Neuromuscular Activity Induces Paracrine Signaling and Triggers Axonal Regrowth after Injury in Microfluidic Lab-On-Chip Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Compartmentalized Microfluidic Devices
2.3. C2C12 Myoblast Culture and Generation of a Stable ChR2-eYFP-Expressing C2C12 Cell Line
2.4. C2C12 Myotube Differentiation in Microfluidic Platforms
2.5. Spinal Cord Explant Cultures in Compartmentalized Microfluidic Devices
2.6. In Vitro Axotomy in Microfluidic Platforms
2.7. Optogenetic Setup and Stimulation Procedures
2.8. Analysis of ChR2-Positive Activity in Spinal Cord Slides with mRuby-Based RCaMP (jRCaMP1b)
2.9. Analysis of Myotube Contractility
2.10. Immunocytochemical Techniques
2.11. Quantitative Real-Time PCR
2.12. Functional Roles of Selected Candidates in Spinal Cord Explants
2.13. Quantification and Statistical Analysis
3. Results
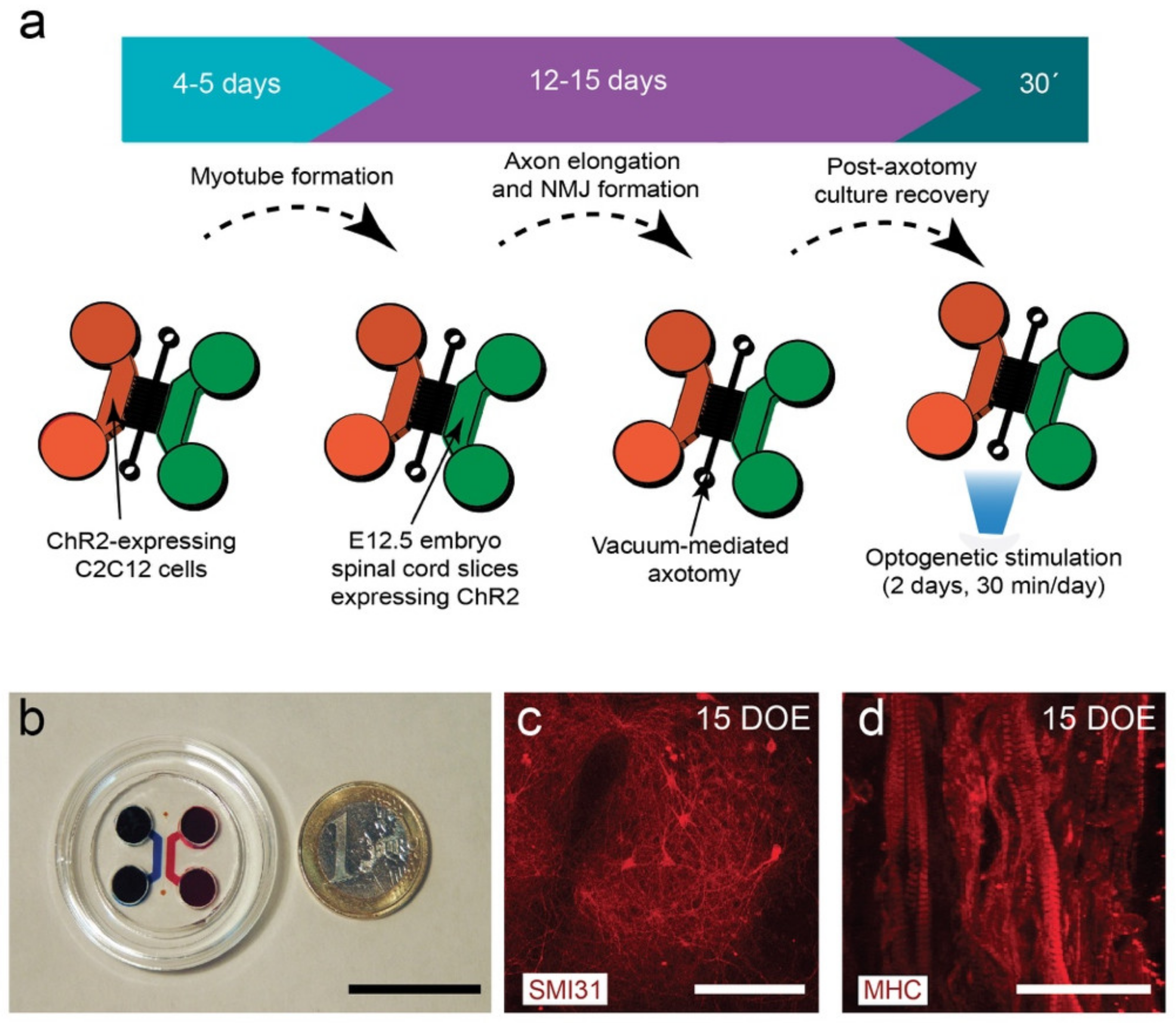
3.1. Design and Fabrication of the NMJ Microfluidic Axotomy/Co-Culture Platform
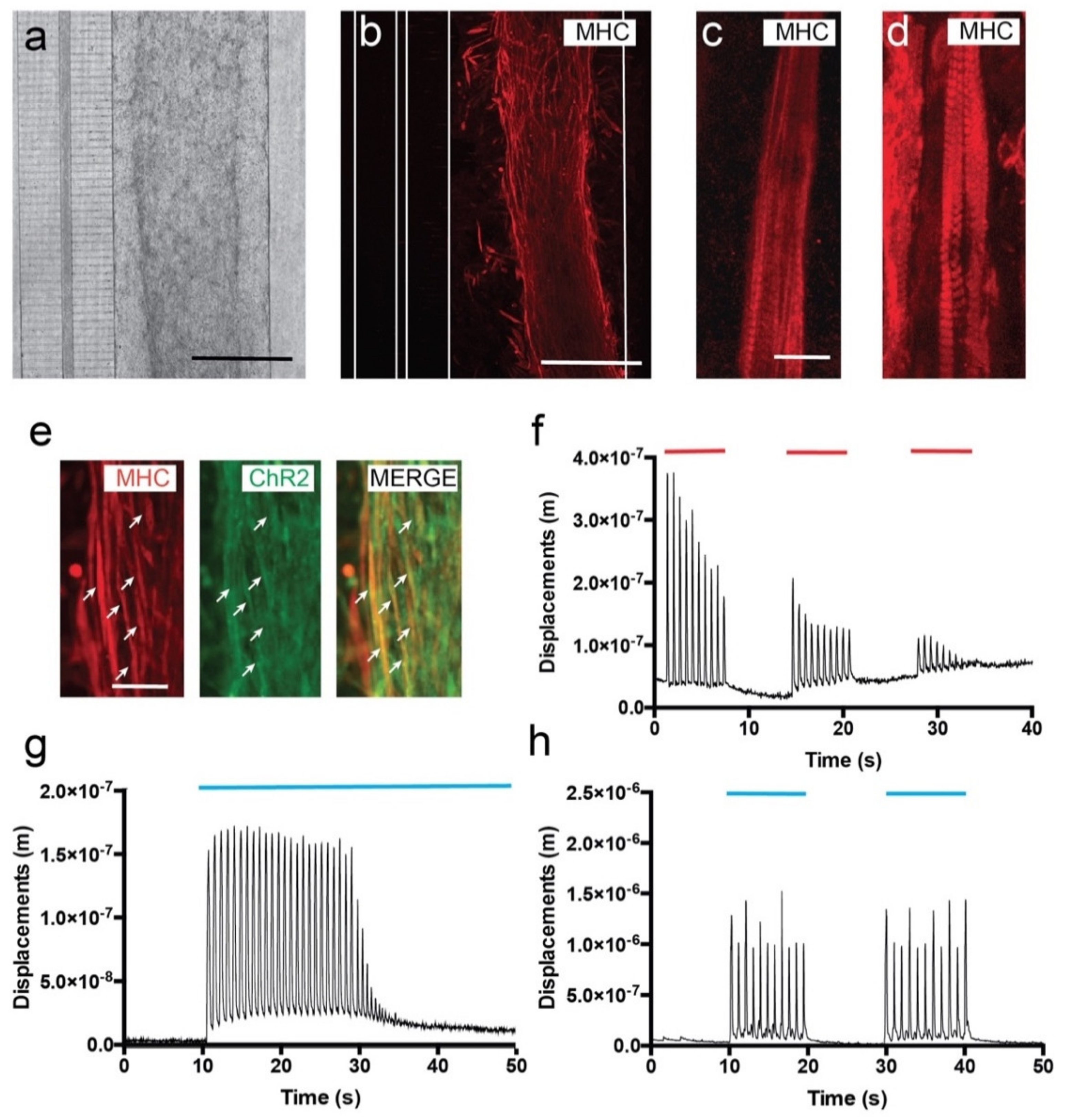
3.2. C2C12-ChR2 Myoblasts Differentiate into Light-Inducible Contractile Myotubes
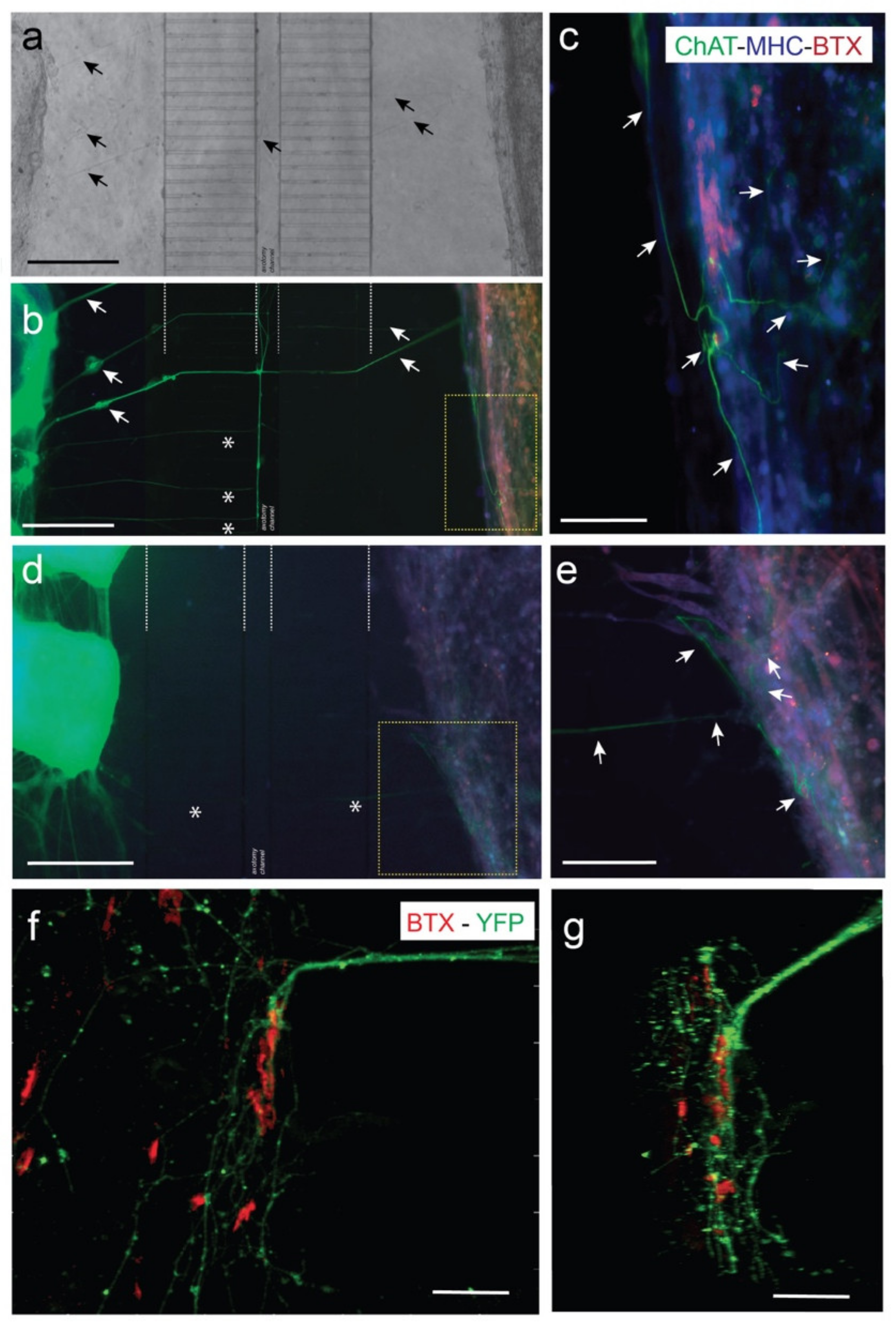
3.3. Viable Co-Culture and Formation of NMJ in Compartmentalized Microfluidic Devices
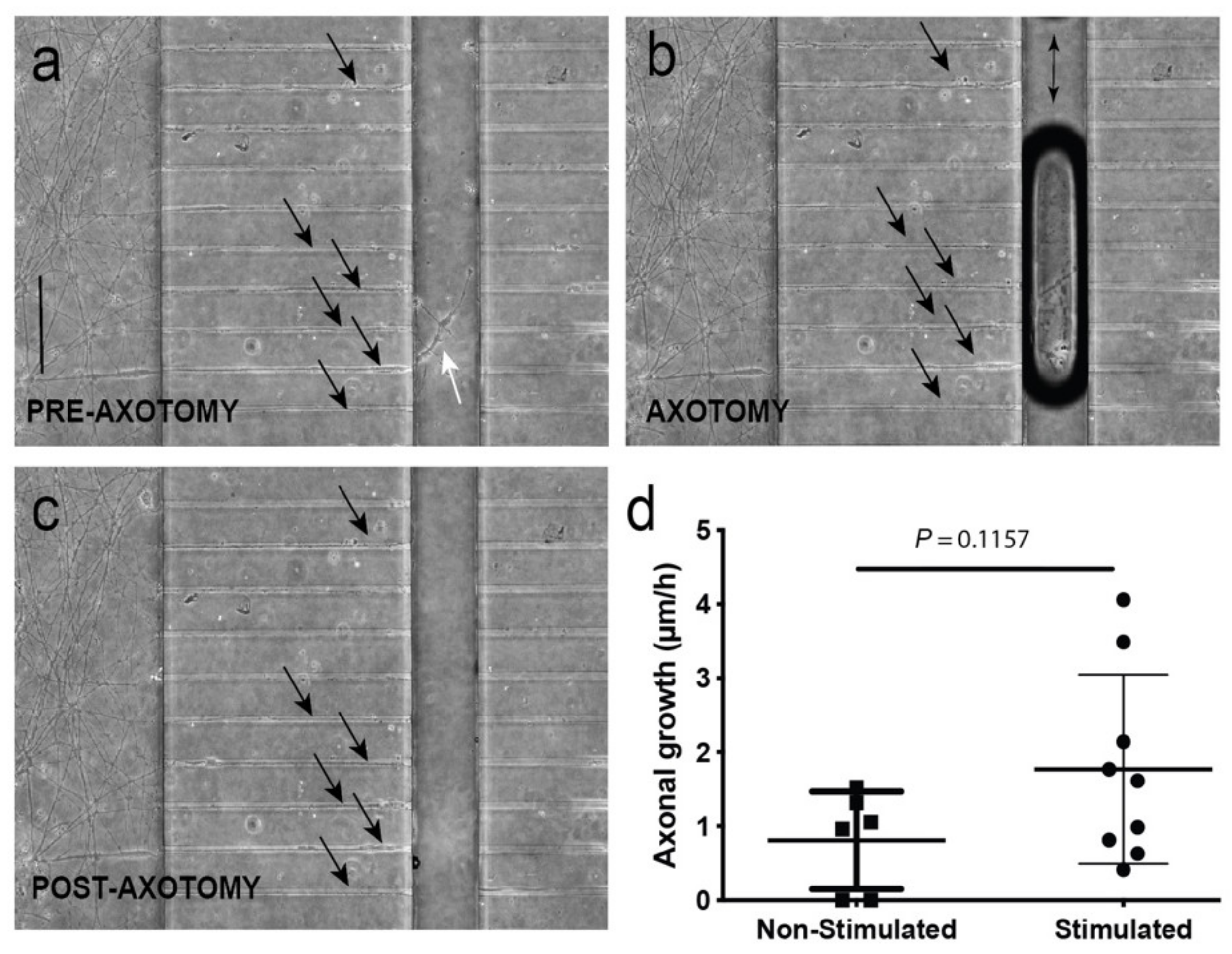
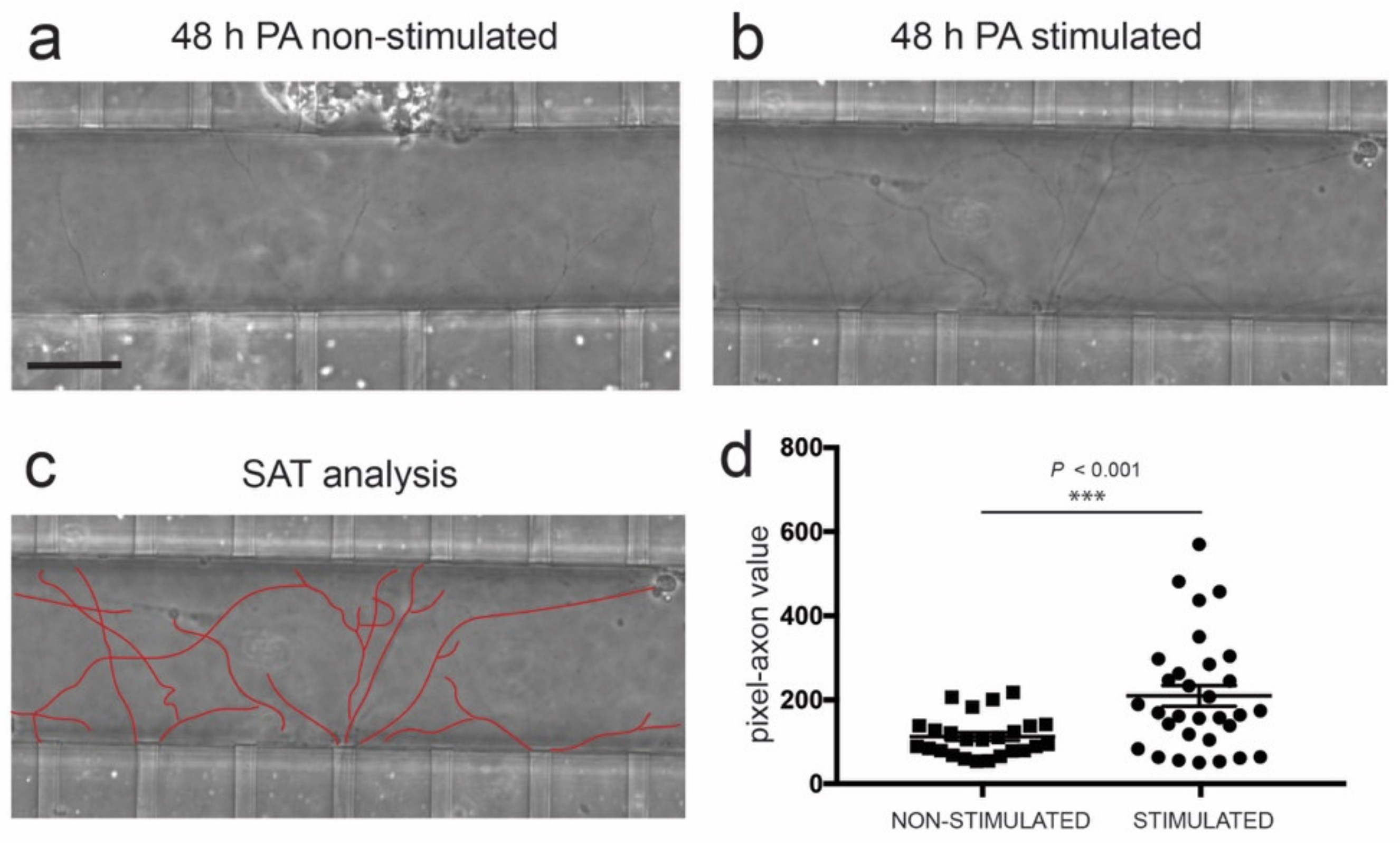
3.4. Optogenetic Modulation of MN Activity Increases Axonal Outgrowth after Microfluidic Induced Axotomy
3.5. Optogenetic Modulation of Muscular Activity Induces Paracrine Signaling, Triggering Axonal Growth after Lesion on MNs
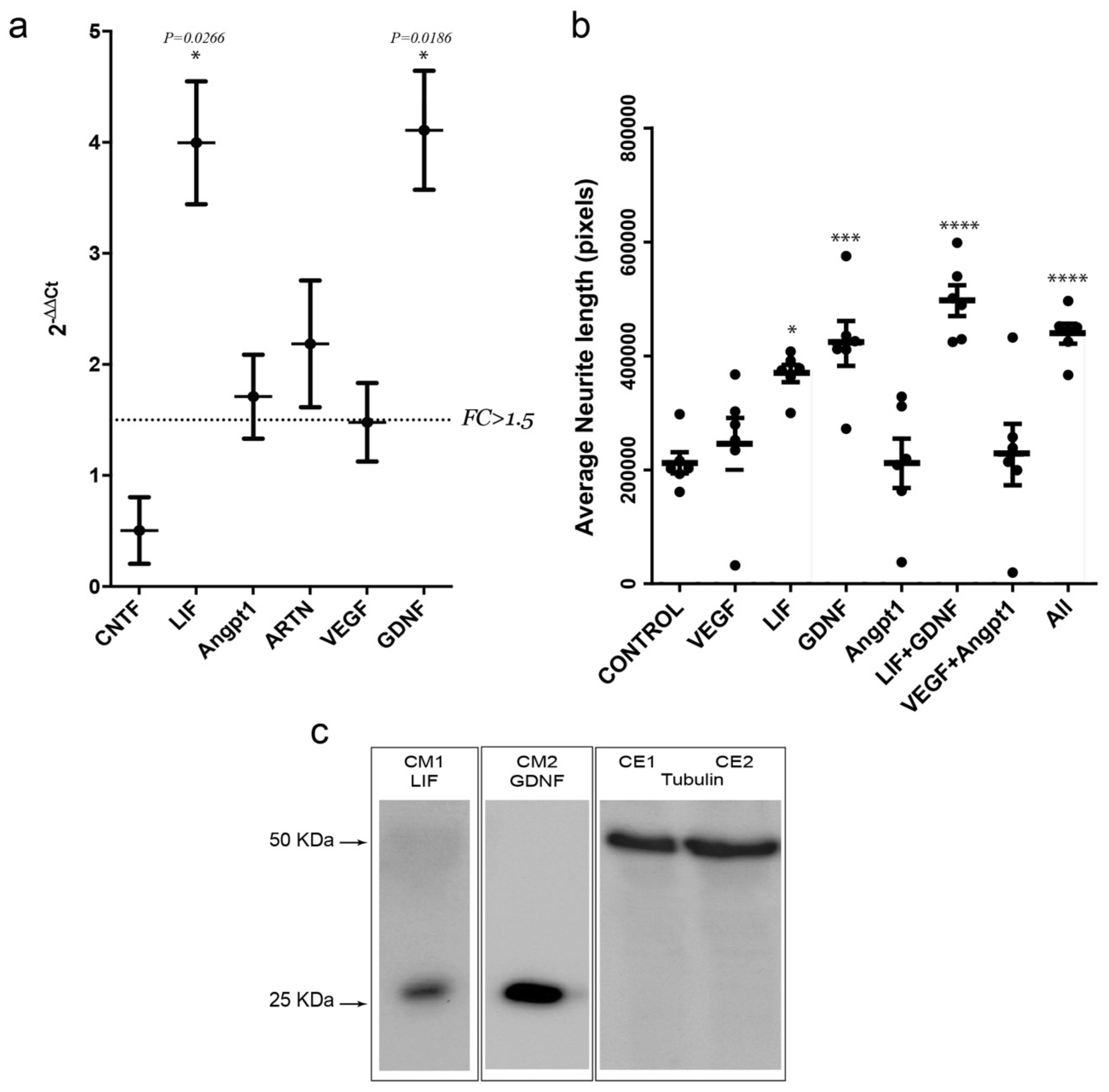
3.6. Activity-Dependent Muscle-Derived LIF and GDNF Induce Axonal Growth on MN
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, A.M.; Rhee, S.W.; Tu, C.H.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. Microfluidic Multicompartment Device for Neuroscience Research. Langmuir 2003, 19, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Campenot, R.B. Independent control of the local environment of somas and neurites. Methods Enzymol. 1979, 58, 302–307. [Google Scholar] [PubMed]
- Campenot, R.B. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. USA 1977, 74, 4516–4519. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Vahidi, B.; Taylor, A.M.; Rhee, S.W.; Jeon, N.L. Microfluidic culture platform for neuroscience research. Nat. Protoc. 2006, 1, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Neto, E.; Leitao, L.; Sousa, D.M.; Alves, C.J.; Alencastre, I.S.; Aguiar, P.; Lamghari, M. Compartmentalized Microfluidic Platforms: The Unrivaled Breakthrough of In Vitro Tools for Neurobiological Research. J. Neurosci. 2016, 36, 11573–11584. [Google Scholar] [CrossRef]
