Role of the TRPV Channels in the Endoplasmic Reticulum Calcium Homeostasis
Abstract
1. Introduction: TRPV Channels Subfamily Overview
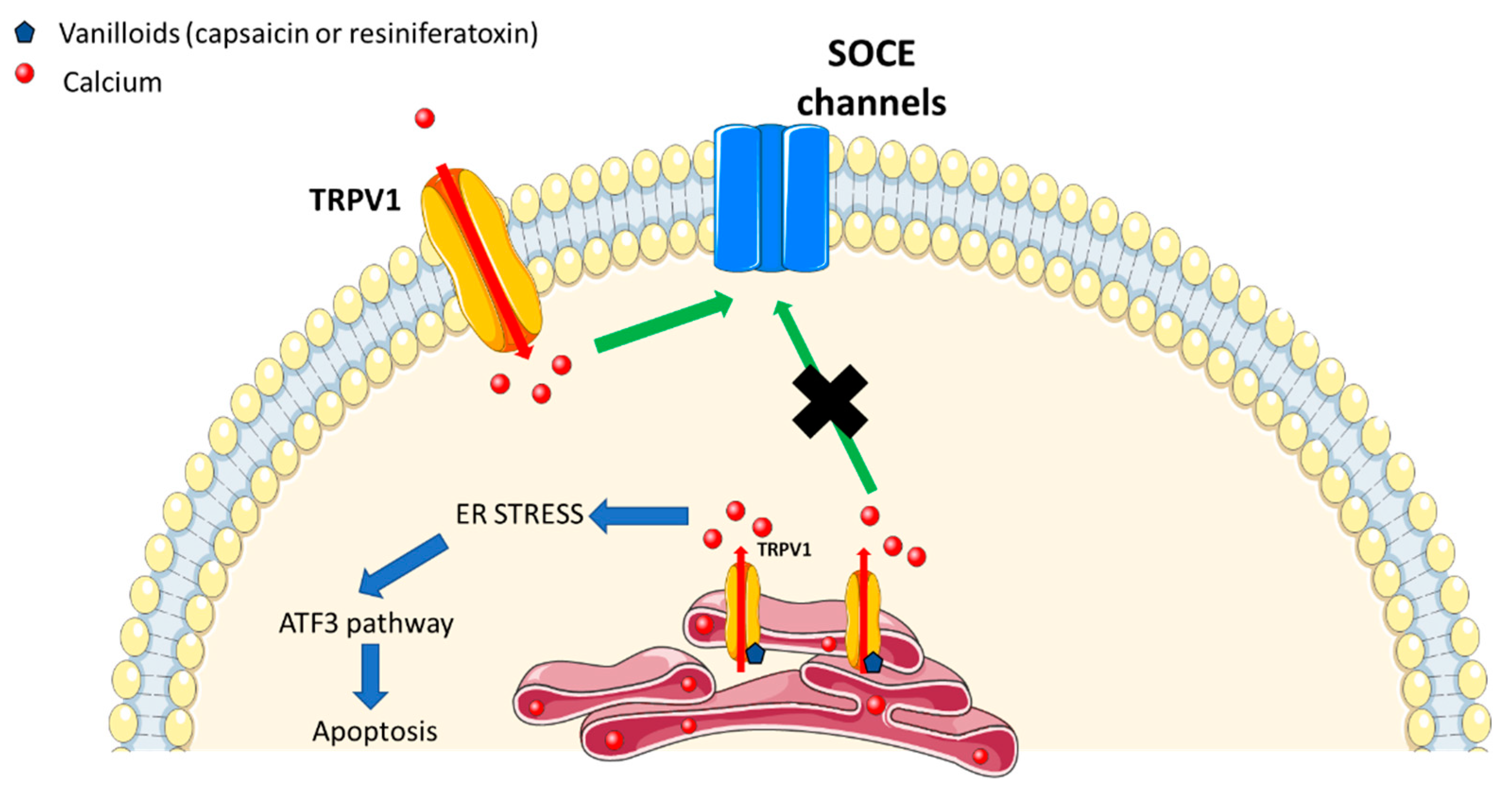
2. TRPV1 Channel in ER
3. TRPV2 Channel in ER
4. TRPV3 Channel in ER
5. TRPV4 Channel in ER
6. TRPV5 and TRPV6 Channels
7. Relationships between Channels
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M.; Annicelli, L.L.; Krause, J.E.; Cortright, D.N. The cloned rat vanilloid receptor VR1 mediates both R-type binding and C-type calcium response in dorsal root ganglion neurons. Mol. Pharmacol. 1999, 56, 581–587. [Google Scholar] [CrossRef]
- Xin, H.; Tanaka, H.; Yamaguchi, M.; Takemori, S.; Nakamura, A.; Kohama, K. Vanilloid receptor expressed in the sarcoplasmic reticulum of rat skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 756–762. [Google Scholar] [CrossRef]
- Sanchez, M.G.; Sanchez, A.M.; Collado, B.; Malagarie-Cazenave, S.; Olea, N.; Carmena, M.J.; Prieto, J.C.; Diaz-Laviada I, I. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur. J. Pharmacol. 2005, 515, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Sabnis, A.S.; Johansen, M.E.; Lanza, D.L.; Moos, P.J.; Yost, G.S.; Reilly, C.A. Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J. Pharmacol. Exp. Ther. 2007, 321, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Kárai, L.J.; Russell, J.T.; Iadarola, M.J.; Oláh, Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J. Biol. Chem. 2004, 279, 16377–16387. [Google Scholar] [CrossRef] [PubMed]
- Wisnoskey, B.J.; Sinkins, W.G.; Schilling, W.P. Activation of vanilloid receptor type I in the endoplasmic reticulum fails to activate store-operated Ca2+ entry. Biochem. J. 2003, 372, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Sandín, S.; Rodríguez-García, A.; Alonso, M.T.; García-Sancho, J. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J. Biol. Chem. 2009, 284, 32591–32601. [Google Scholar] [CrossRef]
- Wong, C.-O.; Chen, K.; Lin, Y.Q.; Chao, Y.; Duraine, L.; Lu, Z.; Yoon, W.H.; Sullivan, J.M.; Broadhead, G.T.; Sumner, C.J.; et al. A TRPV channel in Drosophila motor neurons regulates presynaptic resting Ca2+ levels, synapse growth, and synaptic transmission. Neuron 2014, 84, 764–777. [Google Scholar] [CrossRef]
- Stock, K.; Kumar, J.; Synowitz, M.; Petrosino, S.; Imperatore, R.; Smith, E.S.J.; Wend, P.; Purfürst, B.; Nuber, U.A.; Gurok, U.; et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat. Med. 2012, 18, 1232–1238. [Google Scholar] [CrossRef]
- Ip, S.-W.; Lan, S.-H.; Lu, H.-F.; Huang, A.-C.; Yang, J.-S.; Lin, J.-P.; Huang, H.-Y.; Lien, J.-C.; Ho, C.-C.; Chiu, C.-F.; et al. Capsaicin mediates apoptosis in human nasopharyngeal carcinoma NPC-TW 039 cells through mitochondrial depolarization and endoplasmic reticulum stress. Hum Exp Toxicol 2012, 31, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-S.; Ma, K.-H.; Lee, H.-S.; Liu, P.-S.; Li, Y.-H.; Huang, Y.-S.; Chueh, S.-H. Dual effect of capsaicin on cell death in human osteosarcoma G292 cells. Eur. J. Pharmacol. 