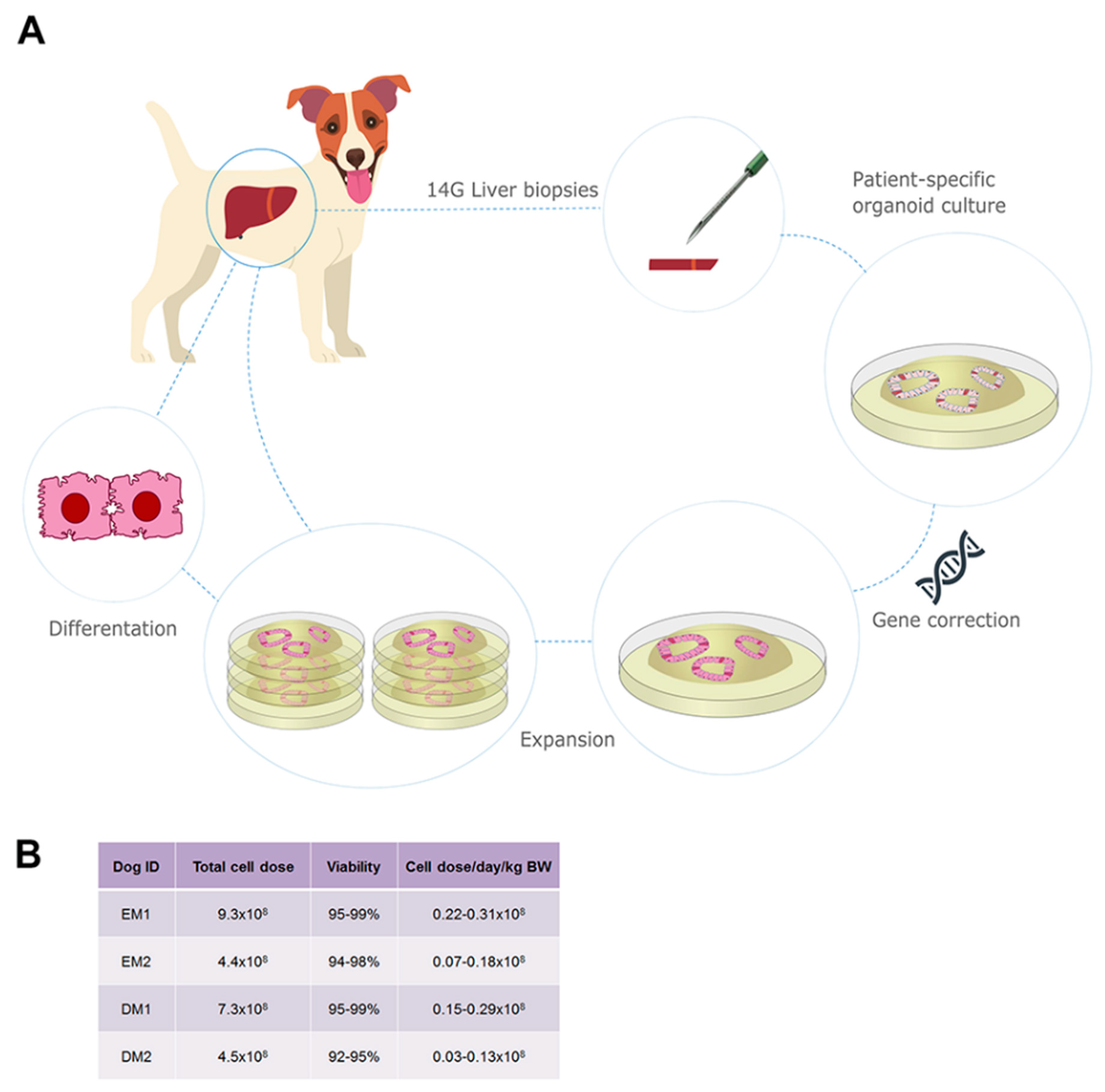
2.1. Study Design
2.2. COMMD1-Deficient Dogs
2.3. Biliary Duct Isolation, Autologous Liver Organoid Culture, Lentiviral Transduction and Harvest
2.4. Microbead Perfusion of Canine Liver
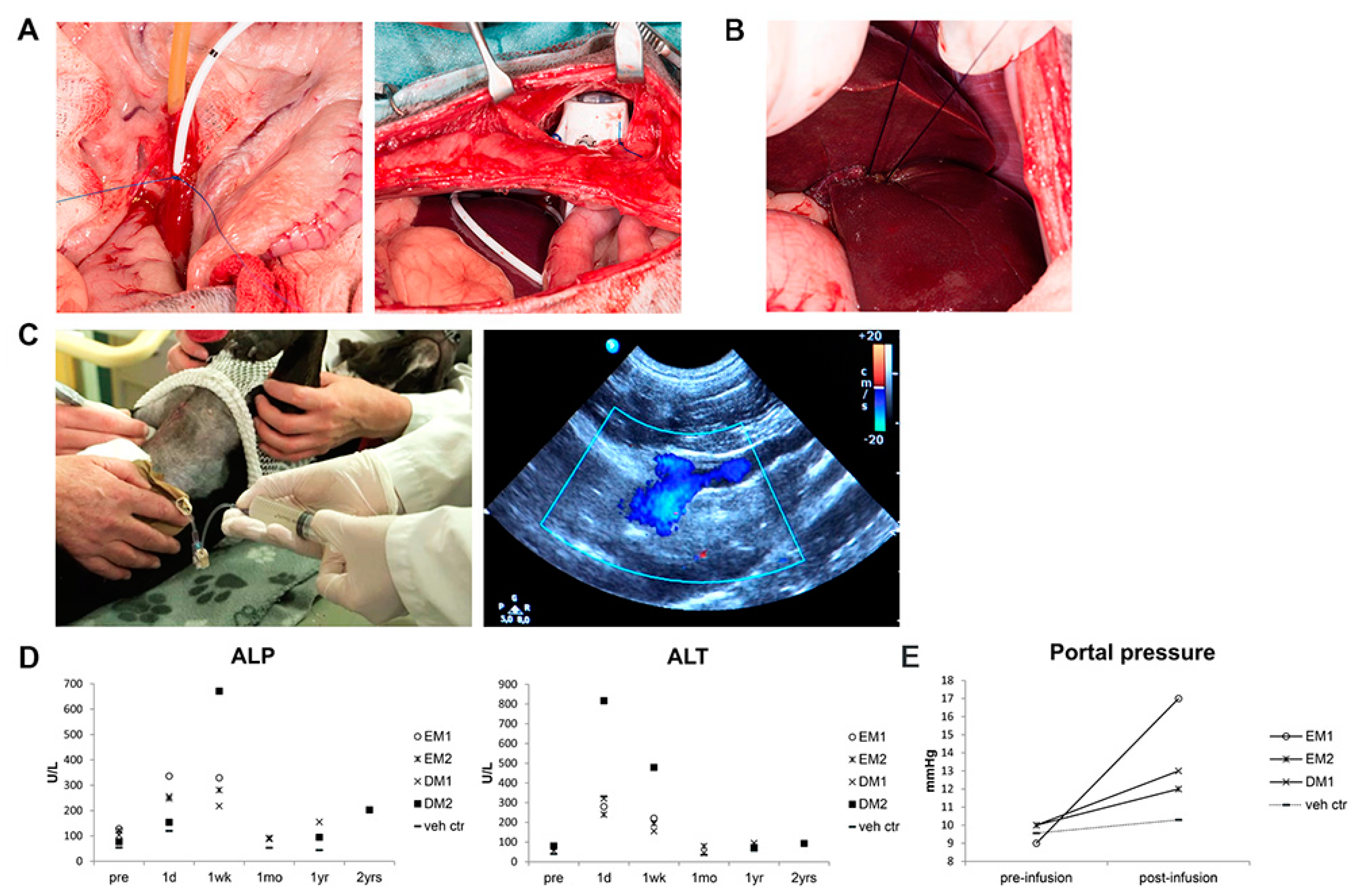
2.5. Partial Hepatectomy and Portal Catheter Implantation
2.6. Transplantation of Organoid-Derived Liver Cells by Intrahepatic Injection
2.7. Transplantation of Organoid-Derived Liver Cells by Intraportal Delivery
2.8. Immune Suppression
2.9. Follow-Up Measurements: Liver Biopsies, Blood Analysis, Staining and Biliary 64Cu Excretion Measurements
2.10. Repopulation Counts
3. Results
3.1. Autologous Gene-Corrected Liver Organoids Can Be Sufficiently Expanded for Transplantation within 12 Weeks
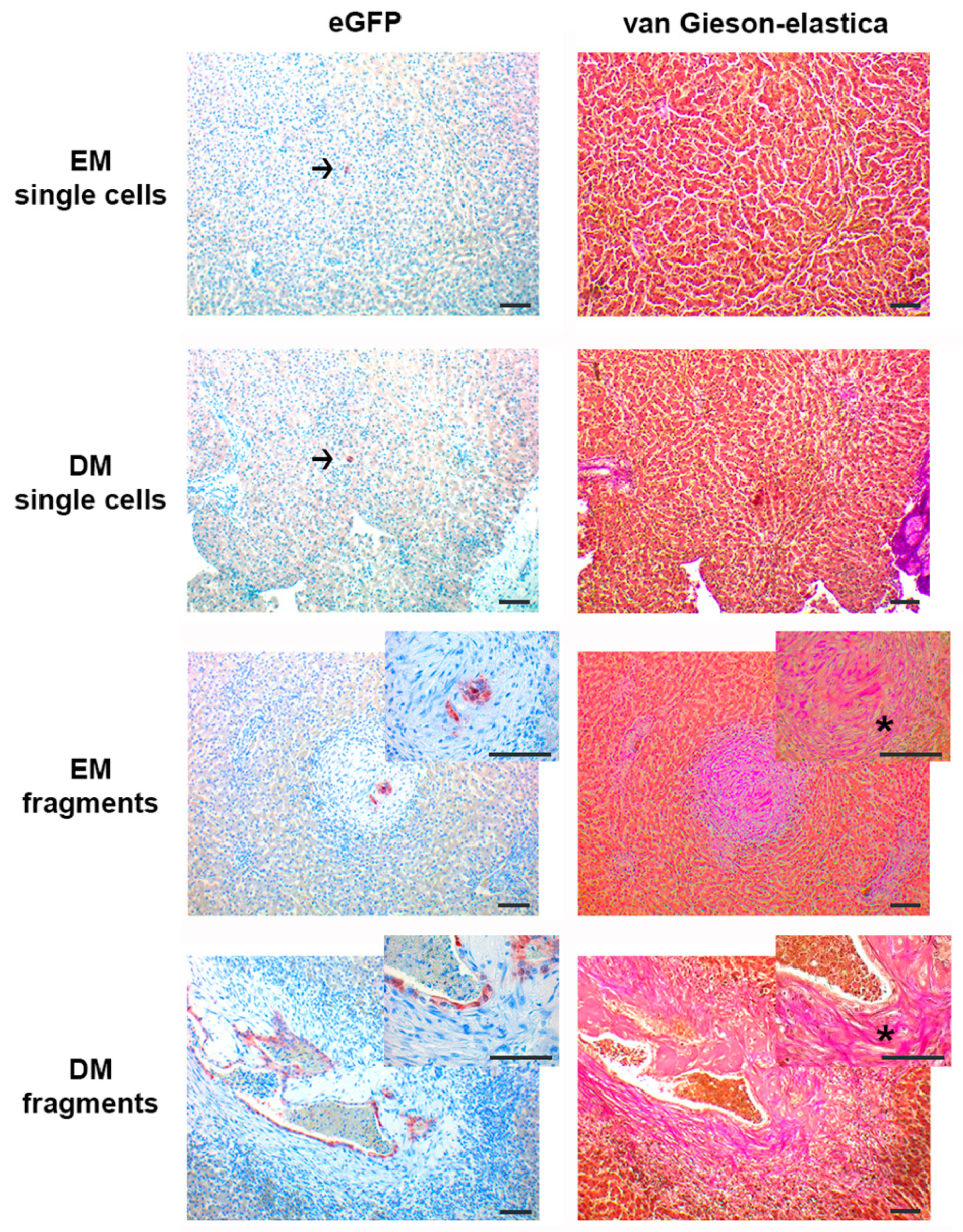
3.2. Organoid Fragments and Single Cells Survive only Short Term (7 days) after Intrahepatic Injection, Irrespective of Differentiation Status; Organoid Fragments but not Single Cells Induce De Novo Stroma Formation
3.3. Organoid-Derived Cells Can Be Safely and Repeatedly Infused via the Portal Vein
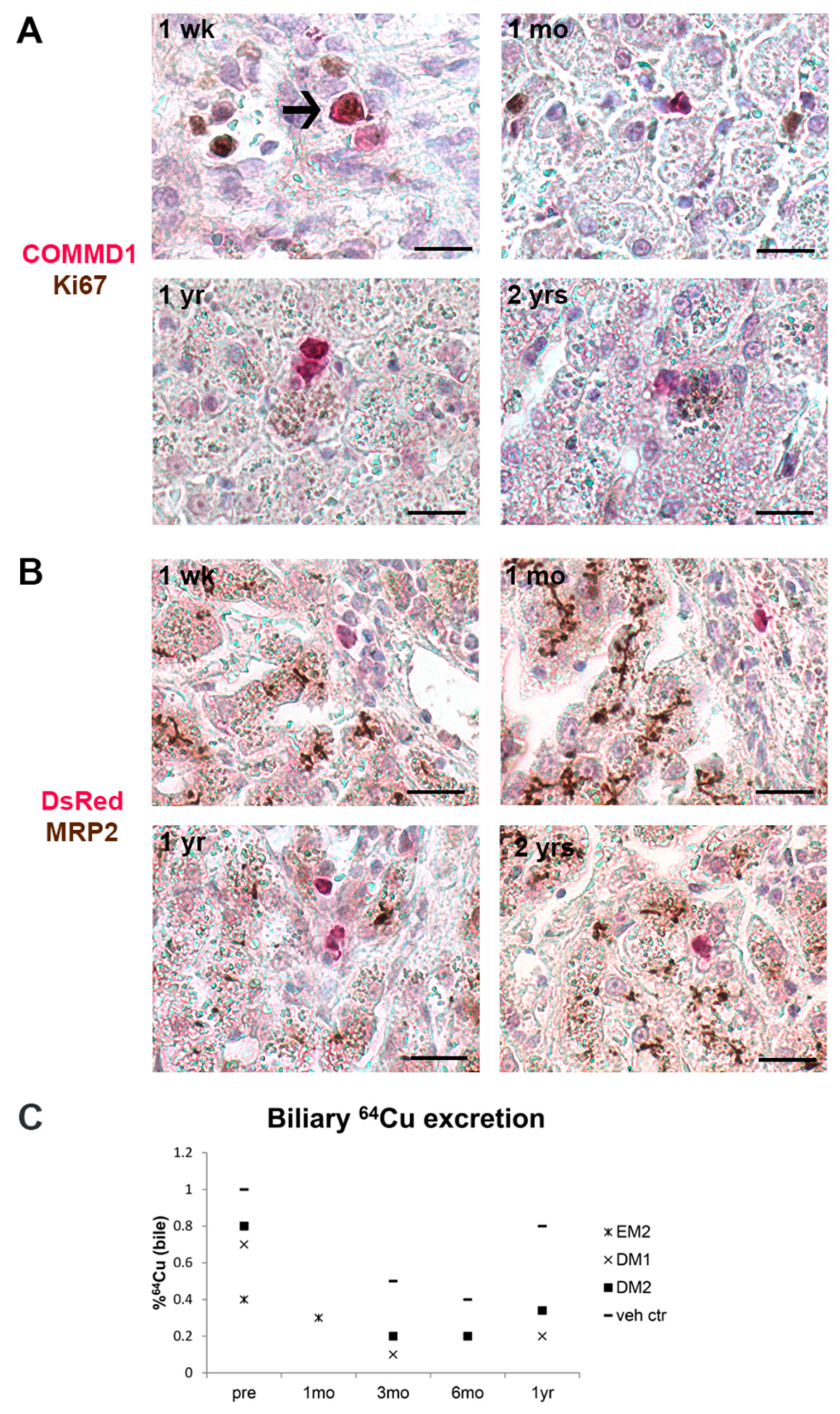
3.4. Gene-Corrected Organoid-Derived Cells Engraft and Survive in the Liver for Up To Two Years after Intraportal Delivery
3.5. Transplanted Organoid-Derived Cells Do Not Show Full Maturation and Functional Integration In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fox, I.J.; Chowdhury, J.R.; Kaufman, S.S.; Goertzen, T.C.; Chowdhury, N.R.; Warkentin, P.I.; Dorko, K.; Sauter, B.V.; Strom, S.C. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N. Engl. J. Med. 1998, 338, 1422–1426. [Google Scholar] [CrossRef]