- Taylor, A.M.; Jeon, N.L. Microfluidic and compartmentalized platforms for neurobiological research. Crit. Rev. Biomed. Eng. 2011, 39, 185–200. [Google Scholar] [CrossRef]
- Siddique, R.; Thakor, N. Investigation of nerve injury through microfluidic devices. J. R. Soc. Interface. 2014, 11, 20130676. [Google Scholar] [CrossRef]
- Rosello-Busquets, C.; de la Oliva, N.; Martinez-Marmol, R.; Hernaiz-Llorens, M.; Pascual, M.; Muhaisen, A.; Navarro, X.; Del Valle, J.; Soriano, E. Cholesterol Depletion Regulates Axonal Growth and Enhances Central and Peripheral Nerve Regeneration. Front. Cell Neurosci. 2019, 13, 40. [Google Scholar] [CrossRef]
- Tourovskaia, A.; Figueroa-Masot, X.; Folch, A. Differentiation-on-a-chip: A microfluidic platform for long-term cell culture studies. Lab Chip 2005, 5, 14–19. [Google Scholar] [CrossRef]
- Tourovskaia, A.; Kosar, T.F.; Folch, A. Local induction of acetylcholine receptor clustering in myotube cultures using microfluidic application of agrin. Biophys. J. 2006, 90, 2192–2198. [Google Scholar] [CrossRef]
- Dennis, R.G.; Kosnik, P.E., 2nd; Gilbert, M.E.; Faulkner, J.A. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am. J. Physiol. Cell Physiol. 2001, 280, C288–C295. [Google Scholar] [CrossRef] [PubMed]
- Southam, K.A.; King, A.E.; Blizzard, C.A.; McCormack, G.H.; Dickson, T.C. Microfluidic primary culture model of the lower motor neuron-neuromuscular junction circuit. J. Neurosci. Methods 2013, 218, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Zahavi, E.E.; Gradus, T.; Ben-Yaakov, K.; Perlson, E. Compartmental microfluidic system for studying muscle-neuron communication and neuromuscular junction maintenance. Eur. J. Cell Biol. 2016, 95, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Liu, S.; McDonald, J.; Thakor, N.; Yang, I.H. Neuromuscular junction in a microfluidic device. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 2013, 2833–2835. [Google Scholar] [CrossRef]
- Uzel, S.G.; Platt, R.J.; Subramanian, V.; Pearl, T.M.; Rowlands, C.J.; Chan, V.; Boyer, L.A.; So, P.T.; Kamm, R.D. Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units. Sci. Adv. 2016, 2, e1501429. [Google Scholar] [CrossRef]
- Osaki, T.; Uzel, S.G.M.; Kamm, R.D. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sc.i Adv. 2018, 4, eaat5847. [Google Scholar] [CrossRef]
- Vila, O.F.; Uzel, S.G.M.; Ma, S.P.; Williams, D.; Pak, J.; Kamm, R.D.; Vunjak-Novakovic, G. Quantification of human neuromuscular function through optogenetics. Theranostics 2019, 9, 1232–1246. [Google Scholar] [CrossRef]
- Nedachi, T.; Fujita, H.; Kanzaki, M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1191–E1204. [Google Scholar] [CrossRef]
