Involvement of TRPC4 and 5 Channels in Persistent Firing in Hippocampal CA1 Pyramidal Cells
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Immunohistochemistry
2.3. In Vitro Patch Clamp Recording
2.3.1. Slice Preparation
2.3.2. Recording Procedures
2.3.3. Chemicals and Antibodies
2.4. Data Analysis
3. Results
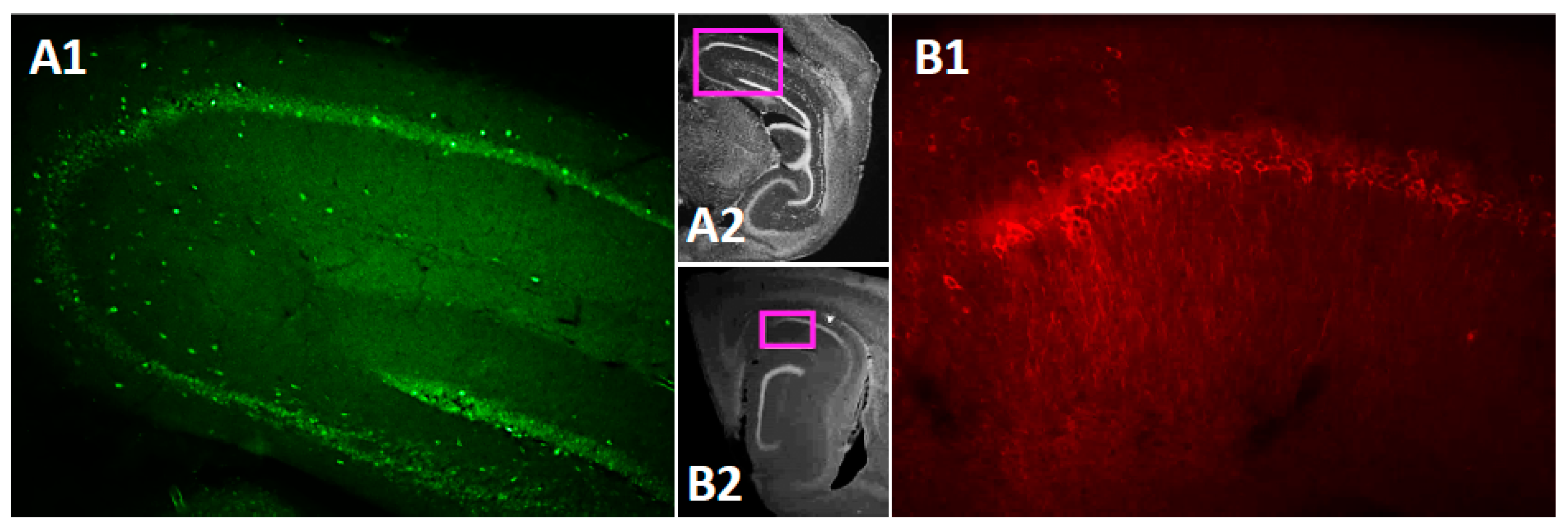
3.1. TRPC4 and TRPC5 Channels Expression in Mouse CA1 Pyramidal Layer
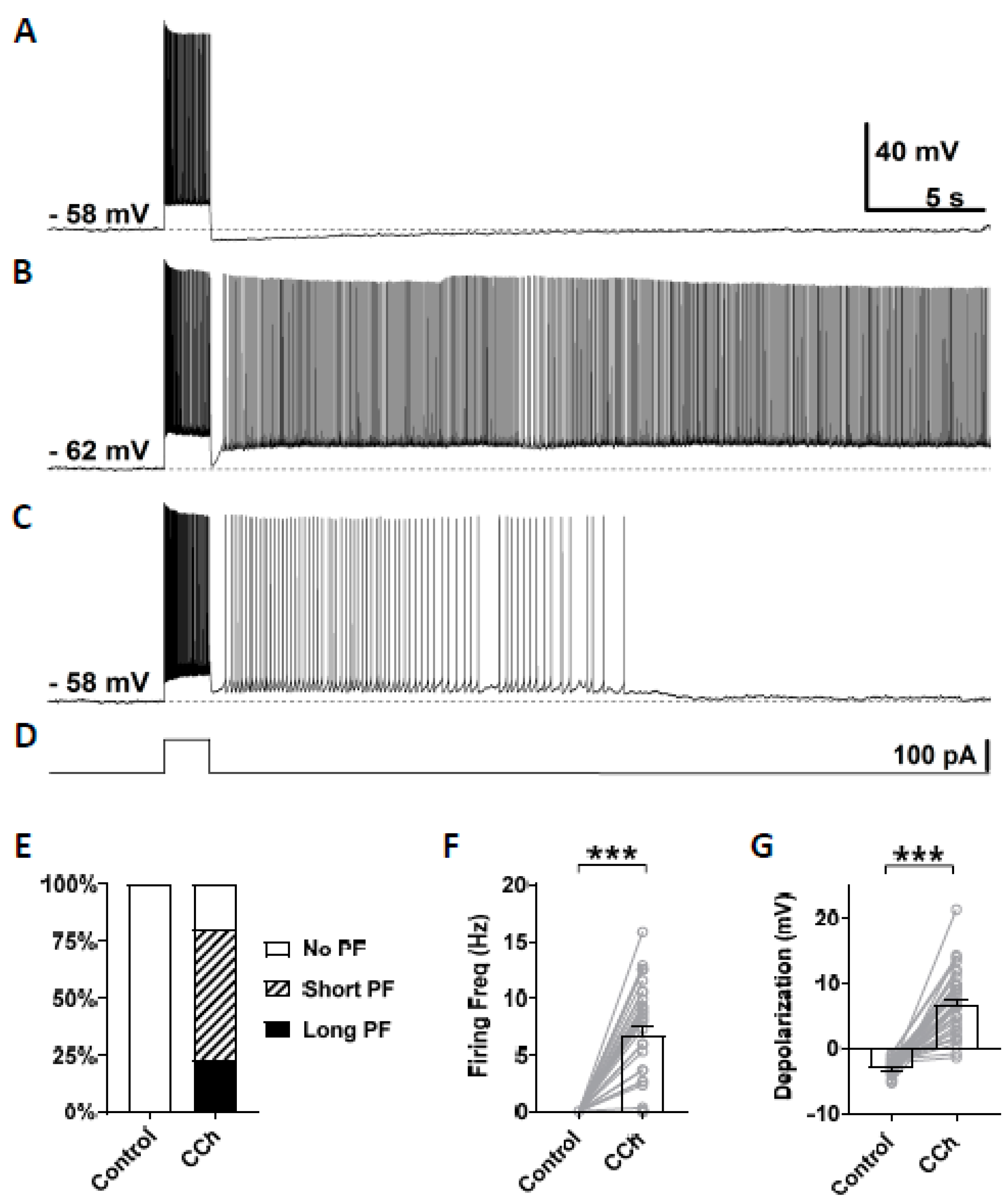
3.2. Cholinergic Agonist Supports Persistent Firing in Mouse CA1 Pyramidal Neurons
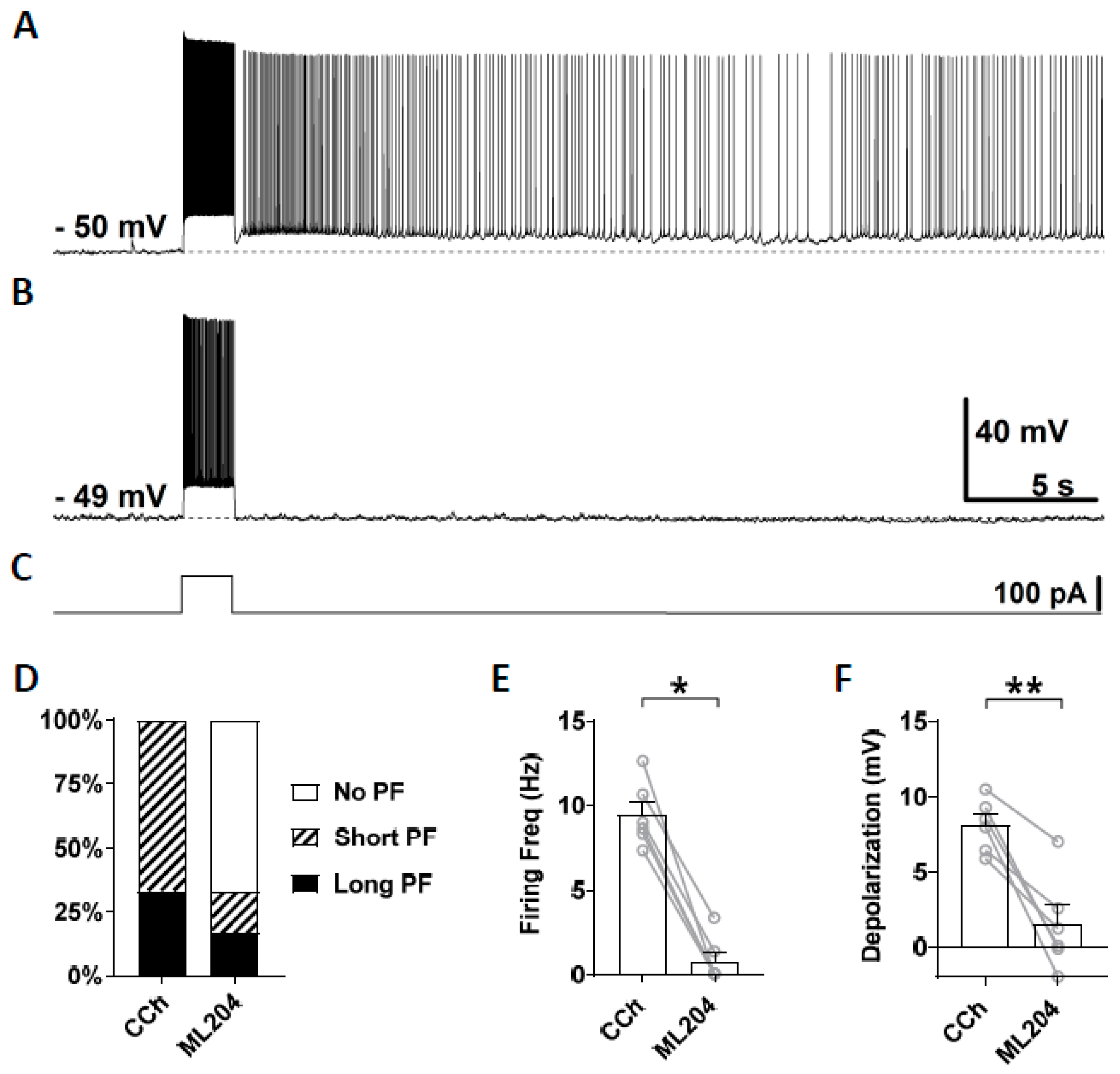
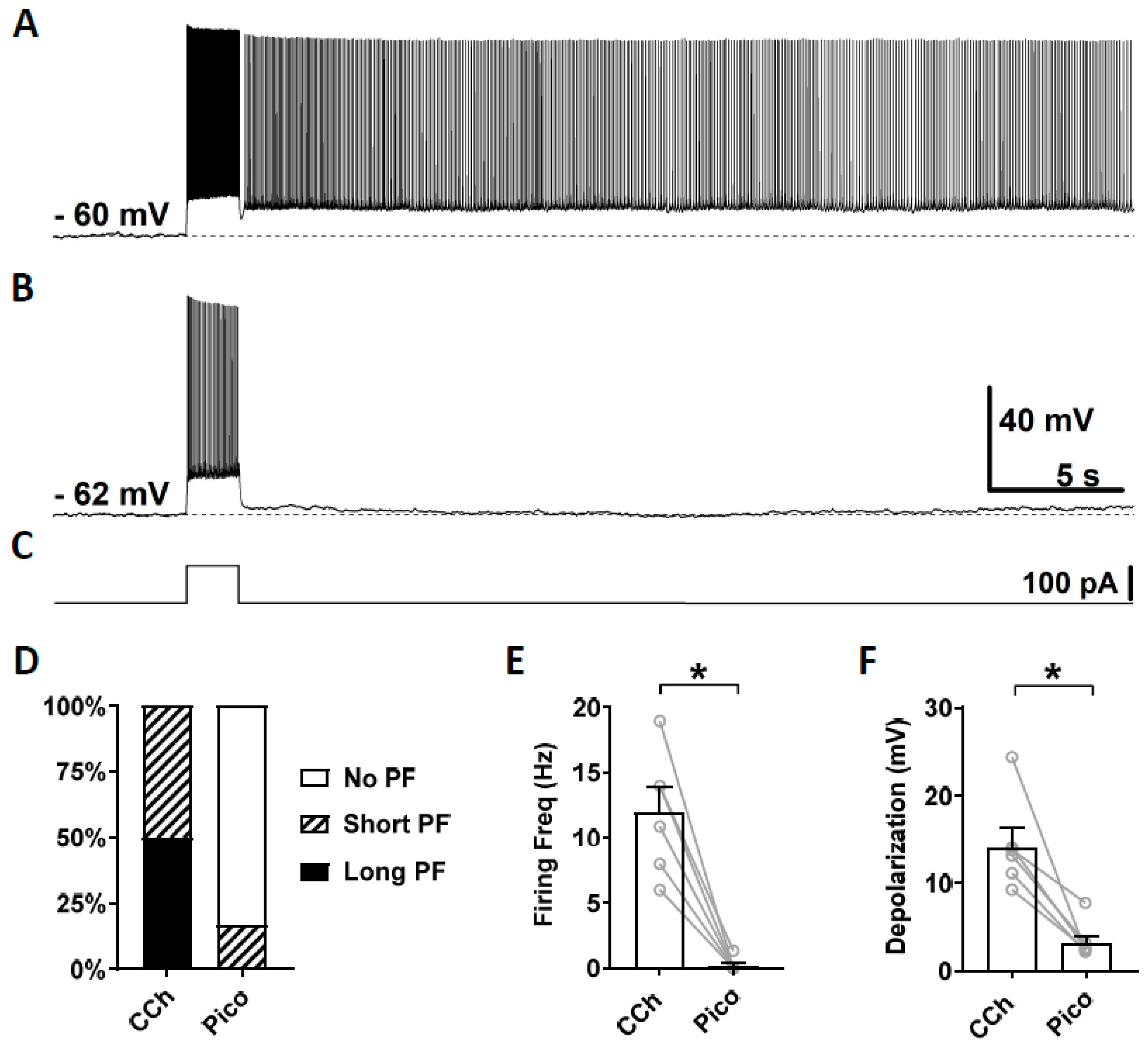
3.3. Novel TRPC Antagonists Suppress Persistent Firing
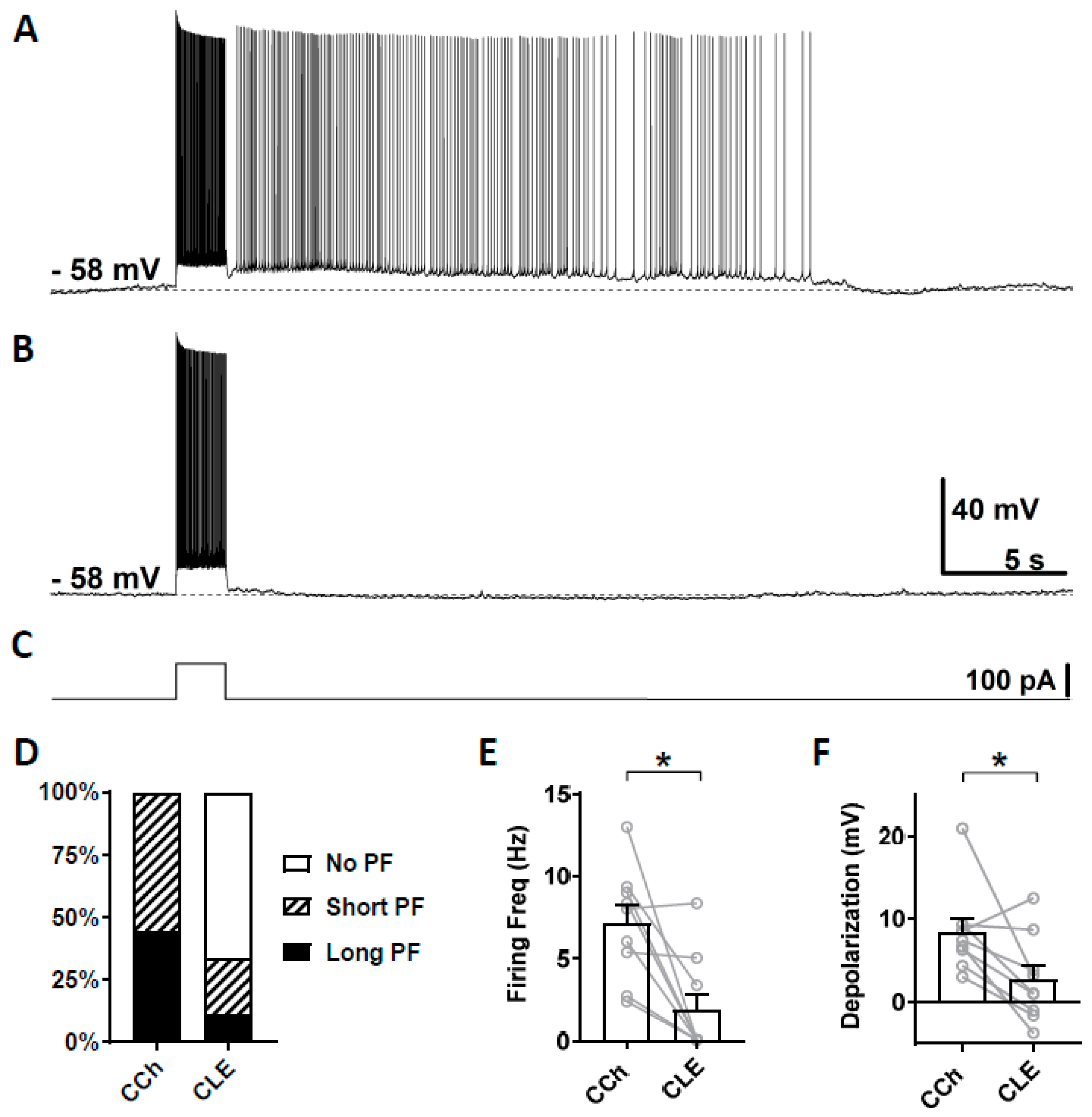
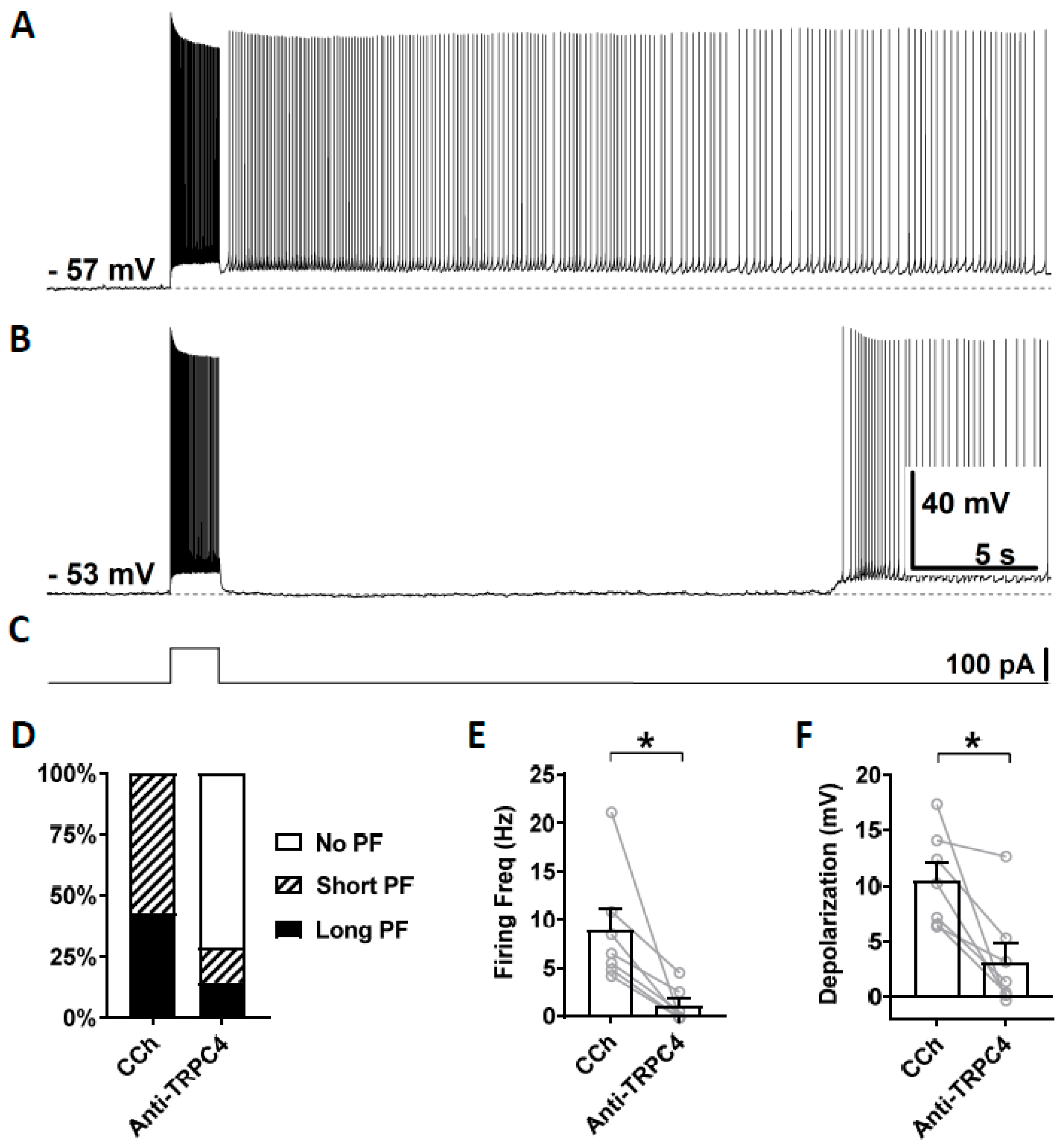
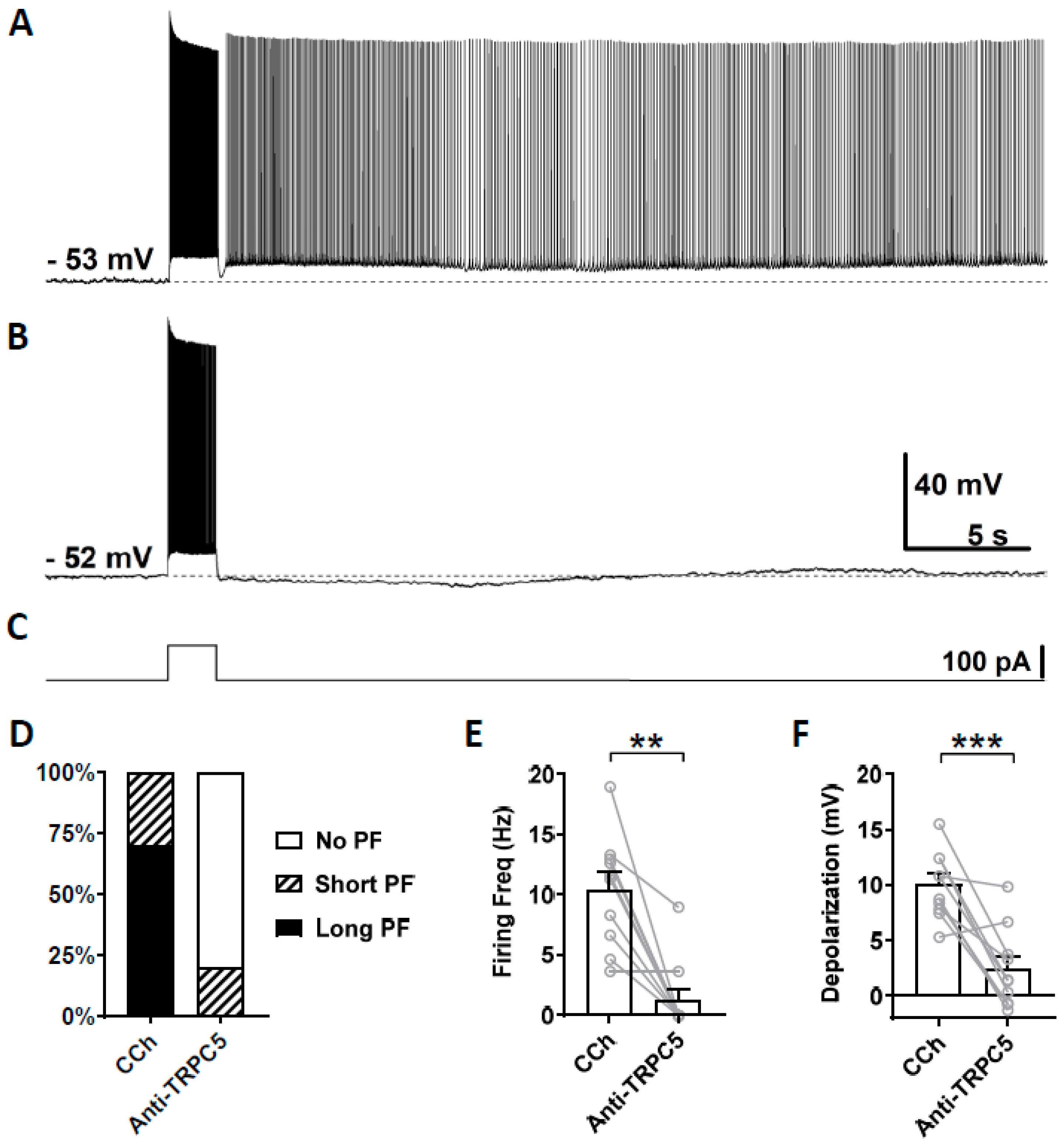
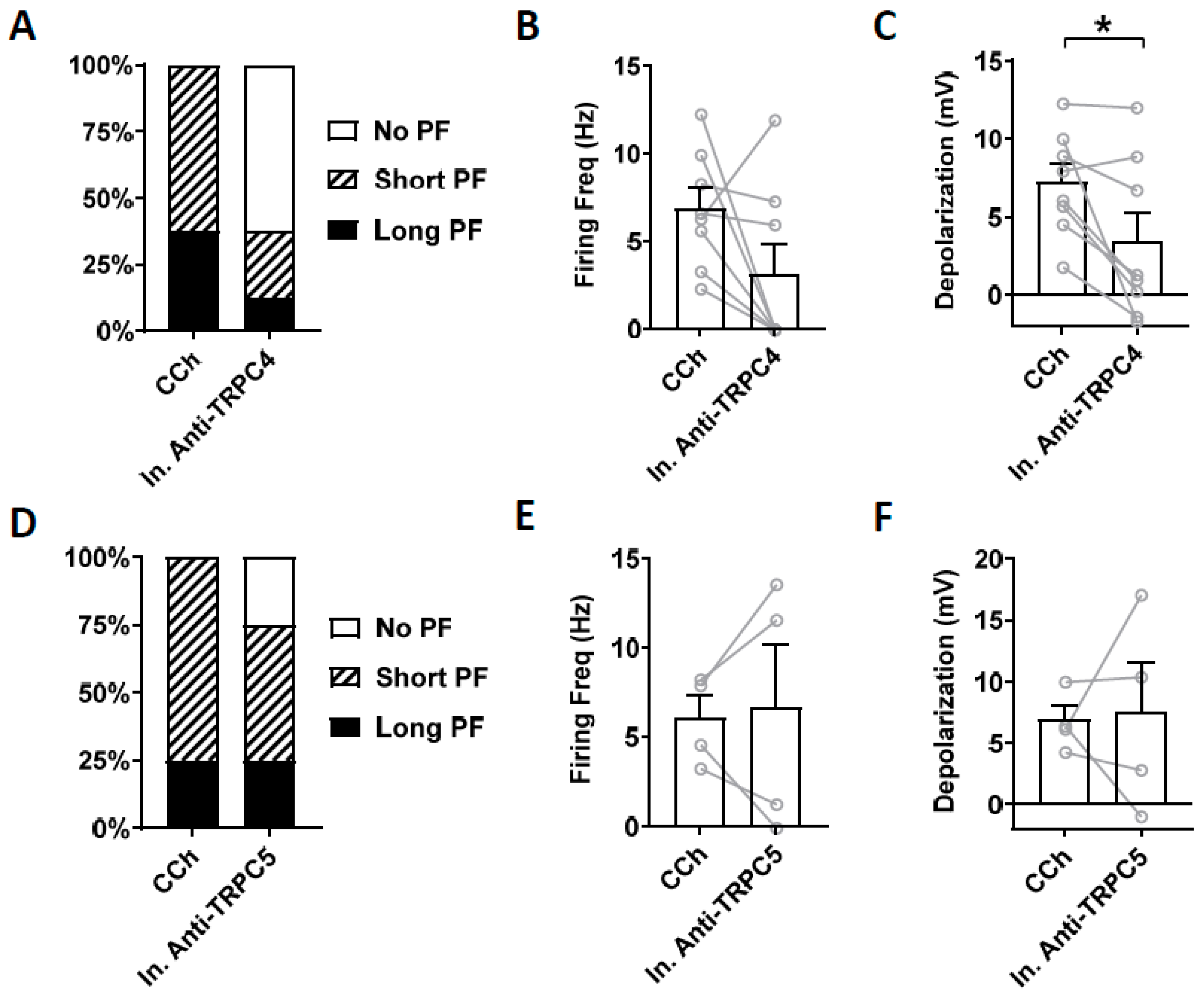
3.4. Effects of TRPC Antibodies on Persistent Firing
4. Discussion
4.1. Immunohistochemistry
4.2. Persistent Firing in CA1 Pyramidal Cells in Mice
4.3. Effects of TRPC4 and 5 Blockers
4.4. Effect of TRPC4 and 5 Antibodies
4.5. Controversies and Limitations
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuster, J.M.; Alexander, G.E. Neuron Activity Related to Short-Term Memory. Science 1971, 173, 652–654. [Google Scholar] [CrossRef]
- Goldman-Rakic Cellular Basis of Working Memory Review. Neuron 1995, 14, 477–485. [CrossRef]
- Solomon, P.R.; Vander Schaaf, E.R.; Thompson, R.F.; Weisz, D.J. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1986, 100, 729–744. [Google Scholar] [CrossRef] [PubMed]
- McEchron, M.D.; Weible, A.P.; Disterhoft, J.F. Aging and Learning-Specific Changes in Single-Neuron Activity in CA1 Hippocampus during Rabbit Trace Eyeblink Conditioning. J. Neurophysiol. 2001, 86, 1839–1857. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Gross, C.G. Responses of inferior temporal cortex and hippocampal neurons during delayed matching to sample in monkeys (Macaca fascicularis). Behav. Neurosci. 1994, 108, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Hampson, R.E.; Pons, T.P.; Stanford, T.R.; Deadwyler, S.A. Categorization in the monkey hippocampus: A possible mechanism for encoding information into memory. Proc. Natl. Acad. Sci. USA 2004, 101, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Kornblith, S.; Quian Quiroga, R.; Koch, C.; Fried, I.; Mormann, F. Persistent Single-Neuron Activity during Working Memory in the Human Medial Temporal Lobe. Curr. Biol. 2017, 27, 1026–1032. [Google Scholar] [CrossRef]