- Puppi, J.; Tan, N.; Mitry, R.R.; Hughes, R.D.; Lehec, S.; Mieli-Vergani, G.; Karani, J.; Champion, M.P.; Heaton, N.; Mohamed, R.; et al. Hepatocyte transplantation followed by auxiliary liver transplantation--a novel treatment for ornithine transcarbamylase deficiency. Am. J. Transpl. 2008, 8, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Stephenne, X.; Najimi, M.; Sibille, C.; Nassogne, M.C.; Smets, F.; Sokal, E.M. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology 2006, 130, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Stephenne, X.; Debray, F.G.; Smets, F.; Jazouli, N.; Sana, G.; Tondreau, T.; Menten, R.; Goffette, P.; Boemer, F.; Schoos, R.; et al. Hepatocyte transplantation using the domino concept in a child with tetrabiopterin nonresponsive phenylketonuria. Cell Transpl. 2012, 21, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Puppi, J.; Strom, S.C.; Hughes, R.D.; Bansal, S.; Castell, J.V.; Dagher, I.; Ellis, E.C.; Nowak, G.; Ericzon, B.G.; Fox, I.J.; et al. Improving the techniques for human hepatocyte transplantation: Report from a consensus meeting in London. Cell Transpl. 2012, 21, 1–10. [Google Scholar] [CrossRef]
- Forbes, S.J.; Gupta, S.; Dhawan, A. Cell therapy for liver disease: From liver transplantation to cell factory. J. Hepatol. 2015, 62, 157. [Google Scholar] [CrossRef]
- Fisher, R.A.; Strom, S.C. Human hepatocyte transplantation: Worldwide results. Transplantation 2006, 82, 441–449. [Google Scholar] [CrossRef]
- Huch, M.; Dorrell, C.; Boj, S.F.; van Es, J.H.; Li, V.S.; van de Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef]