- Pasterkamp, R.J.; Giger, R.J.; Verhaagen, J. Regulation of semaphorin III/collapsin-1 gene expression during peripheral nerve regeneration. Exp. Neurol. 1998, 153, 313–327. [Google Scholar] [CrossRef]
- Spinelli, E.D.; McPhail, L.T.; Oschipok, L.W.; Teh, J.; Tetzlaff, W. Class A plexin expression in axotomized rubrospinal and facial motoneurons. Neuroscience 2007, 144, 1266–1277. [Google Scholar] [CrossRef]
- Martini, R.; Schachner, M.; Brushart, T.M. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J. Neurosci. 1994, 14, 7180–7191. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Gerber, J.; Kessens, P.; Chen, Y.G.; Royall, R.M. Contributions of pathway and neuron to preferential motor reinnervation. J. Neurosci. 1998, 18, 8674–8681. [Google Scholar] [CrossRef] [PubMed]
- Hoke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, K.A.; Irintchev, A.; Al-Majed, A.A.; Simova, O.; Brushart, T.M.; Gordon, T.; Schachner, M. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp. Neurol. 2006, 198, 500–510. [Google Scholar] [CrossRef]
- Madison, R.D.; Sofroniew, M.V.; Robinson, G.A. Schwann cell influence on motor neuron regeneration accuracy. Neuroscience 2009, 163, 213–221. [Google Scholar] [CrossRef]
- Jennische, E.; Ekberg, S.; Matejka, G.L. Expression of hepatocyte growth factor in growing and regenerating rat skeletal muscle. Am. J. Physiol. 1993, 265, C122–C128. [Google Scholar] [CrossRef]
- Lie, D.C.; Weis, J. GDNF expression is increased in denervated human skeletal muscle. Neurosci. Lett. 1998, 250, 87–90. [Google Scholar] [CrossRef]
- Tonra, J.R.; Curtis, R.; Wong, V.; Cliffer, K.D.; Park, J.S.; Timmes, A.; Nguyen, T.; Lindsay, R.M.; Acheson, A.; DiStefano, P.S. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J. Neurosci. 1998, 18, 4374–4383. [Google Scholar] [CrossRef]
- Wallenius, V.; Hisaoka, M.; Helou, K.; Levan, G.; Mandahl, N.; Meis-Kindblom, J.M.; Kindblom, L.G.; Jansson, J.O. Overexpression of the hepatocyte growth factor (HGF) receptor (Met) and presence of a truncated and activated intracellular HGF receptor fragment in locally aggressive/malignant human musculoskeletal tumors. Am. J. Pathol. 2000, 156, 821–829. [Google Scholar] [CrossRef]
- Wehrwein, E.A.; Roskelley, E.M.; Spitsbergen, J.M. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve 2002, 26, 206–211. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Ishii, H.; Morita, I.; Oota, I.; Takeda, H. mRNA expression of fibroblast growth factors and hepatocyte growth factor in rat plantaris muscle following denervation and compensatory overload. Pflugers Arch. 2004, 448, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Veltri, K.; Li, S.; Bain, J.R.; Fahnestock, M. NGF, BDNF, NT-3, and GDNF mRNA expression in rat skeletal muscle following denervation and sensory protection. J. Neurotrauma 2004, 21, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R.; Gifondorwa, D.J.; Newbern, J.M.; Robinson, M.B.; Strupe, J.L.; Prevette, D.; Oppenheim, R.W.; Milligan, C.E. Astrocyte and muscle-derived secreted factors differentially regulate motoneuron survival. J. Neurosci. 