The LisH Domain-Containing N-Terminal Fragment is Important for the Localization, Dimerization, and Stability of Katnal2 in Tetrahymena
Abstract
1. Introduction
2. Materials and Methods
2.1. Tetrahymena Strains and Culture
2.2. Cross-Linkers
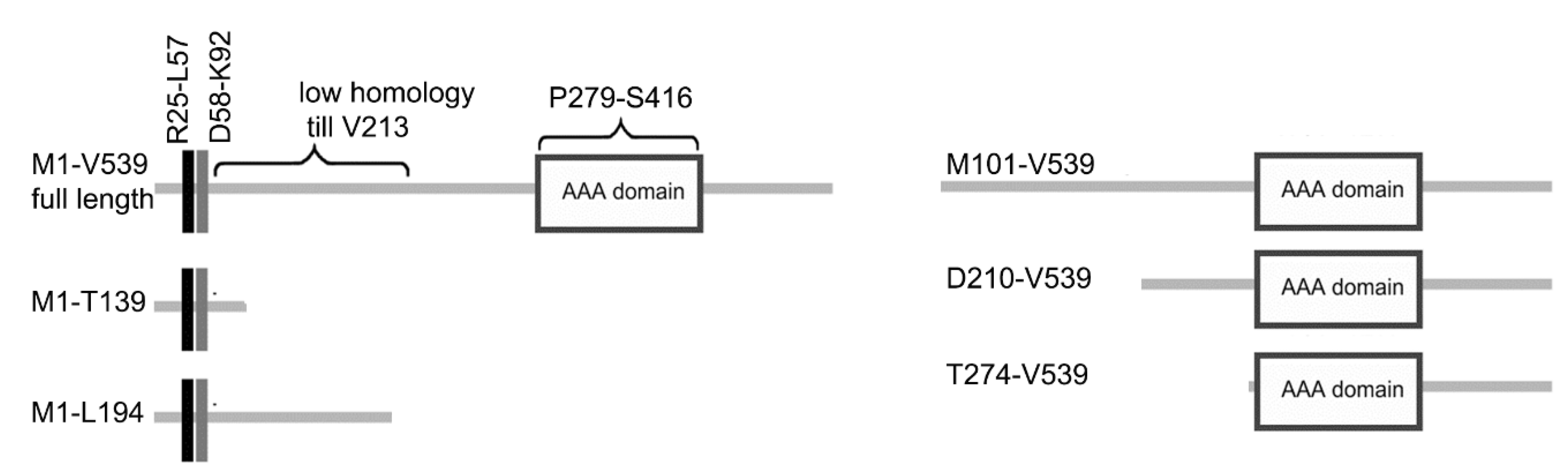
2.3. Protein Tagging and Domain Analysis
2.4. Immunofluorescence and Transmission Electron Microscopy
2.5. Western Blots
2.6. Microtubule Polymerization, Microtubule Binding Assay, Protein Cross-Linking and Immunoprecipitation
2.7. Protein Sequence Analysis
3. Results
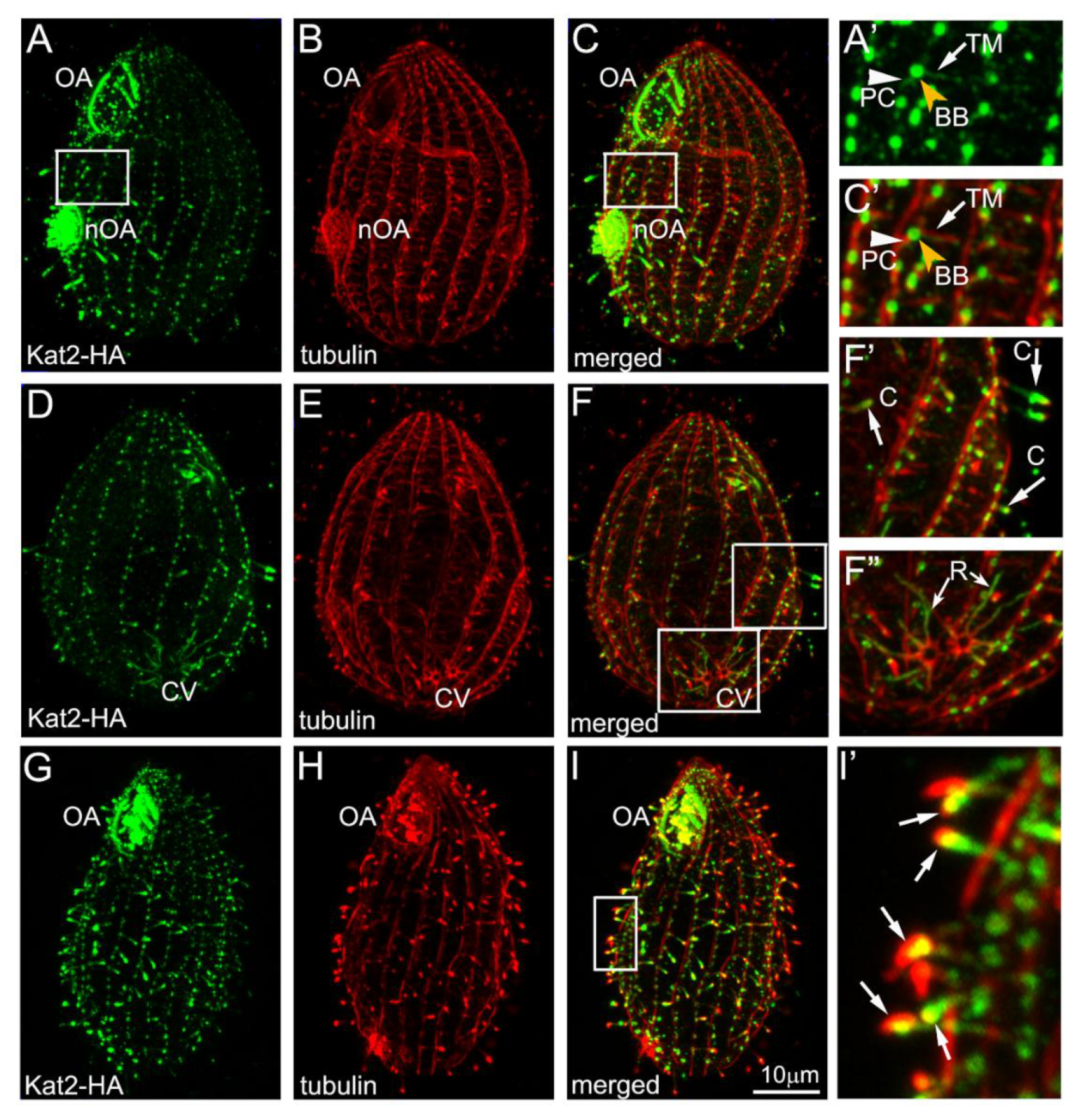
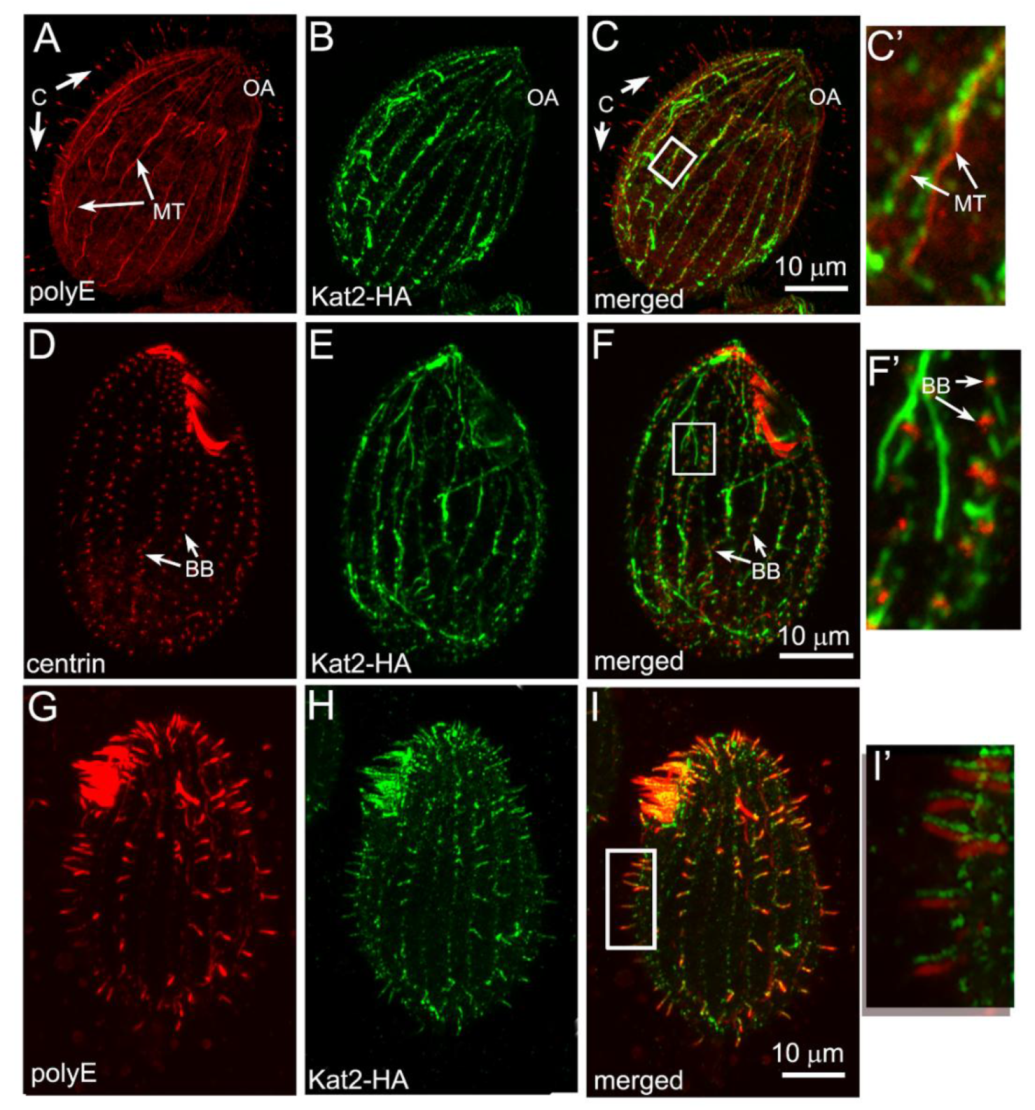
3.1. Kat2, an Ortholog of Mammalian Katnal2, Co-Localizes with Microtubular Structures
3.2. Kat2-HA Preferentially Co-Localizes with Glutamylated Microtubules
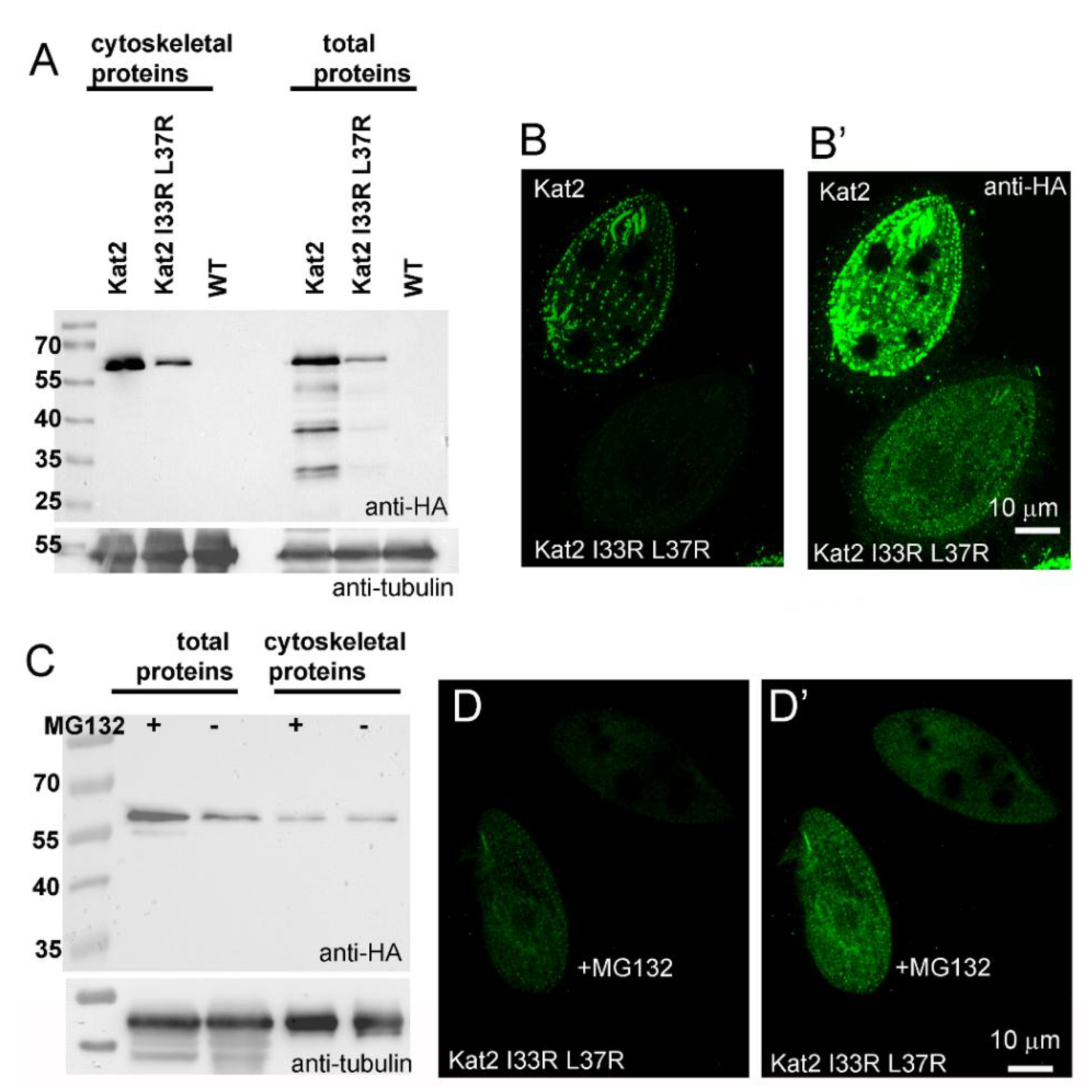
3.3. LisH Domain Plays a Role in Kat2 Basal Body Targeting and Protein Stability
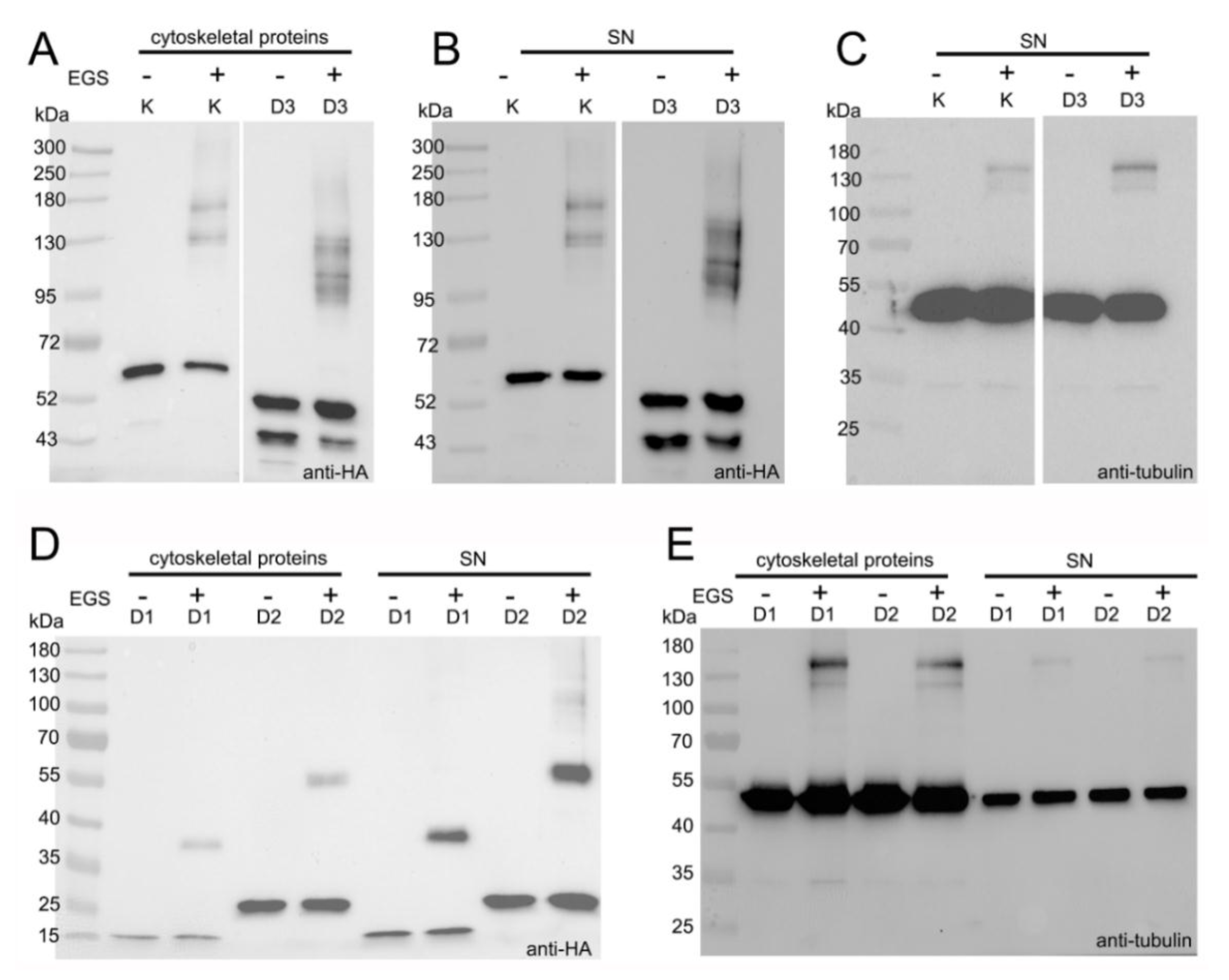
3.4. LisH Domain Mediates Kat2 Dimerization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McNally, F.J.; Roll-Mecak, A. Microtubule-severing enzymes: From cellular functions to molecular mechanism. J. Cell Biol. 2018, 217, 4057–4069. [Google Scholar] [CrossRef]
- Vemu, A.; Szczesna, E.; Zehr, E.A.; Spector, J.O.; Grigorieff, N.; Deaconescu, A.M.; Roll-Mecak, A. Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 2018, 361. [Google Scholar] [CrossRef]