- Kuijk, E.W.; Rasmussen, S.; Blokzijl, F.; Huch, M.; Gehart, H.; Toonen, P.; Begthel, H.; Clevers, H.; Geurts, A.M.; Cuppen, E. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci. Rep. 2016, 6, 22154. [Google Scholar] [CrossRef]
- Huch, M.; Boj, S.F.; Clevers, H. Lgr5(+) liver stem cells, hepatic organoids and regenerative medicine. Regen. Med. 2013, 8, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Bru, P.; Najimi, M.; Caruso, M.; Pauwelyn, K.; Cantz, T.; Forbes, S.; Roskams, T.; Ott, M.; Gehling, U.; Sokal, E.; et al. Stem and progenitor cells for liver repopulation: Can we standardise the process from bench to bedside? Gut 2009, 58, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Theoret, C. Translating stem cell therapies: The role of companion animals in regenerative medicine. Wound Repair Regen. 2013, 21, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Kruitwagen, H.S.; Spee, B.; Schotanus, B.A. Hepatic progenitor cells in canine and feline medicine: Potential for regenerative strategies. BMC Vet. Res. 2014, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Spee, B.; Arends, B.; van den Ingh, T.S.; Roskams, T.; Rothuizen, J.; Penning, L.C. Major HGF-mediated regenerative pathways are similarly affected in human and canine cirrhosis. Comp. Hepatol. 2007, 6, 8. [Google Scholar] [CrossRef]
- Schotanus, B.A.; van den Ingh, T.S.; Penning, L.C.; Rothuizen, J.; Roskams, T.A.; Spee, B. Cross-species immunohistochemical investigation of the activation of the liver progenitor cell niche in different types of liver disease. Liver Int. 2009, 29, 1241–1252. [Google Scholar] [CrossRef]