- Kamiński, J.; Sullivan, S.; Chung, J.M.; Ross, I.B.; Mamelak, A.N.; Rutishauser, U. Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nat. Neurosci. 2017, 20, 590–601. [Google Scholar] [CrossRef]
- Constantinidis, C.; Funahashi, S.; Lee, D.; Murray, J.D.; Qi, X.-L.; Wang, M.; Arnsten, A.F.T. Persistent Spiking Activity Underlies Working Memory. J. Neurosci. 2018, 38, 7020–7028. [Google Scholar] [CrossRef]
- Lundqvist, M.; Herman, P.; Miller, E.K. Working Memory: Delay Activity, Yes! Persistent Activity? Maybe Not. J. Neurosci. 2018, 38, 7013–7019. [Google Scholar] [CrossRef]
- Olson, I.R.; Moore, K.S.; Stark, M.; Chatterjee, A. Visual working memory is impaired when the medial temporal lobe is damaged. J. Cogn. Neurosci. 2006, 18, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Hannula, D.E.; Tranel, D.; Cohen, N.J. The long and the short of it: Relational memory impairments in amnesia, even at short lags. J. Neurosci. 2006, 26, 8352–8359. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Clark, R.E.; Thompson, R.F. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav. Neurosci. 1995, 109, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Bouwmeester, H.; Power, J.M.; Disterhoft, J.F. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav. Brain Res. 1999, 99, 123–132. [Google Scholar] [CrossRef]
- Kesner, R.P.; Hunsaker, M.R.; Gilbert, P.E. The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behav. Neurosci. 2005, 119, 781–786. [Google Scholar] [CrossRef]
- Mikulka, P.J.; Freeman, F.G. The effects of reinforcement delay and hippocampal lesions on the acquisition of a choice response. Behav. Biol. 1975, 15, 473–477. [Google Scholar] [CrossRef]
- Rawlins, J.N.; Feldon, J.; Butt, S. The effects of delaying reward on choice preference in rats with hippocampal or selective septal lesions. Behav. Brain Res. 1985, 15, 191–203. [Google Scholar] [CrossRef]
- Major, G.; Tank, D. Persistent neural activity: Prevalence and mechanisms. Curr. Opin. Neurobiol. 2004, 14, 675–684. [Google Scholar] [CrossRef]
- Zylberberg, J.; Strowbridge, B.W. Mechanisms of Persistent Activity in Cortical Circuits: Possible Neural Substrates for Working Memory. Annu. Rev. Neurosci. 2017, 40, 603–627. [Google Scholar] [CrossRef]