- Delto, C.F.; Heisler, F.F.; Kuper, J.; Sander, B.; Kneussel, M.; Schindelin, H. The LisH motif of muskelin is crucial for oligomerization and governs intracellular localization. Structure 2015, 23, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bryant, J.; Wloga, D.; Donaldson, R.; Davis, R.C.; Jerka-Dziadosz, M.; Gaertig, J. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 2007, 178, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Ververis, A.; Christodoulou, A.; Christoforou, M.; Kamilari, C.; Lederer, C.W.; Santama, N. A novel family of katanin-like 2 protein isoforms (KATNAL2), interacting with nucleotide-binding proteins Nubp1 and Nubp2, are key regulators of different MT-based processes in mammalian cells. Cell Mol. Life Sci. 2016, 73, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Senese, S.; Kuang, J.; Bui, N.; Ongpipattanakul, C.; Gholkar, A.; Cohn, W.; Capri, J.; Whitelegge, J.P.; Torres, J.Z. Proteomic analysis of the mammalian Katanin family of microtubule-severing enzymes defines Katanin p80 subunit B-like 1 (KATNBL1) as a regulator of mammalian Katanin microtubule-severing. Mol. Cell Proteom. 2016, 15, 1658–1669. [Google Scholar] [CrossRef]
- Willsey, H.R.; Walentek, P.; Exner, C.R.T.; Xu, Y.; Lane, A.B.; Harland, R.M.; Heald, R.; Santama, N. Katanin-like protein Katnal2 is required for ciliogenesis and brain development in Xenopus embryos. Dev. Biol. 2018, 442, 276–287. [Google Scholar] [CrossRef]
- Casanova, M.; Crobu, L.; Blaineau, C.; Bourgeois, N.; Bastien, P.; Pagès, M. Microtubule-severing proteins are involved in flagellar length control and mitosis in Trypanosomatids. Mol. Microbiol. 2009, 71, 1353–1370. [Google Scholar] [CrossRef]