2007, 27, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Segura-Feliu, M.; Seira, O.; Homs-Corbera, A.; Del Río, J.A.; Samitier, J. A microfluidic neuronal platform for neuron axotomy and controlled regenerative studies. RSC Adv. 2015, 5, 73457–73466. [Google Scholar] [CrossRef]
- Deleglise, B.; Lassus, B.; Soubeyre, V.; Alleaume-Butaux, A.; Hjorth, J.J.; Vignes, M.; Schneider, B.; Brugg, B.; Viovy, J.L.; Peyrin, J.M. Synapto-protective drugs evaluation in reconstructed neuronal network. PLoS ONE 2013, 8, e71103. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef]
- Dagberg, B.; Alstermark, B. Improved organotypic cell culture model for analysis of the neuronal circuit involved in the monosynaptic stretch reflex. J. Neurosci. Res. 2006, 84, 460–469. [Google Scholar] [CrossRef]
- Gerhardt, K.P.; Olson, E.J.; Castillo-Hair, S.M.; Hartsough, L.A.; Landry, B.P.; Ekness, F.; Yokoo, R.; Gomez, E.J.; Ramakrishnan, P.; Suh, J.; et al. An open-hardware platform for optogenetics and photobiology. Sci. Rep. 2016, 6, 35363. [Google Scholar] [CrossRef]
- Tucker, C.L.; Vrana, J.D.; Kennedy, M.J. Tools for controlling protein interactions using light. Curr. Protoc. Cell Biol. 2014, 64, 17.16.1–17.16.20. [Google Scholar] [CrossRef]
- Huebsch, N.; Loskill, P.; Mandegar, M.A.; Marks, N.C.; Sheehan, A.S.; Ma, Z.; Mathur, A.; Nguyen, T.N.; Yoo, J.C.; Judge, L.M.; et al. Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng. Part. C Methods 2015, 21, 467–479. [Google Scholar] [CrossRef]
- Gotts, J.; Atkinson, L.; Yanagawa, Y.; Deuchars, J.; Deuchars, S.A. Co-expression of GAD67 and choline acetyltransferase in neurons in the mouse spinal cord: A focus on lamina X. Brain Res. 2016, 1646, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.C.; Niu, T.; Alaynick, W.A. Molecular and cellular development of spinal cord locomotor circuitry. Front. Mol. Neurosci. 2015, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, M.; Shimizu-Okabe, C.; Kim, J.; Kobayashi, S.; Kosaka, Y.; Yanagawa, Y.; Matsushita, M.; Okabe, A.; Takayama, C. Distinct development of the glycinergic terminals in the ventral and dorsal horns of the mouse cervical spinal cord. Neuroscience 2017, 343, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Meijering, E.; Jacob, M.; Sarria, J.C.; Steiner, P.; Hirling, H.; Unser, M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 