- Knauer, B.; Jochems, A.; Valero-Aracama, M.J.; Yoshida, M. Long-lasting intrinsic persistent firing in rat CA1 pyramidal cells: A possible mechanism for active maintenance of memory. Hippocampus 2013, 23, 820–831. [Google Scholar] [CrossRef]
- Jochems, A.; Yoshida, M. Persistent firing supported by an intrinsic cellular mechanism in hippocampal CA3 pyramidal cells. Eur. J. Neurosci. 2013, 38, 2250–2259. [Google Scholar] [CrossRef]
- Anderson, R.W.; Strowbridge, B.W. Regulation of persistent activity in hippocampal mossy cells by inhibitory synaptic potentials. Learn. Mem. 2014, 21, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Klink, R.; Alonso, A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. J. Neurophysiol. 1997, 77, 1829–1843. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.V.; Hamam, B.N.; Fransén, E.; Hasselmo, M.E.; Alonso, A.A. Graded persistent activity in entorhinal cortex neurons. Nature 2002, 420, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Navaroli, V.L.; Zhao, Y.; Boguszewski, P.; Brown, T.H. Muscarinic receptor activation enables persistent firing in pyramidal neurons from superficial layers of dorsal perirhinal cortex. Hippocampus 2012, 22, 1392–1404. [Google Scholar] [CrossRef]
- Rahman, J.; Berger, T. Persistent activity in layer 5 pyramidal neurons following cholinergic activation of mouse primary cortices. Eur. J. Neurosci. 2011, 34, 22–30. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Stern, C.E. Mechanisms underlying working memory for novel information. Trends Cogn. Sci. 2006, 10, 487–493. [Google Scholar] [CrossRef]
- Schon, K. Scopolamine Reduces Persistent Activity Related to Long-Term Encoding in the Parahippocampal Gyrus during Delayed Matching in Humans. J. Neurosci. 2005, 25, 9112–9123. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, X.L.; Douglas, K.; Palaninathan, K.; Kang, H.S.; Buccafusco, J.J.; Blake, D.T.; Constantinidis, C. Cholinergic modulation of working memory activity in primate prefrontal cortex. J. Neurophysiol. 2011, 106, 2180–2188. [Google Scholar] [CrossRef]
- Benardo, L.S.; Prince, D.A. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982, 249, 315–331. [Google Scholar] [CrossRef]
- Gahwiler, B.H.; Dreifuss, J.J. Multiple actions of acetylcholine on hippocampal pyramidal cells in organotypic explant cultures. Neuroscience 1982, 7, 1243–1256. [Google Scholar] [CrossRef]