- Dunleavy, J.E.M.; Okuda, H.; O’Connor, A.E.; Merriner, D.J.; O’Donnell, L.; Jamsai, D.; Bergmann, M.; O’Bryan, M.K. Katanin-like 2 (KATNAL2) functions in multiple aspects of haploid male germ cell development in the mouse. PLoS Genet. 2017, 13, e1007078. [Google Scholar] [CrossRef]
- Williams, M.R.; Fricano-Kugler, C.J.; Getz, S.A.; Skelton, P.D.; Lee, J.; Rizzuto, C.P.; Geller, J.S.; Li, M.; Luikart, B.W. A retroviral CRISPR-Cas9 system for cellular autism-associated phenotype discovery in developing neurons. Sci. Rep. 2016, 6, 25611. [Google Scholar] [CrossRef]
- Neale, B.M.; Kou, Y.; Liu, L.; Ma’ayan, A.; Samocha, K.E.; Sabo, A.; Lin, C.F.; Stevens, C.; Wang, L.S.; Makarov, V.; et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 2012, 485, 242–245. [Google Scholar] [CrossRef] [PubMed]
- O’Roak, B.J.; Vives, L.; Fu, W.; Egertson, J.D.; Stanaway, I.B.; Phelps, I.G.; Carvill, G.; Kumar, A.; Lee, C.; Ankenman, K.; et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 2012, 338, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Stessman, H.A.; Xiong, B.; Coe, B.P.; Wang, T.; Hoekzema, K.; Fenckova, M.; Kvarnung, M.; Gerdts, J.; Trinh, S.; Cosemans, N.; et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 2017, 49, 515–526. [Google Scholar] [CrossRef]
- Emes, R.D.; Ponting, C.P. A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum. Mol. Genet. 2001, 10, 2813–2820. [Google Scholar] [CrossRef]
- Kim, M.H.; Cooper, D.R.; Oleksy, A.; Devedjiev, Y.; Derewenda, U.; Reiner, O.; Otlewski, J.; Derewenda, Z.S. The structure of the N-terminal domain of the product of the lissencephaly gene Lis1 and its functional implications. Structure 2004, 12, 987–998. [Google Scholar] [CrossRef]