2004, 58, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Sivathanu, V.; Kamm, R.D. Crosstalk between developing vasculature and optogenetically engineered skeletal muscle improves muscle contraction and angiogenesis. Biomaterials 2018, 156, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Bruegmann, T.; van Bremen, T.; Vogt, C.C.; Send, T.; Fleischmann, B.K.; Sasse, P. Optogenetic control of contractile function in skeletal muscle. Nat. Commun. 2015, 6, 7153. [Google Scholar] [CrossRef]
- Asano, T.; Ishizua, T.; Yawo, H. Optically controlled contraction of photosensitive skeletal muscle cells. Biotechnol. Bioeng. 2012, 109, 199–204. [Google Scholar] [CrossRef]
- Tong, Z.; Seira, O.; Casas, C.; Reginensi, D.; Homs-Corbera, A.; Samitier, J.; Del Río, J.A. Engineering a functional neuro-muscular junction model in a chip. RSC Adv. 2014, 4, 54788–54797. [Google Scholar] [CrossRef]
- Hyung, S.; Lee, S.R.; Kim, Y.J.; Bang, S.; Tahk, D.; Park, J.C.; Suh, J.F.; Jeon, N.L. Optogenetic neuronal stimulation promotes axon outgrowth and myelination of motor neurons in a three-dimensional motor neuron-Schwann cell coculture model on a microfluidic biochip. Biotechnol. Bioeng. 2019, 116, 2425–2438. [Google Scholar] [CrossRef]
- Wong, K.H.; Chan, J.M.; Kamm, R.D.; Tien, J. Microfluidic models of vascular functions. Annu. Rev. Biomed. Eng. 2012, 14, 205–230. [Google Scholar] [CrossRef]
- Badiola-Mateos, M.; Hervera, A.; Del Rio, J.A.; Samitier, J. Challenges and Future Prospects on 3D in-vitro Modeling of the Neuromuscular Circuit. Front. Bioeng. Biotechnol. 2018, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Vila, O.F.; Qu, Y.; Vunjak-Novakovic, G. In vitro models of neuromuscular junctions and their potential for novel drug discovery and development. Expert Opin. Drug Discov. 2019, 1–11. [Google Scholar] [CrossRef]
- Thompson, S.W.; Majithia, A.A. Leukemia inhibitory factor induces sympathetic sprouting in intact dorsal root ganglia in the adult rat in vivo. J. Physiol. 1998, 506, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Sun, F.; Park, K.K.; Cai, B.; Wang, C.; Kuwako, K.; Martinez-Carrasco, I.; Connolly, L.; He, Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 2009, 64, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Hofmann, H.D.; Kirsch, M. Glial reactivity in ciliary neurotrophic factor-deficient mice after optic nerve lesion. J. Neurosci. 2003, 23, 5416–5424. [Google Scholar] [CrossRef]
- Qiu, J.; Cafferty, W.B.; McMahon, S.B.; Thompson, S.W. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J. Nurosci. 2005, 25, 1645–1653. [Google Scholar] [CrossRef]