- Ijzer, J.; Schotanus, B.A.; Vander Borght, S.; Roskams, T.A.; Kisjes, R.; Penning, L.C.; Rothuizen, J.; van den Ingh, T.S. Characterisation of the hepatic progenitor cell compartment in normal liver and in hepatitis: An immunohistochemical comparison between dog and man. Vet. J. 2010, 184, 308–314. [Google Scholar] [CrossRef]
- Su, L.C.; Ravanshad, S.; Owen, C.A., Jr.; McCall, J.T.; Zollman, P.E.; Hardy, R.M. A comparison of copper-loading disease in Bedlington terriers and Wilson’s disease in humans. Am. J. Physiol. 1982, 243, 226. [Google Scholar] [CrossRef]
- van De Sluis, B.; Rothuizen, J.; Pearson, P.L.; van Oost, B.A.; Wijmenga, C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet 2002, 11, 165–173. [Google Scholar] [CrossRef]
- Klomp, A.E.; van de Sluis, B.; Klomp, L.W.; Wijmenga, C. The ubiquitously expressed MURR1 protein is absent in canine copper toxicosis. J. Hepatol. 2003, 39, 703–709. [Google Scholar] [CrossRef]
- Favier, R.P.; Spee, B.; Schotanus, B.A.; van den Ingh, T.S.; Fieten, H.; Brinkhof, B.; Viebahn, C.S.; Penning, L.C.; Rothuizen, J. COMMD1-deficient dogs accumulate copper in hepatocytes and provide a good model for chronic hepatitis and fibrosis. PLoS ONE 2012, 7, e42158. [Google Scholar] [CrossRef] [PubMed]
- Favier, R.P.; Spee, B.; Fieten, H.; van den Ingh, T.S.; Schotanus, B.A.; Brinkhof, B.; Rothuizen, J.; Penning, L.C. Aberrant expression of copper associated genes after copper accumulation in COMMD1-deficient dogs. J. Trace Elem. Med. Biol. 2015, 29, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Nantasanti, S.; Spee, B.; Kruitwagen, H.S.; Chen, C.; Geijsen, N.; Oosterhoff, L.A.; van Wolferen, M.E.; Pelaez, N.; Fieten, H.; Wubbolts, R.W.; et al. Disease Modeling and Gene Therapy of Copper Storage Disease in Canine Hepatic Organoids. Stem Cell Rep. 2015, 5, 895–907. [Google Scholar] [CrossRef] [PubMed]