2013, 718, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Vrechi, T.A.; Crunfli, F.; Costa, A.P.; Torrão, A.S. Cannabinoid Receptor Type 1 Agonist ACEA Protects Neurons from Death and Attenuates Endoplasmic Reticulum Stress-Related Apoptotic Pathway Signaling. Neurotox Res 2018, 33, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Torrao, A.S.; Mestre, L.; Carrillo-Salinas, F.J.; Mechoulam, R.; Guaza, C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis 2012, 3, e331. [Google Scholar] [CrossRef] [PubMed]
- Pumroy, R.A.; Samanta, A.; Liu, Y.; Hughes, T.E.; Zhao, S.; Yudin, Y.; Rohacs, T.; Han, S.; Moiseenkova-Bell, V.Y. Molecular mechanism of TRPV2 channel modulation by cannabidiol. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yang, P.; Qin, P.; Lu, Y.; Li, X.; Tian, Q.; Li, Y.; Xie, C.; Tian, J.; Zhang, C.; et al. Selective potentiation of 2-APB-induced activation of TRPV1-3 channels by acid. Sci Rep 2016, 6, 20791. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Kim, K.Y.; Yoo, S.; Lee, S.-H.; Hwang, S.W. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci. Lett. 2007, 425, 120–125. [Google Scholar] [CrossRef]
- Monet, M. Lehen, kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res 2010, 70, 1225–1235. [Google Scholar] [CrossRef]
- Morelli, M.B.; Nabissi, M.; Amantini, C.; Farfariello, V.; Ricci-Vitiani, L.; di Martino, S.; Pallini, R.; Larocca, L.M.; Caprodossi, S.; Santoni, M.; et al. The transient receptor potential vanilloid-2 cation channel impairs glioblastoma stem-like cell proliferation and promotes differentiation. Int. J. Cancer 2012, 131, 1067–1077. [Google Scholar] [CrossRef]
- Santoni, G.; Amantini, C.; Maggi, F.; Marinelli, O.; Santoni, M.; Nabissi, M.; Morelli, M.B. The TRPV2 cation channels: from urothelial cancer invasiveness to glioblastoma multiforme interactome signature. Lab. Invest. 2019. [Google Scholar] [CrossRef]
- Kowase, T.; Nakazato, Y.; Yoko-O, H.; Morikawa, A.; Kojima, I. Immunohistochemical localization of growth factor-regulated channel (GRC) in human tissues. Endocr. J. 2002, 49, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, M.; Zhang, Y.Q.; Mashima, H.; Li, L.; Shibata, H.; Kojima, I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. 1999, 1, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Nakagawa, Y.; Tanaka, S.; Kojima, I. Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J. Cell. Physiol. 2007, 210, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Harisseh, R.; Chatelier, A.; Magaud, C.; Déliot, N.; Constantin, B. Involvement of TRPV2 and SOCE in calcium influx disorder in DMD primary human myotubes with a specific contribution of α1-syntrophin and PLC/PKC in SOCE regulation. Am. J. Physiol., Cell Physiol. 2013, 304, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.-P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.-K.; Lee, H.; Mizuno, A.; Suzuki, M.; Caterina, M.J. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 2004, 24, 5177–5182. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.-J.; Cho, H.; Hwang, S.W. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J. Biol. Chem. 2010, 285, 19362–19371. [Google Scholar] [CrossRef]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef]