- Yu, S.; Yao, S.; Wen, Y.; Wang, Y.; Wang, H.; Xu, Q. Angiogenic microspheres promote neural regeneration and motor function recovery after spinal cord injury in rats. Sci. Rep. 2016, 6, 33428. [Google Scholar] [CrossRef]
- Kosacka, J.; Figiel, M.; Engele, J.; Hilbig, H.; Majewski, M.; Spanel-Borowski, K. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 2005, 320, 11–19. [Google Scholar] [CrossRef]
- Poss, K.D.; Shen, J.; Nechiporuk, A.; McMahon, G.; Thisse, B.; Thisse, C.; Keating, M.T. Roles for Fgf signaling during zebrafish fin regeneration. Dev. Biol. 2000, 222, 347–358. [Google Scholar] [CrossRef]
- Saera-Vila, A.; Kish, P.E.; Kahana, A. Fgf regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish. Cell. Signal. 2016, 28, 1196–1204. [Google Scholar] [CrossRef]
- Suzuki, M.; McHugh, J.; Tork, C.; Shelley, B.; Hayes, A.; Bellantuono, I.; Aebischer, P.; Svendsen, C.N. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol. Ther. 2008, 16, 2002–2010. [Google Scholar] [CrossRef]
- Oppenheim, R.W.; Houenou, L.J.; Johnson, J.E.; Lin, L.F.; Li, L.; Lo, A.C.; Newsome, A.L.; Prevette, D.M.; Wang, S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature 1995, 373, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.E.; Phillips, H.S.; Pollock, R.A.; Davies, A.M.; Lemeulle, C.; Armanini, M.; Simmons, L.; Moffet, B.; Vandlen, R.A.; Simpson, L.C.; et al. GDNF: A potent survival factor for motoneurons present in peripheral nerve and muscle. Science 1994, 266, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Glat, M.J.; Benninger, F.; Barhum, Y.; Ben-Zur, T.; Kogan, E.; Steiner, I.; Yaffe, D.; Offen, D. Ectopic Muscle Expression of Neurotrophic Factors Improves Recovery After Nerve Injury. J. Mol. Neurosci. 2016, 58, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Vianney, J.M.; Miller, D.A.; Spitsbergen, J.M. Effects of acetylcholine and electrical stimulation on glial cell line-derived neurotrophic factor production in skeletal muscle cells. Brain Res. 2014, 1588, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Montoya, G.J.; Sutachan, J.J.; Chan, W.S.; Sideris, A.; Blanck, T.J.; Recio-Pinto, E. Muscle-conditioned media and cAMP promote survival and neurite outgrowth of adult spinal cord motor neurons. Exp. Neurol. 2009, 220, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Angka, H.E.; Geddes, A.J.; Kablar, B. Differential survival response of neurons to exogenous GDNF depends on the presence of skeletal muscle. Dev. Dyn. 2008, 237, 3169–3178. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Ball, A.K. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: Combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience 2002, 110, 555–567. [Google Scholar] [CrossRef]