- de Nies, K.S.; Kruitwagen, H.S.; van Straten, G.; van Bruggen, L.W.L.; Robben, J.H.; Schotanus, B.A.; Akkerdaas, I.; Kummeling, A. Innovative application of an implantable venous access system in the portal vein: Technique, results and complications in three dogs. BMC Vet. Res. 2019, 15, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.A.; Sokal, E.; Stephenne, X.; Najimi, M.; de Goyet Jde, V.; Reding, R. Permanent access to the portal system for cellular transplantation using an implantable port device. Liver Transpl. 2004, 10, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Guha, C.; Deb, N.J.; Sappal, B.S.; Ghosh, S.S.; Roy-Chowdhury, N.; Roy-Chowdhury, J. Amplification of engrafted hepatocytes by preparative manipulation of the host liver. Artif. Organs 2001, 25, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Lidbury, J.A.; Rodrigues Hoffmann, A.; Ivanek, R.; Cullen, J.M.; Porter, B.F.; Oliveira, F.; Van Winkle, T.J.; Grinwis, G.C.; Sucholdolski, J.S.; Steiner, J.M. Interobserver Agreement Using Histological Scoring of the Canine Liver. J. Vet. Int. Med. 2017, 31, 778–783. [Google Scholar] [CrossRef]
- Sohlenius-Sternbeck, A.K. Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol. In Vitro 2006, 20, 1582–1586. [Google Scholar] [CrossRef]
