Impact of Truncated O-glycans in Gastric-Cancer-Associated CD44v9 Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Lectins
2.2. Cell Culture
2.3. Immunofluorescence
2.4. Flow Cytometry
2.5. RNA Isolations and Real-time (RT)-qPCR
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
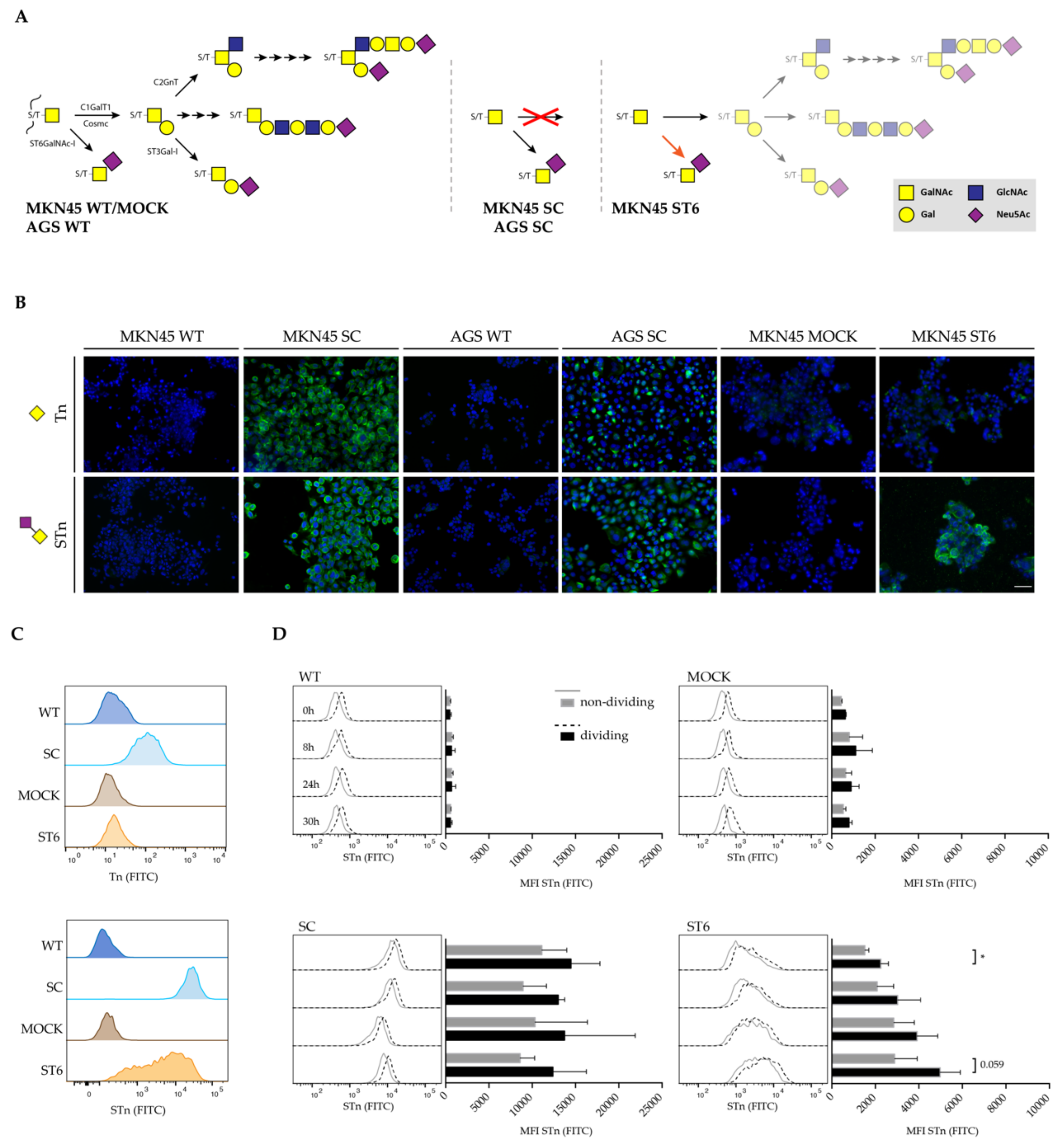
3.1. Glycoengineered Gastric Cancer Cell Lines Enable In Vitro Study of O-glycosylation Truncation
3.2. Total CD44 and CD44v9 Expression in Gastric Cancer Cell Line Models of O-glycosylation Truncation
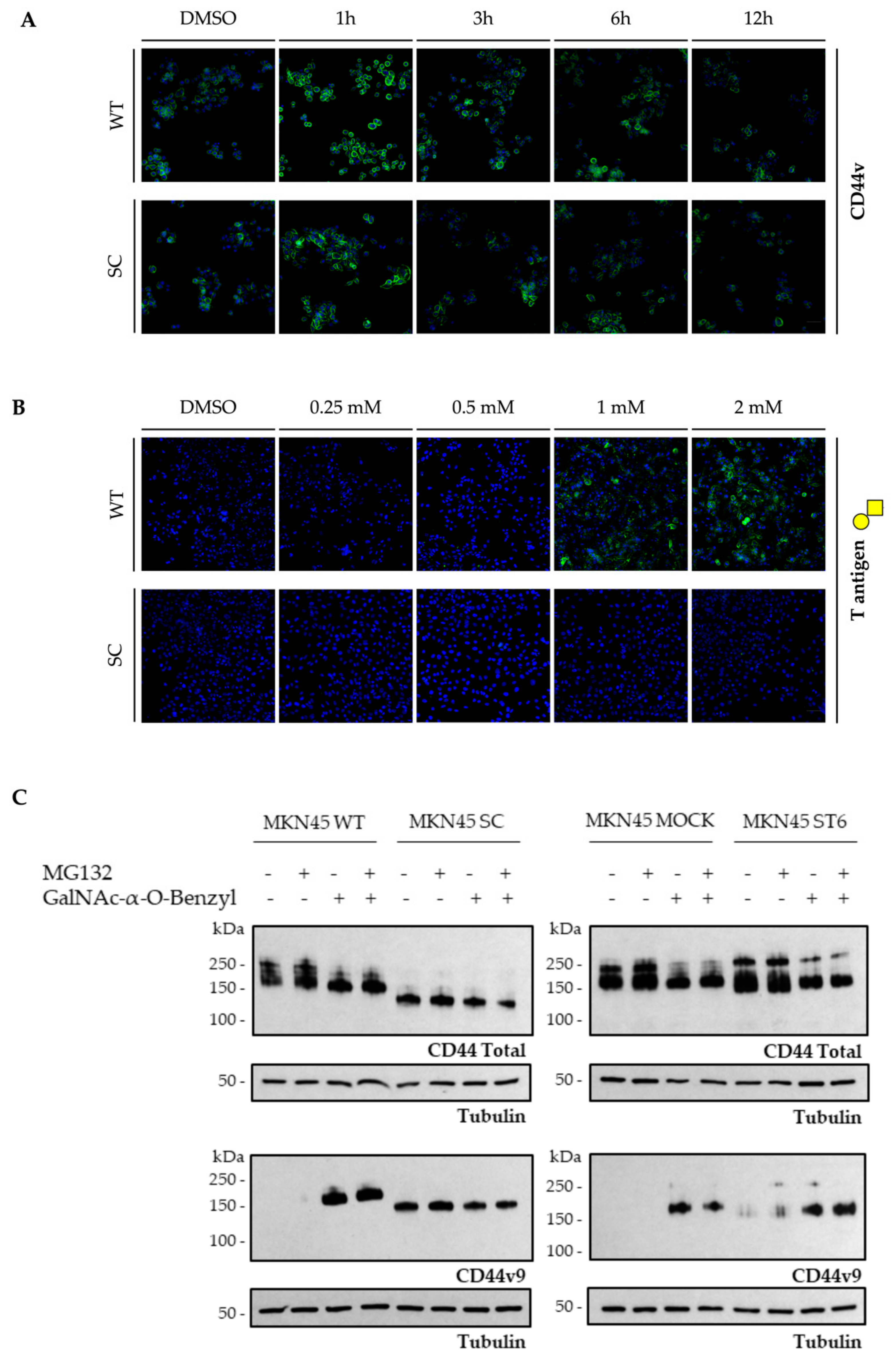
3.3. Cancer-Related O-glycosylation Truncation Enhances CD44v9 Detection by a Monoclonal Antibody in Human Gastric Cancer Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, J.; Maciejewski, R.; Polkowski, W. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef]
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Irvin, D.K.; Jouanneau, E.; Duvall, G.; Zhang, X.X.; Zhai, Y.; Sarayba, D.; Seksenyan, A.; Panwar, A.; Black, K.L.; Wheeler, C.J. T cells enhance stem-like properties and conditional malignancy in gliomas. PLoS ONE 2010, 5, e10974. [Google Scholar] [CrossRef] [PubMed]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.; Shimada, Y.; Wang, T.C. Identification of Gastric Cancer Stem Cells Using the Cell Surface Marker CD44. Stem Cells 2009, 27, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the era of cancer-targeted therapy: Where are we heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Martins, Á.M.; Gomes, C.; Balmaña, M.; Macedo, J.A.; Polom, K.; Roviello, F.; Magalhães, A.; Reis, C.A. O-glycan truncation enhances cancer-related functions of CD44 in gastric cancer. FEBS Lett. 2019, 593, 1675–1689. [Google Scholar]
- Lau, W.M.; Teng, E.; Chong, H.S.; Lopez, K.A.P.; Tay, A.Y.L.; Salto-Tellez, M.; Shabbir, A.; So, J.B.Y.; Chan, S.L. CD44v8-10 Is a Cancer-Specific Marker for Gastric Cancer Stem Cells. Cancer Res. 2014, 74, 2630–2641. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc- and Thereby Promotes Tumor Growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef]
- Tanabe, K.K.; Ellis, L.M.; Saya, H. Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet 1993, 341, 725–726. [Google Scholar] [CrossRef]