- Andrade, R. Cell excitation enhances muscarinic cholinergic responses in rat association cortex. Brain Res. 1991, 548, 81–93. [Google Scholar] [CrossRef]
- Cole, A.E.; Nicoll, R.A. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science 1983, 221, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, J.V.; Adams, P.R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982, 250, 71–92. [Google Scholar] [CrossRef]
- Madison, D.V.; Lancaster, B.; Nicoll, R.A. Voltage clamp analysis of cholinergic action in the hippocampus. J. Neurosci. 1987, 7, 733–741. [Google Scholar] [CrossRef]
- Caeser, M.; Brown, D.A.; Gahwiler, B.H.; Knopfel, T. Characterization of a calcium-dependent current generating a slow afterdepolarization of CA3 pyramidal cells in rat hippocampal slice cultures. Eur. J. Neurosci. 1993, 5, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.D.; MacVicar, B.A. Cholinergic-Dependent Plateau Potential in Hippocampal CA1 Pyramidal Neurons. J. Neurosci. 1996, 16, 4113–4128. [Google Scholar] [CrossRef] [PubMed]
- Haj-Dahmane, S.; Andrade, R. Ionic mechanism of the slow afterdepolarization induced by muscarinic receptor activation in rat prefrontal cortex. J. Neurophysiol. 1998, 80, 1197–1210. [Google Scholar] [CrossRef]
- Kawasaki, H.; Palmieri, C.; Avoli, M. Muscarinic receptor activation induces depolarizing plateau potentials in bursting neurons of the rat subiculum. J. Neurophysiol. 1999, 82, 2590–2601. [Google Scholar] [CrossRef]
- Fransen, E.; Alonso, A.A.; Hasselmo, M.E. Simulations of the role of the muscarinic-activated calcium-sensitive nonspecific cation current INCM in entorhinal neuronal activity during delayed matching tasks. J. Neurosci. 2002, 22, 1081–1097. [Google Scholar] [CrossRef]
- Reboreda, A.; Jiménez-Díaz, L.; Navarro-López, J.D. TRP Channels and Neural Persistent Activity; Springer: Dordrecht, The Netherlands, 2011; Volume 704. [Google Scholar]
- Reboreda, A.; Theissen, F.M.; Valero-Aracama, M.J.; Arboit, A.; Corbu, M.A.; Yoshida, M. Do TRPC channels support working memory? Comparing modulations of TRPC channels and working memory through G-protein coupled receptors and neuromodulators. Behav. Brain Res. 2018, 354, 64–83. [Google Scholar] [CrossRef]
- Vennekens, R.; Menigoz, A.; Nilius, B. TRPs in the Brain. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; p. 49. [Google Scholar]