- Bilak, M.M.; Shifrin, D.A.; Corse, A.M.; Bilak, S.R.; Kuncl, R.W. Neuroprotective utility and neurotrophic action of neurturin in postnatal motor neurons: Comparison with GDNF and persephin. Mol. Cell. Neurosci. 1999, 13, 326–336. [Google Scholar] [CrossRef]
- Widenfalk, J.; Wu, W.; Hao, J.; Person, J.K.; Wiesenfeldt-Hallin, Z.; Risling, M. Treatment of transected peripheral nerves with artemin improved motor neuron regeneration, but did not reduce nerve injury-induced pain behaviour. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2009, 43, 245–250. [Google Scholar] [CrossRef]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef]
- Mousavi, K.; Jasmin, B.J. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J. Neurosci. 2006, 26, 5739–5749. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Watanabe, K.; Sano, M.; Uramoto, I.; Totsuka, T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim. Biophys. Acta 2000, 1497, 77–88. [Google Scholar] [CrossRef]
- Weis, J.; Lie, D.C.; Ragoss, U.; Zuchner, S.L.; Schroder, J.M.; Karpati, G.; Farruggella, T.; Stahl, N.; Yancopoulos, G.D.; DiStefano, P.S. Increased expression of CNTF receptor alpha in denervated human skeletal muscle. J. Neuropathol. Exp. Neurol. 1998, 57, 850–857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurek, J.B.; Austin, L.; Cheema, S.S.; Bartlett, P.F.; Murphy, M. Up-regulation of leukaemia inhibitory factor and interleukin-6 in transected sciatic nerve and muscle following denervation. Neuromuscul. Disord. 1996, 6, 105–114. [Google Scholar] [CrossRef]
- Davis, S.; Aldrich, T.H.; Ip, N.Y.; Stahl, N.; Scherer, S.; Farruggella, T.; DiStefano, P.S.; Curtis, R.; Panayotatos, N.; Gascan, H.; et al. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science 1993, 259, 1736–1739. [Google Scholar] [CrossRef]
- Hunt, L.C.; Anthea Coles, C.; Gorman, C.M.; Tudor, E.M.; Smythe, G.M.; White, J.D. Alterations in the expression of leukemia inhibitory factor following exercise: Comparisons between wild-type and mdx muscles. PLoS Curr. 2011, 3, RRN1277. [Google Scholar] [CrossRef]
- Niemi, J.P.; DeFrancesco-Lisowitz, A.; Cregg, J.M.; Howarth, M.; Zigmond, R.E. Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism. Exp. Neurol. 2016, 275 Pt 1, 25–37. [Google Scholar] [CrossRef]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003, 27, 277–324. [Google Scholar] [CrossRef]
- Leibinger, M.; Muller, A.; Andreadaki, A.; Hauk, T.G.; Kirsch, M.; Fischer, D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J. Neurosci. 2009, 29, 14334–14341. [Google Scholar] [CrossRef]
- Paratcha, G.; Ledda, F. GDNF and GFRalpha: A versatile molecular complex for developing neurons. Trends Neurosci. 2008, 31, 384–391. [Google Scholar] [CrossRef]
