Fibronectin and Its Receptors in Hematopoiesis
Abstract
1. Introduction
2. The Rationale for Evaluating Fibronectin and Its Receptors
3. Fibronectin Receptors Relevant for Hematopoiesis
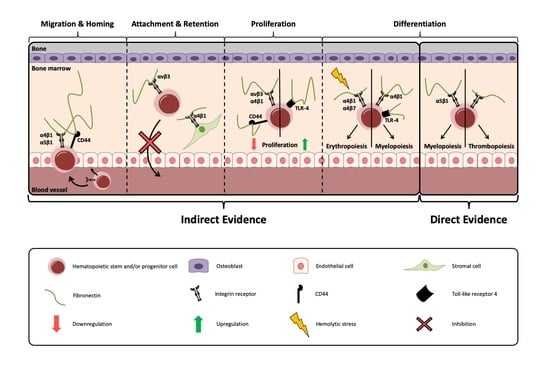
4. Steps of Hematopoiesis
5. Fibronectin and Fibronectin Receptors in Hematopoietic Stem Cells
5.1. Evidence for A Role of Integrins in Hematopoiesis
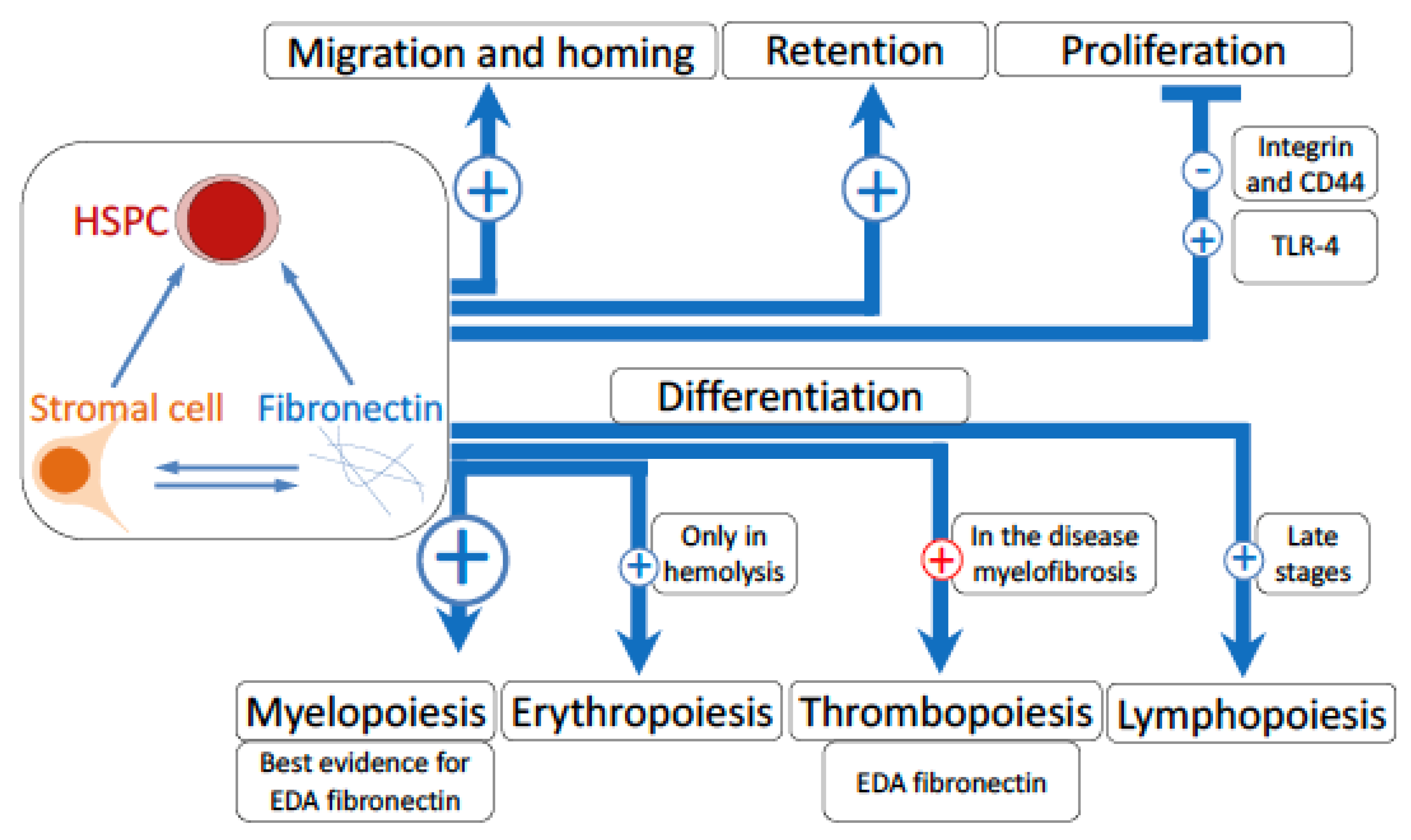
5.2. Migration and Homing
5.3. Attachment and Retention
5.4. Proliferation
5.5. Differentiation
5.5.1. Myelopoiesis
5.5.2. Erythropoiesis
5.5.3. Thrombopoiesis
5.5.4. Lymphopoiesis
6. Fibronectin and Malignancy
7. Discussion and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef]
- Szade, K.; Gulati, G.S.; Chan, C.K.F.; Kao, K.S.; Miyanishi, M.; Marjon, K.D.; Sinha, R.; George, B.M.; Chen, J.Y.; Weissman, I.L. Where Hematopoietic Stem Cells Live: The Bone Marrow Niche. Antioxid. Redox Signal. 2018, 29, 191–204. [Google Scholar] [CrossRef]
- Ding, L.; Morrison, S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013, 495, 231–235. [Google Scholar] [CrossRef]
- Dallas, S.L.; Sivakumar, P.; Jones, C.J.; Chen, Q.; Peters, D.M.; Mosher, D.F.; Humphries, M.J.; Kielty, C.M. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 2005, 280, 18871–18880. [Google Scholar] [CrossRef]
- Granito, R.N.; Bouleftour, W.; Sabido, O.; Lescale, C.; Thomas, M.; Aubin, J.E.; Goodhardt, M.; Vico, L.; Malaval, L. Absence of bone sialoprotein (BSP) alters profoundly hematopoiesis and upregulates osteopontin. J. Cell. Physiol. 2015, 230, 1342–1351. [Google Scholar] [CrossRef]
- Cao, H.; Cao, B.; Heazlewood, C.K.; Domingues, M.; Sun, X.; Debele, E.; McGregor, N.E.; Sims, N.A.; Heazlewood, S.Y.; Nilsson, S.K. Osteopontin is an important regulative component of the fetal bone marrow hematopoietic stem cell niche. Cells 2019, 8, 985. [Google Scholar] [CrossRef]
- Siewe, B.T.; Kalis, S.L.; Le, P.T.; Witte, P.L.; Choi, S.; Conway, S.J.; Druschitz, L.; Knight, K.L. In vitro requirement for periostin in B lymphopoiesis. Blood 2011, 117, 3770–3779. [Google Scholar] [CrossRef][Green Version]
- Turner, C.J.; Badu-Nkansah, K.; Hynes, R.O. Endothelium-derived fibronectin regulates neonatal vascular morphogenesis in an autocrine fashion. Angiogenesis 2017, 20, 519–531. [Google Scholar] [CrossRef]
- Von Au, A.; Vasel, M.; Kraft, S.; Sens, C.; Hackl, N.; Marx, A.; Stroebel, P.; Hennenlotter, J.; Todenhofer, T.; Stenzl, A.; et al. Circulating fibronectin controls tumor growth. Neoplasia 2013, 15, 925–938. [Google Scholar] [CrossRef]
- Zobel, K.; Hansen, U.; Galla, H.J. Blood-brain barrier properties in vitro depend on composition and assembly of endogenous extracellular matrices. Cell Tissue Res. 2016, 365, 233–245. [Google Scholar] [CrossRef]
- Bentmann, A.; Kawelke, N.; Moss, D.; Zentgraf, H.; Bala, Y.; Berger, I.; Gasser, J.A.; Nakchbandi, I.A. Circulating fibronectin affects bone matrix, whereas osteoblast fibronectin modulates osteoblast function. J. Bone Min. Res. 2010, 25, 706–715. [Google Scholar]