- Fowler, M.A.; Sidiropoulou, K.; Ozkan, E.D.; Phillips, C.W.; Cooper, D.C. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS ONE 2007, 2, e573. [Google Scholar] [CrossRef]
- Okada, T.; Shimizu, S.; Wakamori, M.; Maeda, A.; Kurosaki, T.; Takada, N.; Imoto, K.; Mori, Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J. Biol. Chem. 1998, 273, 10279–10287. [Google Scholar] [CrossRef]
- Schaefer, M.; Plant, T.D.; Obukhov, A.G.; Hofmann, T.; Gudermann, T.; Schultz, G. Receptor-mediated Regulation of the Nonselective Cation Channels TRPC4 and TRPC5. J. Biol. Chem. 2000, 275, 17517–17526. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Riley, A.; Soliman, M.; Chakraborty, S.; Stamatkin, C.; Obukhov, A. Molecular Determinants of the Sensitivity to Gq/11-Phospholipase C-dependent Gating, Gd3+ Potentiation, and Ca2+ Permeability in the Transient Receptor Potential Canonical Type 5 (TRPC5) Channel. J. Biol. Chem. 2017, 292, 898–911. [Google Scholar] [CrossRef]
- Jung, S.; Mühle, A.; Schaefer, M.; Strotmann, R.; Schultz, G.; Plant, T.D. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J. Biol. Chem. 2003, 278, 3562–3571. [Google Scholar] [CrossRef]
- Blair, N.T.; Kaczmarek, J.S.; Clapham, D.E. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J. Gen. Physiol. 2009, 133, 525–546. [Google Scholar] [CrossRef]
- Obukhov, A.G.; Nowycky, M.C. TRPC5 channels undergo changes in gating properties during the activation-deactivation cycle. J. Cell. Physiol. 2008, 216, 162–171. [Google Scholar] [CrossRef]
- Haj-Dahmane, S.; Andrade, R. Muscarinic receptors regulate two different calcium-dependent non-selective cation currents in rat prefrontal cortex. Eur. J. Neurosci. 1999, 11, 1973–1980. [Google Scholar] [CrossRef]
- Yan, H.-D.; Villalobos, C.; Andrade, R. TRPC Channels Mediate a Muscarinic Receptor-Induced Afterdepolarization in Cerebral Cortex. J. Neurosci. 2009, 29, 10038–10046. [Google Scholar] [CrossRef]
- Tahvildari, B.; Alonso, A.A.; Bourque, C.W. Ionic basis of ON and OFF persistent activity in layer III lateral entorhinal cortical principal neurons. J. Neurophysiol. 