- Kocken, J.M.; Borel Rinkes, I.H.; Bijma, A.M.; de Roos, W.K.; Bouwman, E.; Terpstra, O.T.; Sinaasappel, M. Correction of an inborn error of metabolism by intraportal hepatocyte transplantation in a dog model. Transplantation 1996, 62, 358–364. [Google Scholar] [CrossRef]
- Meyer, H.P.; Rothuizen, J.; van den Brom, W.E.; Voorhout, G.; van Sluijs, F.J.; How, K.L.; Pollak, Y.W. Quantitation of portosystemic shunting in dogs by ultrasound-guided injection of 99MTc-macroaggregates into a splenic vein. Res. Vet. Sci. 1994, 57, 58–62. [Google Scholar] [CrossRef]
- Irani, A.N.; Malhi, H.; Slehria, S.; Gorla, G.R.; Volenberg, I.; Schilsky, M.L.; Gupta, S. Correction of liver disease following transplantation of normal rat hepatocytes into Long-Evans Cinnamon rats modeling Wilson’s disease. Mol. Ther. 2001, 3, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Mandigers, P.J.; Bode, P.; van Wees, A.M.; van den Brom, W.E.; van den Ingh, T.S.; Rothuizen, J. Hepatic (64)Cu excretion in Dobermanns with subclinical hepatitis. Res. Vet. Sci. 2007, 83, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, J.; Bolleyn, J.; Vanhaecke, T.; Rogiers, V.; Vinken, M. Primary hepatocyte cultures for pharmaco-toxicological studies: At the busy crossroad of various anti-dedifferentiation strategies. Arch. Toxicol. 2013, 87, 577–610. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.; Lim, R.; Yeoh, G.C.; Olynyk, J.K. Interferon-gamma exacerbates liver damage, the hepatic progenitor cell response and fibrosis in a mouse model of chronic liver injury. J. Hepatol. 2007, 47, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Kuramitsu, K.; Sverdlov, D.Y.; Liu, S.B.; Csizmadia, E.; Burkly, L.; Schuppan, D.; Hanto, D.W.; Otterbein, L.E.; Popov, Y. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 2013, 183, 182–194. [Google Scholar] [CrossRef]