- Sens, C.; Huck, K.; Pettera, S.; Uebel, S.; Wabnitz, G.; Moser, M.; Nakchbandi, I.A. Fibronectins containing extradomain A or B enhance osteoblast differentiation via distinct integrins. J. Biol. Chem. 2017, 292, 7745–7760. [Google Scholar] [CrossRef]
- Rossnagl, S.; Ghura, H.; Groth, C.; Altrock, E.; Jakob, F.; Schott, S.; Wimberger, P.; Link, T.; Kuhlmann, J.D.; Stenzl, A.; et al. A subpopulation of stromal cells controls cancer cell homing to the bone marrow. Cancer Res. 2018, 78, 129–142. [Google Scholar] [CrossRef]
- Kawelke, N.; Bentmann, A.; Hackl, N.; Hager, H.D.; Feick, P.; Geursen, A.; Singer, M.V.; Nakchbandi, I.A. Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J. Bone Min. Res. 2008, 23, 1278–1286. [Google Scholar] [CrossRef]
- Kawelke, N.; Vasel, M.; Sens, C.; von Au, A.; Dooley, S.; Nakchbandi, I.A. Fibronectin protects from excessive liver fibrosis by modulating the availability of and responsiveness of stellate cells to active TGF-beta. PLoS ONE 2011, 6, e28181. [Google Scholar] [CrossRef]
- Leiss, M.; Beckmann, K.; Giros, A.; Costell, M.; Fassler, R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr. Opin. Cell Biol. 2008, 20, 502–507. [Google Scholar] [CrossRef]
- Sens, C.; Altrock, E.; Rau, K.; Klemis, V.; von Au, A.; Pettera, S.; Uebel, S.; Damm, T.; Tiwari, S.; Moser, M.; et al. An O-glycosylation of fibronectin mediates hepatic osteodystrophy through alpha4beta1 integrin. J. Bone Min. Res. 2017, 32, 70–81. [Google Scholar] [CrossRef]
- George, E.L.; Georges-Labouesse, E.N.; Patel-King, R.S.; Rayburn, H.; Hynes, R.O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993, 119, 1079–1091. [Google Scholar]
- Rossnagl, S.; Altrock, E.; Sens, C.; Kraft, S.; Rau, K.; Milsom, M.D.; Giese, T.; Samstag, Y.; Nakchbandi, I.A. EDA-fibronectin originating from osteoblasts inhibits the immune response against cancer. PLoS Biol. 2016, 14, e1002562. [Google Scholar]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef]
- Wierenga, P.K.; Weersing, E.; Dontje, B.; de Haan, G.; van Os, R. Differential role for very late antigen-5 in mobilization and homing of hematopoietic stem cells. Bone Marrow Transpl. 2006, 38, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Grassinger, J.; Haylock, D.N.; Storan, M.J.; Haines, G.O.; Williams, B.; Whitty, G.A.; Vinson, A.R.; Be, C.L.; Li, S.; Sorensen, E.S.; et al. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood 2009, 114, 49–59. [Google Scholar] [CrossRef]