2008, 99, 2006–2011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tai, C.; Hines, D.J.; Choi, H.B.; MacVicar, B.A. Plasma membrane insertion of TRPC5 channels contributes to the cholinergic plateau potential in hippocampal CA1 pyramidal neurons. Hippocampus 2010, 967, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reboreda, A.; Alonso, A.; Barker, P.A.; Séguéla, P. TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus 2011, 21, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Phelan, K.D.; Mock, M.M.; Kretz, O.; Shwe, U.T.; Kozhemyakin, M.; Greenfield, L.J.; Dietrich, A.; Birnbaumer, L.; Freichel, M.; Flockerzi, V.; et al. Heteromeric canonical transient receptor potential 1 and 4 channels play a critical role in epileptiform burst firing and seizure-induced neurodegeneration. Mol. Pharmacol. 2012, 81, 384–392. [Google Scholar] [CrossRef]
- Phelan, K.D.; Shwe, U.T.; Abramowitz, J.; Birnbaumer, L.; Zheng, F. Critical role of canonical transient receptor potential channel 7 in initiation of seizures. Proc. Natl. Acad. Sci. USA 2014, 111, 11533–11538. [Google Scholar] [CrossRef]
- Dasari, S.; Abramowitz, J.; Birnbaumer, L.; Gulledge, A.T. Do canonical transient receptor potential channels mediate cholinergic excitation of cortical pyramidal neurons? Neuroreport 2013, 24, 550–554. [Google Scholar] [CrossRef]
- Egorov, A.V.; Schumacher, D.; Medert, R.; Birnbaumer, L.; Freichel, M.; Draguhn, A. TRPC channels are not required for graded persistent activity in entorhinal cortex neurons. Hippocampus 2019, 29, 1038–1048. [Google Scholar] [CrossRef]
- Bröker-Lai, J.; Kollewe, A.; Schindeldecker, B.; Pohle, J.; Nguyen Chi, V.; Mathar, I.; Guzman, R.; Schwarz, Y.; Lai, A.; Weißgerber, P.; et al. Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory. EMBO J. 2017, 36, 2770–2789. [Google Scholar] [CrossRef]
- Lepannetier, S.; Gualdani, R.; Tempesta, S.; Schakman, O.; Seghers, F.; Kreis, A.; Yerna, X.; Slimi, A.; de Clippele, M.; Tajeddine, N.; et al. Activation of TRPC1 Channel by Metabotropic Glutamate Receptor mGluR5 Modulates Synaptic Plasticity and Spatial Working Memory. Front. Cell. Neurosci. 2018, 12, 318. [Google Scholar] [CrossRef]
- Cui, E.D.; Strowbridge, B.W. Modulation of Ether-à-Go-Go Related Gene (ERG) Current Governs Intrinsic Persistent Activity in Rodent Neocortical Pyramidal Cells. J. Neurosci. 2018, 38, 423–440. [Google Scholar] [CrossRef]