- Osaki, T.; Shin, Y.; Sivathanu, V.; Campisi, M.; Kamm, R.D. In Vitro Microfluidic Models for Neurodegenerative Disorders. Adv. Healthc. Mater 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Chauvet, S.; Burk, K.; Mann, F. Navigation rules for vessels and neurons: Cooperative signaling between VEGF and neural guidance cues. Cell Mol. Life Sci. 2013, 70, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; Del Rio, J.A. Functions of Plexins/Neuropilins and Their Ligands during Hippocampal Development and Neurodegeneration. Cells 2019, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Eichmann, A. Axon guidance molecules in vascular patterning. Cold Spring Harb. Perspect. Biol. 2010, 2, a001875. [Google Scholar] [CrossRef]
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| CNTF | TTAGGGGATGGCTTTCGCAG | GGAGGTTCTCTTGGAGTCGC |
| LIF | GGTGGAGCTGTATCGGATGG | ATTGAGCTTGACCTGGAGGC |
| Angpt1 | GGAACCGAGCCTACTCACAG | CAAGCTGCTCTGTTTGCCTG |
| ARTN | GAGCCTACTGCATTGTCCCA | CAAATGCGCAGTGTGTCCC |
| NRTN | CTACACGTCGGATGAGACCG | GACACCTCGTCCTCATAGGC |
| BDNF | AGTCTCCAGGACAGCAAAGC | TCGTCAGACCTCTCGAACCT |
| VEGF | GCAGACTATTCAGCGGACTCA | GGGAGTGAAGAACCAACCTCC |
| GDNF | GCATTCCTGCTACAGTGCGA | CACCCTGAAGTGCTCAGACG |
| Fgfb | TCAGTCCAGGCACCCTGT | GGGGCTCTCTTCACTCCACT |
| NT3 | GGAAGTCCTTCAAAGGGATCGT | GCAGAAGTAACCATGGCATCC |
| GAPDH | ACCCTGTTGCTGTAGCCGTATCA | TCAACAGCAACTCCCACTCTCCA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sala-Jarque, J.; Mesquida-Veny, F.; Badiola-Mateos, M.; Samitier, J.; Hervera, A.; del Río, J.A. Neuromuscular Activity Induces Paracrine Signaling and Triggers Axonal Regrowth after Injury in Microfluidic Lab-On-Chip Devices. Cells 2020, 9, 302. https://doi.org/10.3390/cells9020302
Sala-Jarque J, Mesquida-Veny F, Badiola-Mateos M, Samitier J, Hervera A, del Río JA. Neuromuscular Activity Induces Paracrine Signaling and Triggers Axonal Regrowth after Injury in Microfluidic Lab-On-Chip Devices. Cells. 2020; 9(2):302. https://doi.org/10.3390/cells9020302
Chicago/Turabian StyleSala-Jarque, Julia, Francina Mesquida-Veny, Maider Badiola-Mateos, Josep Samitier, Arnau Hervera, and José Antonio del Río. 2020. "Neuromuscular Activity Induces Paracrine Signaling and Triggers Axonal Regrowth after Injury in Microfluidic Lab-On-Chip Devices" Cells 9, no. 2: 302. https://doi.org/10.3390/cells9020302
APA StyleSala-Jarque, J., Mesquida-Veny, F., Badiola-Mateos, M., Samitier, J., Hervera, A., & del Río, J. A. (2020). Neuromuscular Activity Induces Paracrine Signaling and Triggers Axonal Regrowth after Injury in Microfluidic Lab-On-Chip Devices. Cells, 9(2), 302. https://doi.org/10.3390/cells9020302