- Mikolajka, A.; Yan, X.; Popowicz, G.M.; Smialowski, P.; Nigg, E.A.; Holak, T.A. Structure of the N-terminal domain of the FOP (FGFR1OP) protein and implications for its dimerization and centrosomal localization. J. Mol. Biol. 2006, 359, 863–875. [Google Scholar] [CrossRef]
- Ahn, J.; Novince, Z.; Concel, J.; Byeon, C.H.; Makhov, A.M.; Byeon, I.J.; Zhang, P.; Gronenborn, A.M. The Cullin-RING E3 ubiquitin ligase CRL4-DCAF1 complex dimerizes via a short helical region in DCAF1. Biochemistry 2011, 50, 1359–1367. [Google Scholar] [CrossRef]
- Ulrich, A.K.C.; Schulz, J.F.; Kamprad, A.; Schütze, T.; Wahl, M.C. Structural basis for the functional coupling of the alternative splicing factors smu1 and RED. Structure 2016, 24, 762–773. [Google Scholar] [CrossRef]
- Gerlitz, G.; Darhin, E.; Giorgio, G.; Franco, B.; Reiner, O. Novel functional features of the Lis-H domain: Role in protein dimerization, half-life and cellular localization. Cell Cycle 2005, 4, 1632–1640. [Google Scholar] [CrossRef]
- Gorovsky, M.A.; Yao, M.C.; Keevert, J.B.; Pleger, G.L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975, 9, 311–327. [Google Scholar] [PubMed]
- Gaertig, J.; Thatcher, T.H.; Gu, L.; Gorovsky, M.A. Electroporation-mediated replacement of a positively and negatively selectable beta-tubulin gene in Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 1994, 91, 4549–4553. [Google Scholar] [CrossRef]
- Janke, C.; Rogowski, K.; Wloga, D.; Regnard, C.; Kajava, A.V.; Strub, J.M.; Temurak, N.; van Dijk, J.; Boucher, D.; van Dorsselaer, A.; et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 2005, 308, 1758–1762. [Google Scholar] [CrossRef]