- Bungartz, G.; Stiller, S.; Bauer, M.; Muller, W.; Schippers, A.; Wagner, N.; Fassler, R.; Brakebusch, C. Adult murine hematopoiesis can proceed without beta1 and beta7 integrins. Blood 2006, 108, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T.D.; Steinl, C.; Essl, M.; Abele, H.; Geiger, K.; Muller, C.A.; Aicher, W.K.; Klein, G. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: Involvement in cell adhesion, proliferation and differentiation. Haematologica 2009, 94, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Yamato, M.; Ishihara, J.; Shiratsuchi, Y.; Utsumi, M.; Morita, Y.; Tsukui, H.; Terasawa, M.; Shibata, T.; Nishida, K.; et al. Integrin-alphavbeta3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood 2012, 119, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Garrett, K.P.; Ohta, S.; Bahrun, U.; Kouro, T.; Akira, S.; Takatsu, K.; Kincade, P.W. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 2006, 24, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Esplin, B.L.; Shimazu, T.; Welner, R.S.; Garrett, K.P.; Nie, L.; Zhang, Q.; Humphrey, M.B.; Yang, Q.; Borghesi, L.A.; Kincade, P.W. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J. Immunol. 2011, 186, 5367–5375. [Google Scholar] [CrossRef]
- Cao, H.; Heazlewood, S.Y.; Williams, B.; Cardozo, D.; Nigro, J.; Oteiza, A.; Nilsson, S.K. The role of CD44 in fetal and adult hematopoietic stem cell regulation. Haematologica 2016, 101, 26–37. [Google Scholar] [CrossRef]
- Manabe, R.; Ohe, N.; Maeda, T.; Fukuda, T.; Sekiguchi, K. Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J. Cell Biol. 1997, 139, 295–307. [Google Scholar] [CrossRef]
- Liao, Y.F.; Gotwals, P.J.; Koteliansky, V.E.; Sheppard, D.; Van De Water, L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 2002, 277, 14467–14474. [Google Scholar] [CrossRef]
- Shinde, A.V.; Bystroff, C.; Wang, C.; Vogelezang, M.G.; Vincent, P.A.; Hynes, R.O.; Van De Water, L. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J. Biol. Chem. 2008, 283, 2858–2870. [Google Scholar] [CrossRef] [PubMed]
- Kraft, S.; Klemis, V.; Sens, C.; Lenhard, T.; Jacobi, C.; Samstag, Y.; Wabnitz, G.; Kirschfink, M.; Wallich, R.; Hansch, G.M.; et al. Identification and characterization of a unique role for EDB fibronectin in phagocytosis. J. Mol. Med. 2016, 94, 567–581. [Google Scholar] [CrossRef]
- Adair, B.D.; Xiong, J.P.; Maddock, C.; Goodman, S.L.; Arnaout, M.A.; Yeager, M. Three-dimensional EM structure of the ectodomain of integrin {alpha}V{beta}3 in a complex with fibronectin. J. Cell Biol. 2005, 168, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Curnis, F.; Longhi, R.; Crippa, L.; Cattaneo, A.; Dondossola, E.; Bachi, A.; Corti, A. Spontaneous formation of L-isoaspartate and gain of function in fibronectin. J. Biol. Chem. 2006, 281, 36466–36476. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Watari, M.; Jerud, E.S.; Young, D.W.; Ishizaka, S.T.; Rose, J.; Chow, J.C.; Strauss, J.F., 3rd. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001, 276, 10229–10233. [Google Scholar] [CrossRef]
- You, R.; Zheng, M.; McKeown-Longo, P.J. The first type III repeat in fibronectin activates an inflammatory pathway in dermal fibroblasts. J. Biol. Chem. 2010, 285, 36255–36259. [Google Scholar] [CrossRef] [PubMed]