- Yamakawa, Y.; Kusuhara, M.; Terashima, M.; Kinugasa, Y.; Sugino, T.; Abe, M.; Mochizuki, T.; Hatakeyma, K.; Kami, K.; Yamaguchi, K. CD44 variant 9 expression as a predictor for gastric cancer recurrence: Immunohistochemical and metabolomic analysis of surgically resected tissues. Biomed. Res. Tokyo 2017, 38, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Kim, H.; Kim, H.P.; Choi, Y.; Goh, S.H. CD44v8-10 as a potential theranostic biomarker for targeting disseminated cancer cells in advanced gastric cancer. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Baldus, S.E.; Hanisch, F.G. Biochemistry and pathological importance of mucin-associated antigens in gastrointestinal neoplasia. Adv. Cancer Res. 2000, 79, 201–248. [Google Scholar]
- David, L.; Nesland, J.M.; Clausen, H.; Carneiro, F.; Sobrinho-Simoes, M. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS Suppl. 1992, 27, 162–172. [Google Scholar]
- Victorzon, M.; Nordling, S.; Nilsson, O.; Roberts, P.J.; Haglund, C. Sialyl Tn antigen is an independent predictor of outcome in patients with gastric cancer. Int. J. Cancer 1996, 65, 295–300. [Google Scholar] [CrossRef]
- De Oliveira, F.M.S.; Mereiter, S.; Lönn, P.; Siart, B.; Shen, Q.; Heldin, J.; Raykova, D.; Karlsson, N.G.; Polom, K.; Roviello, F.; et al. Detection of post-translational modifications using solid-phase proximity ligation assay. New Biotechnol. 2018, 45, 51–59. [Google Scholar] [CrossRef]
- Nagano, O.; Saya, H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004, 95, 930–935. [Google Scholar] [CrossRef]
- Campos, D.; Freitas, D.; Gomes, J.; Magalhaes, A.; Steentoft, C.; Gomes, C.; Vester-Christensen, M.B.; Ferreira, J.A.; Afonso, L.P.; Santos, L.L.; et al. Probing the O-glycoproteome of gastric cancer cell lines for biomarker discovery. Mol. Cell Proteom. 2015, 14, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Colcher, D.; Hand, P.H.; Nuti, M.; Schlom, J.A. Spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3199–3203. [Google Scholar] [CrossRef] [PubMed]
- Clausen, H.; Stroud, M.; Parker, J.; Springer, G.; Hakomori, S. Monoclonal antibodies directed to the blood group A associated structure, galactosyl-a: Specificity and relation to the thomsen-friedenreich antigen. Mol. Immunol. 1988, 25, 199–204. [Google Scholar] [CrossRef]