- Wloga, D.; Dave, D.; Meagley, J.; Rogowski, K.; Jerka-Dziadosz, M.; Gaertig, J. Hyperglutamylation of tubulin can either stabilize or destabilize microtubules in the same cell. Eukaryot. Cell 2010, 9, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Bregier, C.; Krzemień-Ojak, L.; Włoga, D.; Jerka-Dziadosz, M.; Joachimiak, E.; Batko, K.; Filipiuk, I.; Smietanka, U.; Gaertig, J.; Fabczak, S.; et al. PHLP2 is essential and plays a role in ciliogenesis and microtubule assembly in Tetrahymena thermophila. J. Cell Physiol. 2013, 228, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, P.; Joachimiak, E.; Bazan, R.; Fu, G.; Poprzeczko, M.; Fabczak, H.; Nicastro, D.; Wloga, D. Ciliary proteins Fap43 and Fap44 interact with each other and are essential for proper cilia and flagella beating. Cell Mol. Life Sci. 2018, 75, 4479–4493. [Google Scholar] [CrossRef] [PubMed]
- Waclawek, E.; Joachimiak, E.; Hall, M.H.; Fabczak, H.; Wloga, D. Regulation of katanin activity in the ciliate Tetrahymena thermophila. Mol. Microbiol. 2017, 103, 134–150. [Google Scholar] [CrossRef]
- Urbanska, P.; Song, K.; Joachimiak, E.; Krzemien-Ojak, L.; Koprowski, P.; Hennessey, T.; Jerka-Dziadosz, M.; Fabczak, H.; Gaertig, J.; Nicastro, D.; et al. The CSC proteins FAP61 and FAP251 build the basal substructures of radial spoke 3 in cilia. Mol. Biol. Cell 2015, 26, 1463–1475. [Google Scholar] [CrossRef]
- Shang, Y.; Li, B.; Gorovsky, M.A. Tetrahymena thermophila contains a conventional gamma-tubulin that is differentially required for the maintenance of different microtubule-organizing centers. J. Cell Biol. 2002, 158, 1195–1206. [Google Scholar] [CrossRef]