- Gupta, S.; Rajvanshi, P.; Lee, C.D. Integration of transplanted hepatocytes into host liver plates demonstrated with dipeptidyl peptidase IV-deficient rats. Proc. Natl. Acad. Sci. USA 1995, 92, 5860–5864. [Google Scholar] [CrossRef]
- Gupta, S.; Rajvanshi, P.; Sokhi, R.; Slehria, S.; Yam, A.; Kerr, A.; Novikoff, P.M. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology 1999, 29, 509–519. [Google Scholar] [CrossRef]
- Jorns, C.; Ellis, E.C.; Nowak, G.; Fischler, B.; Nemeth, A.; Strom, S.C.; Ericzon, B.G. Hepatocyte transplantation for inherited metabolic diseases of the liver. J. Int. Med. 2012, 272, 201–223. [Google Scholar] [CrossRef]
- Cheng, K.; Benten, D.; Bhargava, K.; Inada, M.; Joseph, B.; Palestro, C.; Gupta, S. Hepatic targeting and biodistribution of human fetal liver stem/progenitor cells and adult hepatocytes in mice. Hepatology 2009, 50, 1194–1203. [Google Scholar] [CrossRef]
- Riehle, K.J.; Dan, Y.Y.; Campbell, J.S.; Fausto, N. New concepts in liver regeneration. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 1), 203–212. [Google Scholar] [CrossRef]
- Ochiya, T.; Yamamoto, Y.; Banas, A. Commitment of stem cells into functional hepatocytes. Differentiation 2010, 79, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Soto-Gutierrez, A.; Baptista, P.M.; Spee, B. Biotechnology Challenges to In Vitro Maturation of Hepatic Stem Cells. Gastroenterology 2018, 154, 1258–1272. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruitwagen, H.S.; Oosterhoff, L.A.; van Wolferen, M.E.; Chen, C.; Nantasanti Assawarachan, S.; Schneeberger, K.; Kummeling, A.; van Straten, G.; Akkerdaas, I.C.; Vinke, C.R.; et al. Long-Term Survival of Transplanted Autologous Canine Liver Organoids in a COMMD1-Deficient Dog Model of Metabolic Liver Disease. Cells 2020, 9, 410. https://doi.org/10.3390/cells9020410
Kruitwagen HS, Oosterhoff LA, van Wolferen ME, Chen C, Nantasanti Assawarachan S, Schneeberger K, Kummeling A, van Straten G, Akkerdaas IC, Vinke CR, et al. Long-Term Survival of Transplanted Autologous Canine Liver Organoids in a COMMD1-Deficient Dog Model of Metabolic Liver Disease. Cells. 2020; 9(2):410. https://doi.org/10.3390/cells9020410
Chicago/Turabian StyleKruitwagen, Hedwig S., Loes A. Oosterhoff, Monique E. van Wolferen, Chen Chen, Sathidpak Nantasanti Assawarachan, Kerstin Schneeberger, Anne Kummeling, Giora van Straten, Ies C. Akkerdaas, Christel R. Vinke, and et al. 2020. "Long-Term Survival of Transplanted Autologous Canine Liver Organoids in a COMMD1-Deficient Dog Model of Metabolic Liver Disease" Cells 9, no. 2: 410. https://doi.org/10.3390/cells9020410
APA StyleKruitwagen, H. S., Oosterhoff, L. A., van Wolferen, M. E., Chen, C., Nantasanti Assawarachan, S., Schneeberger, K., Kummeling, A., van Straten, G., Akkerdaas, I. C., Vinke, C. R., van Steenbeek, F. G., van Bruggen, L. W. L., Wolfswinkel, J., Grinwis, G. C. M., Fuchs, S. A., Gehart, H., Geijsen, N., Vries, R. G., Clevers, H., ... Spee, B. (2020). Long-Term Survival of Transplanted Autologous Canine Liver Organoids in a COMMD1-Deficient Dog Model of Metabolic Liver Disease. Cells, 9(2), 410. https://doi.org/10.3390/cells9020410