- Vriens, J.; Janssens, A.; Prenen, J.; Nilius, B.; Wondergem, R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium 2004, 36, 19–28. [Google Scholar] [CrossRef]
- Jariwala, U.; Prescott, J.; Jia, L.; Barski, A.; Pregizer, S.; Cogan, J.P.; Arasheben, A.; Tilley, W.D.; Scher, H.I.; Gerald, W.L.; et al. Identification of novel androgen receptor target genes in prostate cancer. Mol. Cancer 2007, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Lo, I.C.; Chan, H.C.; Qi, Z.; Ng, K.L.; So, C.; Tsang, S.Y. TRPV3 Channel Negatively Regulates Cell Cycle Progression and Safeguards the Pluripotency of Embryonic Stem Cells. J. Cell. Physiol. 2016, 231, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Yan, M.; Zhang, J.; Cheng, R.; Liang, J.; Deng, D.; Wang, Z.; Li, M.; Yao, Z. A novel mutation in TRPV3 gene causes atypical familial Olmsted syndrome. Sci Rep 2016, 6, 21815. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.P.; Liu, J.; Han, J.W.; Huang, Y.P.; Gao, Z.Q.; Yang, Y.; Wu, R.N. Two familial cases of Olmsted-like syndrome with a G573V mutation of the TRPV3 gene. Clin. Exp. Dermatol. 2016, 41, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, G.; Sun, X.; Wang, K. Inhibition of the Warm Temperature-Activated Ca2+-Permeable Transient Receptor Potential Vanilloid TRPV3 Channel Attenuates Atopic Dermatitis. Mol. Pharmacol. 2019, 96, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Goswami, C. TRPV3 mutants causing Olmsted Syndrome induce impaired cell adhesion and nonfunctional lysosomes. Channels (Austin) 2017, 11, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Delany, N.S.; Hurle, M.; Facer, P.; Alnadaf, T.; Plumpton, C.; Kinghorn, I.; See, C.G.; Costigan, M.; Anand, P.; Woolf, C.J.; et al. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol. Genomics 2001, 4, 165–174. [Google Scholar] [CrossRef]
- Arniges, M.; Fernández-Fernández, J.M.; Albrecht, N.; Schaefer, M.; Valverde, M.A. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J. Biol. Chem. 2006, 281, 1580–1586. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, E.J.; Chun, J.; Hyun, S.; Kang, S.S. Phosphorylation on TRPV4 Serine 824 Regulates Interaction with STIM1. Open Biochem J 2015, 9, 24–33. [Google Scholar] [CrossRef]
- Becker, D.; Müller, M.; Leuner, K.; Jendrach, M. The C-terminal domain of TRPV4 is essential for plasma membrane localization. Mol. Membr. Biol. 2008, 25, 139–151. [Google Scholar] [CrossRef]
- Lei, L.; Cao, X.; Yang, F.; Shi, D.-J.; Tang, Y.-Q.; Zheng, J.; Wang, K. A TRPV4 channel C-terminal folding recognition domain critical for trafficking and function. J. Biol. Chem. 2013, 288, 10427–10439. [Google Scholar] [CrossRef] [PubMed]
- Beraud, E. T cell vaccination in autoimmune diseases. Ann. N. Y. Acad. Sci. 1991, 636, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. (Lond.) 2007, 578, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.N.; Rincon, S.; Singh, J.; Matthewman, C.; Pasos, J.; Barrett, E.F.; Rajguru, S.M. Pulsed infrared releases Ca2+ from the endoplasmic reticulum of cultured spiral ganglion neurons. J. Neurophysiol. 2018, 120, 509–524. [Google Scholar] [CrossRef]