- Lauren, P. The Two Histololgica Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Steentoft, C.; Vakhrushev, S.Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Kong, Y.; Bennett, E.P.; Mandel, U.; Wandall, H.; Levery, S.B.; Clausen, H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 2011, 8, 977–982. [Google Scholar] [CrossRef]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 64, 7050–7057. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Harduin-Lepers, A.; Magalhães, A.; Machado, E.; Mendes, N.; Costa, L.T.; Matthiesen, R.; Almeida, R.; Costa, J.; Reis, C.A. Differential expression of alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42, 80–89. [Google Scholar] [CrossRef]
- Marcos, N.T.; Bennett, E.P.; Gomes, J.; Magalhães, A.; Gomes, C.; David, L.; Dar, I.; Jeanneau, C.; DeFrees, S.; Krustrup, D.; et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastroinstestinal tissues. Front. Biosci. 2011, 3, 1443–1455. [Google Scholar]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.A.; Matos, R.; Mereiter, S.; Pinto, M.T.; Polónia, A.; Gartner, F.; et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine 2019, 40, 349–362. [Google Scholar] [CrossRef]
- Sin, A.T.W.; Harrison, R.E. Growth of the Mammalian Golgi Apparatus during Interphase. Mol. Cell. Biol. 2016, 36, 2344–2359. [Google Scholar] [CrossRef]
- Da Cunha, C.B.; Oliveira, C.; Wen, X.; Gomes, B.; Sousa, S.; Suriano, G.; Grellir, M.; Huntsman, D.G.; Carneiro, F.; Granja, P.L.; et al. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab. Investig. 2010, 90, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Jauch, K.W.; Gunthert, U.; Fidgor, C.G.; Schildberg, F.W.; Funke, I.; Johnson, J.P. De-novo expression of CD44 and survival in gastric cancer. Lancet 1993, 342, 1019–1022. [Google Scholar] [CrossRef]
- Jayaprakash, N.G.; Surolia, A. Role of glycosylation in nucleating protein folding and stability. Biochem. J. 2017, 474, 2333–2347. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.H.; Chan, L.C.; Kuo, C.-W.; Khoo, K.H.; Chang, S.S.; Cha, J.H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tijink, B.; Buter, J.; de Bree, R.; Giabbone, G.; Lang, M.; Staab, A.; Leemans, C.; van Dongen, G. A phase I dose escalation study with anti-CD44v6 Bivatuzumab Mertansine in patients with incurable squamous cell carcinoma of head and neck or esophagus. Clin. Cancer Res. 2006, 12, 6064–6072. [Google Scholar] [CrossRef]