- Hackl, N.J.; Bersch, C.; Feick, P.; Antoni, C.; Franke, A.; Singer, M.V.; Nakchbandi, I.A. Circulating fibronectin isoforms predict the degree of fibrosis in chronic hepatitis C. Scand. J. Gastroenterol. 2010, 45, 349–356. [Google Scholar] [CrossRef]
- Sackstein, R. The biology of CD44 and HCELL in hematopoiesis: The ’step 2-bypass pathway’ and other emerging perspectives. Curr. Opin. Hematol. 2011, 18, 239–248. [Google Scholar] [CrossRef]
- Verfaillie, C.M.; Benis, A.; Iida, J.; McGlave, P.B.; McCarthy, J.B. Adhesion of committed human hematopoietic progenitors to synthetic peptides from the C-terminal heparin-binding domain of fibronectin: Cooperation between the integrin alpha 4 beta 1 and the CD44 adhesion receptor. Blood 1994, 84, 1802–1811. [Google Scholar] [CrossRef]
- Tumova, S.; Woods, A.; Couchman, J.R. Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences. J. Biol. Chem. 2000, 275, 9410–9417. [Google Scholar] [CrossRef]
- Morgan, M.R.; Hamidi, H.; Bass, M.D.; Warwood, S.; Ballestrem, C.; Humphries, M.J. Syndecan-4 phosphorylation is a control point for integrin recycling. Dev. Cell 2013, 24, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Kadomatsu, K.; Kojima, T.; Muramatsu, H.; Iwase, M.; Yoshikai, Y.; Yanada, M.; Yamamoto, K.; Matsushita, T.; Nishimura, M.; et al. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J. Biol. Chem. 2001, 276, 47483–47488. [Google Scholar] [CrossRef] [PubMed]
- Kaneider, N.C.; Reinisch, C.M.; Dunzendorfer, S.; Romisch, J.; Wiedermann, C.J. Syndecan-4 mediates antithrombin-induced chemotaxis of human peripheral blood lymphocytes and monocytes. J. Cell Sci. 2002, 115, 227–236. [Google Scholar]
- Weissman, I.L. Developmental switches in the immune system. Cell 1994, 76, 207–218. [Google Scholar] [CrossRef]
- Bryder, D.; Rossi, D.J.; Weissman, I.L. Hematopoietic stem cells: The paradigmatic tissue-specific stem cell. Am. J. Pathol. 2006, 169, 338–346. [Google Scholar] [CrossRef]
- Ruppert, R.; Moser, M.; Sperandio, M.; Rognoni, E.; Orban, M.; Liu, W.H.; Schulz, A.S.; Oostendorp, R.A.; Massberg, S.; Fassler, R. Kindlin-3-mediated integrin adhesion is dispensable for quiescent but essential for activated hematopoietic stem cells. J. Exp. Med. 2015, 212, 1415–1432. [Google Scholar] [CrossRef]
- Ma, Q.; Jones, D.; Springer, T.A. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity 1999, 10, 463–471. [Google Scholar] [CrossRef]
- Broxmeyer, H.E. Regulation of hematopoiesis by chemokine family members. Int. J. Hematol. 2001, 74, 9–17. [Google Scholar] [CrossRef]
- Imai, K.; Kobayashi, M.; Wang, J.; Ohiro, Y.; Hamada, J.; Cho, Y.; Imamura, M.; Musashi, M.; Kondo, T.; Hosokawa, M.; et al. Selective transendothelial migration of hematopoietic progenitor cells: A role in homing of progenitor cells. Blood 1999, 93, 149–156. [Google Scholar]
- Sagar, B.M.; Rentala, S.; Gopal, P.N.; Sharma, S.; Mukhopadhyay, A. Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells. Biochem. Biophys. Res. Commun. 2006, 350, 1000–1005. [Google Scholar] [CrossRef]