- Akella, J.S.; Wloga, D.; Kim, J.; Starostina, N.G.; Lyons-Abbott, S.; Morrissette, N.S.; Dougan, S.T.; Kipreos, E.T.; Gaertig, J. MEC-17 is an alpha-tubulin acetyltransferase. Nature 2010, 467, 218–222. [Google Scholar] [CrossRef]
- Cubillos-Rojas, M.; Schneider, T.; Sánchez-Tena, S.; Bartrons, R.; Ventura, F.; Rosa, J.L. Tris-acetate polyacrylamide gradient gel electrophoresis for the analysis of protein oligomerization. Anal. Bioanal. Chem. 2016, 408, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Jeanmougin, F.; Thompson, J.D.; Gouy, M.; Higgins, D.G.; Gibson, T.J. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 1998, 23, 403–405. [Google Scholar] [CrossRef]
- Galtier, N.; Gouy, M.; Gautier, C. SEAVIEW and PHYLO_WIN: Two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 1996, 12, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.M.; Tegenfeldt, F.; Li, J.; Zdobnov, E.M.; Kriventseva, E.V. OrthoDB: A hierarchical catalog of animal, fungal and bacterial orthologs. Nucleic Acids Res. 2013, 41, D358–D365. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Shang, Y.; Song, X.; Bowen, J.; Corstanje, R.; Gao, Y.; Gaertig, J.; Gorovsky, M.A. A robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 2002, 99, 3734–3739. [Google Scholar] [CrossRef]
- Hartman, J.J.; Vale, R.D. Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science 1999, 286, 782–785. [Google Scholar] [CrossRef]