- Teye, K.; Numata, S.; Ishii, N.; Krol, R.P.; Tsuchisaka, A.; Hamada, T.; Koga, H.; Karashima, T.; Ohata, C.; Tsuruta, D.; et al. Isolation of all CD44 transcripts in human epidermis and regulation of their expression by various agents. PLoS ONE 2016, 11, e0160952. [Google Scholar] [CrossRef]
- Menke-van der Houven van Oordt, C.W.; Gomez-Roca, C.; van Herpen, C.; Coveler, A.L.; Mahalingam, D.; Verheul, H.M.; van der Graaf, W.T.; Christen, R.; Rüttinger, D.; Weigand, S.; et al. First-in-human phase I clinical trial of RG7356, an anti-CD44 humanized antibody, in patients with advanced, CD44-expressing solid tumors. Oncotarget 2016, 7, 80046–80058. [Google Scholar]
- Baaten, B.J.; Li, C.R.; Deiro, M.F.; Lin, M.M.; Linton, P.J.; Bradley, L.M. CD44 regulates survival and memory development in Th1 cells. Immunity 2010, 32, 104–115. [Google Scholar] [CrossRef]
- Reis, C.A.; Sørensen, T.; Mandel, U.; David, L.; Mirgorodskaya, E.; Roepstorff, P.; Kihlberg, J.; Hansen, J.E.; Clausen, H. Development and characterization of na antibody directed to na alfa-N-acetyl-D-galactosamine glycosylated MUC2 peptide. Glycoconjug. J. 1998, 15, 51–62. [Google Scholar] [CrossRef]
- Sørensen, T.; Reis, C.A.; Mandel, U.; Ramachandran, K.; Sankaranarayanan, V.; Schwientek, T.; Graham, R.; Taylor-Papadimitriou, J.; Hollingsworth, M.A.; Burchell, J.; et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptide elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology 2006, 16, 96–107. [Google Scholar] [CrossRef]
- Posey, A.D., Jr.; Clausen, H.; June, C.H. Distinguishing Truncated and Normal MUC1 Glycoform Targeting from Tn-MUC1-Specific CAR T Cells: Specificity Is the Key to Safety. Immunity 2016, 45, 947–948. [Google Scholar] [CrossRef] [PubMed]
- Posey, A.D.; Schwab, R.D.; Boesteanu, A.C.; Steentoft, C.; Mandel, U.; Engels, B.; Stone, J.D.; Madsen, T.D.; Schreiber, K.; Haines, K.M.; et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016, 44, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, W.L.; Kocman, I.; Agrawal, V.; Rahn, H.P.; Besser, D.; Gossen, M. Homogeneity and persistence of transgene expression by omitting antibiotic selection in cell line isolation. Nucleic Acids Res. 2008, 36, e111. [Google Scholar] [CrossRef] [PubMed]