- Singh, A.; Garcia, E. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br. J. Pharmacol. 2010, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Guinamard, R.; Simard, C.; Del Negro, C. Flufenamic acid as an ion channel modulator. Pharmacol. Ther. 2013, 138, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Minard, A.; Bauer, C.C.; Wright, D.J.; Rubaiy, H.N.; Muraki, K.; Beech, D.J.; Bon, R.S. Remarkable Progress with Small-Molecule Modulation of TRPC1/4/5 Channels: Implications for Understanding the Channels in Health and Disease. Cells 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Rubaiy, H.N. Treasure troves of pharmacological tools to study transient receptor potential canonical 1/4/5 channels. Br. J. Pharmacol. 2019, 176, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Shi, J.; Zhu, Y.; Kustov, M.; Tian, J.; Stevens, A.; Wu, M.; Xu, J.; Long, S.; Yang, P.; et al. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J. Biol. Chem. 2011, 286, 33436–33446. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.M.; Schaefer, M.; Hill, K. Clemizole hydrochloride is a novel and potent inhibitor of transient receptor potential channel TRPC5. Mol. Pharmacol. 2014, 86, 514–521. [Google Scholar] [CrossRef]
- Rubaiy, H.N.; Ludlow, M.J.; Henrot, M.; Gaunt, H.J.; Miteva, K.; Cheung, S.Y.; Tanahashi, Y.; Hamzah, N.; Musialowski, K.E.; Blythe, N.M.; et al. Picomolar, selective, and subtype-specific small-molecule inhibition of TRPC1/4/5 channels. J. Biol. Chem. 2017, 292, 8158–8173. [Google Scholar] [CrossRef]
- Faber, E.S.L.; Sedlak, P.; Vidovic, M. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience 2006, 137, 781–794. [Google Scholar] [CrossRef]
- Amaral, M.D.; Pozzo-Miller, L. TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J. Neurosci. 2007, 27, 5179–5189. [Google Scholar] [CrossRef]
- El-Hassar, L.; Hagenston, A.M.; D’Angelo, L.B.; Yeckel, M.F. Metabotropic glutamate receptors regulate hippocampal CA1 pyramidal neuron excitability via Ca2+ wave-dependent activation of SK and TRPC channels. J. Physiol. 2011, 589, 3211–3229. [Google Scholar] [CrossRef]
- Von Bohlen und Halbach, O.; Hinz, U.; Unsicker, K.; Egorov, A.V. Distribution of TRPC1 and TRPC5 in medial temporal lobe structures of mice. Cell Tissue Res. 2005, 322, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Shi, J.; Wu, M.; Engers, J.; Hopkins, C.; Lindsley, C. Novel Chemical Inhibitor of TRPC4 Channels; National Center for Biotechnology Information: Bethesda, MD, USA, 2010; Volume 50, pp. 1–35.