- Ding, Y.H.; Fan, S.B.; Li, S.; Feng, B.Y.; Gao, N.; Ye, K.; He, S.M.; Dong, M.Q. Increasing the depth of mass-spectrometry-based structural analysis of protein complexes through the use of multiple cross-linkers. Anal. Chem. 2016, 88, 4461–4469. [Google Scholar] [CrossRef]
- Shi, Y.; Fernandez-Martinez, J.; Tjioe, E.; Pellarin, R.; Kim, S.J.; Williams, R.; Schneidman-Duhovny, D.; Sali, A.; Rout, M.P.; Chait, B.T. Structural characterization by cross-linking reveals the detailed architecture of a coatomer-related heptameric module from the nuclear pore complex. Mol. Cell Proteom. 2014, 13, 2927–2943. [Google Scholar] [CrossRef]
- Mateja, A.; Cierpicki, T.; Paduch, M.; Derewenda, Z.S.; Otlewski, J. The dimerization mechanism of LIS1 and its implication for proteins containing the LisH motif. J. Mol. Biol. 2006, 357, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Romio, L.; Fry, A.M.; Winyard, P.J.; Malcolm, S.; Woolf, A.S.; Feather, S.A. OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J. Am. Soc. Nephrol. 2004, 15, 2556–2568. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Evans, K.J.; Lary, J.; Cole, J.L.; Lauring, B. Recognition of C-terminal amino acids in tubulin by pore loops in Spastin is important for microtubule severing. J. Cell Biol. 2007, 176, 995–1005. [Google Scholar] [CrossRef]
- Roll-Mecak, A.; Vale, R.D. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 2008, 451, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.F.; Geimer, S. Distribution of polyglutamylated tubulin in the flagellar apparatus of green flagellates. Cell Motil. Cytoskelet. 2000, 47, 219–235. [Google Scholar] [CrossRef]