- Nanbu, T.; Umemura, N.; Ohkoshi, E.; Nanbu, K.; Sakagami, H.; Shimada, J. Combined SN-38 and gefitinib treatment promotes CD44 degradation in head and neck squamous cell carcinoma cells. Oncol. Rep. 2018, 39, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Spadiut, O.; Capone, S.; Krainer, F.; Glieder, A.; Herwig, C. Microbials for the production of monoclonal antibodies and antibody fragments. Trends Biotechnol. 2014, 32, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kagami, T.; Yamade, M.; Suzuki, T.; Uotani, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; Sugimoto, K.; Baba, S.; et al. High expression level of CD44v8-10 in cancer stem-like cells is associated with poor prognosis in esophageal squamous cell carcinoma patients treated with chemoradiotherapy. Oncotarget 2018, 9, 34876–34888. [Google Scholar] [CrossRef]
- Kodama, H.; Murata, S.; Ishida, M.; Yamamoto, H.; Yamaguchi, T.; Kaida, S.; Miyake, T.; Takebayashi, K.; Kushima, R.; Tani, M. Prognostic impact of CD44-positive cancer stem-like cells at the invasive front of gastric cancer. Br. J. Cancer 2016, 116, 186–194. [Google Scholar] [CrossRef]
- Lee, H.H.; Wang, Y.N.; Xia, W.; Chen, C.H.; Rau, K.M.; Ye, L.; Wei, Y.; Chou, C.K.; Wang, S.C.; Yan, M.; et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019, 36, 168–178. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, I.B.; Pinto, F.; Gomes, C.; Campos, D.; Reis, C.A. Impact of Truncated O-glycans in Gastric-Cancer-Associated CD44v9 Detection. Cells 2020, 9, 264. https://doi.org/10.3390/cells9020264
Moreira IB, Pinto F, Gomes C, Campos D, Reis CA. Impact of Truncated O-glycans in Gastric-Cancer-Associated CD44v9 Detection. Cells. 2020; 9(2):264. https://doi.org/10.3390/cells9020264
Chicago/Turabian StyleMoreira, Inês B., Filipe Pinto, Catarina Gomes, Diana Campos, and Celso A. Reis. 2020. "Impact of Truncated O-glycans in Gastric-Cancer-Associated CD44v9 Detection" Cells 9, no. 2: 264. https://doi.org/10.3390/cells9020264
APA StyleMoreira, I. B., Pinto, F., Gomes, C., Campos, D., & Reis, C. A. (2020). Impact of Truncated O-glycans in Gastric-Cancer-Associated CD44v9 Detection. Cells, 9(2), 264. https://doi.org/10.3390/cells9020264