- Du, J.; Ma, X.; Shen, B.; Huang, Y.; Birnbaumer, L.; Yao, X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J. 2014, 28, 4677–4685. [Google Scholar] [CrossRef]
- Wegierski, T.; Steffl, D.; Kopp, C.; Tauber, R.; Buchholz, B.; Nitschke, R.; Kuehn, E.W.; Walz, G.; Köttgen, M. TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. EMBO J. 2009, 28, 490–499. [Google Scholar] [CrossRef]
- Raphaël, M.; Lehen’kyi, V.; Vandenberghe, M.; Beck, B.; Khalimonchyk, S.; Vanden Abeele, F.; Farsetti, L.; Germain, E.; Bokhobza, A.; Mihalache, A.; et al. TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 3870–3879. [Google Scholar] [CrossRef]
- Van de Graaf, S.F.J.; Hoenderop, J.G.J.; Gkika, D.; Lamers, D.; Prenen, J.; Rescher, U.; Gerke, V.; Staub, O.; Nilius, B.; Bindels, R.J.M. Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J. 2003, 22, 1478–1487. [Google Scholar] [CrossRef]
- Lau, J.K.; Brown, K.C.; Dom, A.M.; Witte, T.R.; Thornhill, B.A.; Crabtree, C.M.; Perry, H.E.; Brown, J.M.; Ball, J.G.; Creel, R.G.; et al. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis 2014, 19, 1190–1201. [Google Scholar] [CrossRef]
- Chow, J.; Norng, M.; Zhang, J.; Chai, J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells--Mechanisms behind a possible new “hot” cancer treatment. Biochim. Biophys. Acta 2007, 1773, 565–576. [Google Scholar] [CrossRef]
- Lehen’kyi, V.; Flourakis, M.; Skryma, R.; Prevarskaya, N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene 2007, 26, 7380–7385. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, Z.; Lu, J.; Chen, F.M.; Wong, W.-T.; Tse, G.; Zheng, C.; Keung, W.; Tse, K.; Li, R.A.; et al. TRPV6 protects ER stress-induced apoptosis via ATF6α-TRPV6-JNK pathway in human embryonic stem cell-derived cardiomyocytes. J. Mol. Cell. Cardiol. 2018, 120, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Que, K.; Zang, C.; Wen, J.; Sun, G.; Zhao, Z.; Li, Y. Expression and distribution of three transient receptor potential vanilloid(TRPV) channel proteins in human odontoblast-like cells. J. Mol. Histol. 2017, 48, 367–377. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. Role of the TRPV Channels in the Endoplasmic Reticulum Calcium Homeostasis. Cells 2020, 9, 317. https://doi.org/10.3390/cells9020317
Haustrate A, Prevarskaya N, Lehen’kyi V. Role of the TRPV Channels in the Endoplasmic Reticulum Calcium Homeostasis. Cells. 2020; 9(2):317. https://doi.org/10.3390/cells9020317
Chicago/Turabian StyleHaustrate, Aurélien, Natalia Prevarskaya, and V’yacheslav Lehen’kyi. 2020. "Role of the TRPV Channels in the Endoplasmic Reticulum Calcium Homeostasis" Cells 9, no. 2: 317. https://doi.org/10.3390/cells9020317
APA StyleHaustrate, A., Prevarskaya, N., & Lehen’kyi, V. (2020). Role of the TRPV Channels in the Endoplasmic Reticulum Calcium Homeostasis. Cells, 9(2), 317. https://doi.org/10.3390/cells9020317