- Kubo, T.; Yanagisawa, H.A.; Yagi, T.; Hirono, M.; Kamiya, R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr. Biol. 2010, 20, 441–445. [Google Scholar] [CrossRef]
- Suryavanshi, S.; Eddé, B.; Fox, L.A.; Guerrero, S.; Hard, R.; Hennessey, T.; Kabi, A.; Malison, D.; Pennock, D.; Sale, W.S.; et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr. Biol. 2010, 20, 435–440. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joachimiak, E.; Waclawek, E.; Niziolek, M.; Osinka, A.; Fabczak, H.; Gaertig, J.; Wloga, D. The LisH Domain-Containing N-Terminal Fragment is Important for the Localization, Dimerization, and Stability of Katnal2 in Tetrahymena. Cells 2020, 9, 292. https://doi.org/10.3390/cells9020292
Joachimiak E, Waclawek E, Niziolek M, Osinka A, Fabczak H, Gaertig J, Wloga D. The LisH Domain-Containing N-Terminal Fragment is Important for the Localization, Dimerization, and Stability of Katnal2 in Tetrahymena. Cells. 2020; 9(2):292. https://doi.org/10.3390/cells9020292
Chicago/Turabian StyleJoachimiak, Ewa, Ewa Waclawek, Michal Niziolek, Anna Osinka, Hanna Fabczak, Jacek Gaertig, and Dorota Wloga. 2020. "The LisH Domain-Containing N-Terminal Fragment is Important for the Localization, Dimerization, and Stability of Katnal2 in Tetrahymena" Cells 9, no. 2: 292. https://doi.org/10.3390/cells9020292
APA StyleJoachimiak, E., Waclawek, E., Niziolek, M., Osinka, A., Fabczak, H., Gaertig, J., & Wloga, D. (2020). The LisH Domain-Containing N-Terminal Fragment is Important for the Localization, Dimerization, and Stability of Katnal2 in Tetrahymena. Cells, 9(2), 292. https://doi.org/10.3390/cells9020292