- Knauer, B.; Yoshida, M. Switching between persistent firing and depolarization block in individual rat CA1 pyramidal neurons. Hippocampus 2019, 29, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Zechel, S.; Werner, S.; von Bohlen Und Halbach, O. Distribution of TRPC4 in developing and adult murine brain. Cell Tissue Res. 2007, 328, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Franzius, D.; Hoth, M.; Penner, R. Non-specific effects of calcium entry antagonists in mast cells. Pflügers Arch. Eur. J. Physiol. 1994, 428, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Liu, H.; Yang, L.; Jin, M.W.; Li, G.R. SKF-96365 strongly inhibits voltage-gated sodium current in rat ventricular myocytes. Pflugers Arch. Eur. J. Physiol. 2015, 467, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Yau, H.-J.; Baranauskas, G.; Martina, M. Flufenamic acid decreases neuronal excitability through modulation of voltage-gated sodium channel gating. J. Physiol. 2010, 588, 3869–3882. [Google Scholar] [CrossRef]
- Rae, M.G.; Hilton, J.; Sharkey, J. Putative TRP channel antagonists, SKF 96365, flufenamic acid and 2-APB, are non-competitive antagonists at recombinant human α1β2γ2 GABAA receptors. Neurochem. Int. 2012, 60, 543–554. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Chen, K.H.; Sun, H.Y.; Jin, M.W.; Xiao, G.S.; Wang, Y.; Li, G.R. SKF-96365 blocks human ether-à-go-go-related gene potassium channels stably expressed in HEK 293 cells. Pharmacol. Res. 2016, 104, 61–69. [Google Scholar] [CrossRef]
- Just, S.; Chenard, B.L.; Ceci, A.; Strassmaier, T.; Chong, J.A.; Blair, N.T.; Gallaschun, R.J.; Del Camino, D.; Cantin, S.; D’Amours, M.; et al. Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice. PLoS ONE 2018, 13, e0191225. [Google Scholar] [CrossRef]
- Chenard, B.; Gallaschun, R. Substituted Xanthines and Methods of Use Thereof. Patent No. WO2014/143799, 18 September 2014. [Google Scholar]
- Chenard, B.; Gallaschun, R. Substituted Xanthines and Methods of Use Thereof. U.S. Patent No. 9,359,359, 7 June 2016. [Google Scholar]
- Araneda, R.; Andrade, R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 1991, 40, 399–412. [Google Scholar] [CrossRef]
- Greene, C.C.; Schwindt, P.C.; Crill, W.E. Properties and ionic mechanisms of a metabotropic glutamate receptor-mediated slow afterdepolarization in neocortical neurons. J. Neurophysiol. 1994, 72, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, K.; Lu, F.-M.; Fowler, M.A.; Xiao, R.; Phillips, C.; Ozkan, E.D.; Zhu, M.X.; White, F.J.; Cooper, D.C. Dopamine modulates an mGluR5-mediated depolarization underlying prefrontal persistent activity. Nat. Neurosci. 2009, 12, 190–199. [Google Scholar] [CrossRef] [PubMed]
- El-Brolosy, M.A.; Stainier, D.Y.R. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet. 2017, 13, e1006780. [Google Scholar] [CrossRef]
- Phelan, K.D.; Shwe, U.T.; Abramowitz, J.; Wu, H.; Rhee, S.W.; Howell, M.D.; Gottschall, P.E.; Freichel, M.; Flockerzi, V.; Birnbaumer, L.; et al. Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol. Pharmacol. 2013, 83, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-T.; Thuault, S.J.; Launay, P.; Margolskee, R.F.; Kandel, E.R.; Siegelbaum, S.A. Differential contribution of TRPM4 and TRPM5 nonselective cation channels to the slow afterdepolarization in mouse prefrontal cortex neurons. Front. Cell. Neurosci. 2014, 8, 267. [Google Scholar] [CrossRef]
- Alom, F.; Miyakawa, M.; Matsuyama, H.; Nagano, H.; Tanahashi, Y.; Unno, T. Possible antagonistic effects of the TRPC4 channel blocker ML204 on M2 and M3 muscarinic receptors in mouse ileal and detrusor smooth muscles and atrial myocardium. J. Vet. Med. Sci. 2018, 80, 1407–1415. [Google Scholar] [CrossRef]
- Jie, L.-J.; Wu, W.-Y.; Li, G.; Xiao, G.-S.; Zhang, S.; Li, G.-R.; Wang, Y. Clemizole hydrochloride blocks cardiac potassium currents stably expressed in HEK 293 cells. Br. J. Pharmacol. 2017, 174, 254–266. [Google Scholar] [CrossRef]
- Hofmann, T.; Schaefer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef]
- Strübing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 2001, 29, 645–655. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arboit, A.; Reboreda, A.; Yoshida, M. Involvement of TRPC4 and 5 Channels in Persistent Firing in Hippocampal CA1 Pyramidal Cells. Cells 2020, 9, 365. https://doi.org/10.3390/cells9020365
Arboit A, Reboreda A, Yoshida M. Involvement of TRPC4 and 5 Channels in Persistent Firing in Hippocampal CA1 Pyramidal Cells. Cells. 2020; 9(2):365. https://doi.org/10.3390/cells9020365
Chicago/Turabian StyleArboit, Alberto, Antonio Reboreda, and Motoharu Yoshida. 2020. "Involvement of TRPC4 and 5 Channels in Persistent Firing in Hippocampal CA1 Pyramidal Cells" Cells 9, no. 2: 365. https://doi.org/10.3390/cells9020365
APA StyleArboit, A., Reboreda, A., & Yoshida, M. (2020). Involvement of TRPC4 and 5 Channels in Persistent Firing in Hippocampal CA1 Pyramidal Cells. Cells, 9(2), 365. https://doi.org/10.3390/cells9020365