- Potocnik, A.J.; Brakebusch, C.; Fassler, R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 2000, 12, 653–663. [Google Scholar] [CrossRef]
- Arroyo, A.G.; Yang, J.T.; Rayburn, H.; Hynes, R.O. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity 1999, 11, 555–566. [Google Scholar] [CrossRef]
- Papayannopoulou, T.; Craddock, C.; Nakamoto, B.; Priestley, G.V.; Wolf, N.S. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc. Natl. Acad. Sci. USA 1995, 92, 9647–9651. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Hidalgo, A.; Peired, A.; Frenette, P.S. Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood 2004, 104, 2020–2026. [Google Scholar] [CrossRef]
- Scott, L.M.; Priestley, G.V.; Papayannopoulou, T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol. Cell Biol. 2003, 23, 9349–9360. [Google Scholar] [CrossRef]
- Sackstein, R.; Merzaban, J.S.; Cain, D.W.; Dagia, N.M.; Spencer, J.A.; Lin, C.P.; Wohlgemuth, R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008, 14, 181–187. [Google Scholar] [CrossRef]
- Cancelas, J.A. Adhesion, migration, and homing of murine hematopoietic stem cells and progenitors. Methods Mol. Biol. 2011, 750, 187–196. [Google Scholar]
- Papayannopoulou, T.; Nakamoto, B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc. Natl. Acad. Sci. USA 1993, 90, 9374–9378. [Google Scholar] [CrossRef]
- Khurana, S.; Schouteden, S.; Manesia, J.K.; Santamaria-Martinez, A.; Huelsken, J.; Lacy-Hulbert, A.; Verfaillie, C.M. Outside-in integrin signalling regulates haematopoietic stem cell function via Periostin-Itgav axis. Nat. Commun. 2016, 7, 13500. [Google Scholar]
- Liu, A.; Wang, Y.; Ding, Y.; Baez, I.; Payne, K.J.; Borghesi, L. Cutting edge: Hematopoietic stem cell expansion and common lymphoid progenitor depletion require hematopoietic-derived, cell-autonomous TLR4 in a model of chronic endotoxin. J. Immunol. 2015, 195, 2524–2528. [Google Scholar] [CrossRef]
- Dao, M.A.; Nolta, J.A. Cytokine and integrin stimulation synergize to promote higher levels of GATA-2, c-myb, and CD34 protein in primary human hematopoietic progenitors from bone marrow. Blood 2007, 109, 2373–2379. [Google Scholar] [CrossRef][Green Version]
- Abbuehl, J.P.; Tatarova, Z.; Held, W.; Huelsken, J. Long-Term Engraftment of Primary Bone Marrow Stromal Cells Repairs Niche Damage and Improves Hematopoietic Stem Cell Transplantation. Cell Stem Cell 2017, 21, 241–255.e6. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.A.; Hashino, K.; Kato, I.; Nolta, J.A. Adhesion to fibronectin maintains regenerative capacity during ex vivo culture and transduction of human hematopoietic stem and progenitor cells. Blood 1998, 92, 4612–4621. [Google Scholar] [CrossRef] [PubMed]
- Brakebusch, C.; Fillatreau, S.; Potocnik, A.J.; Bungartz, G.; Wilhelm, P.; Svensson, M.; Kearney, P.; Korner, H.; Gray, D.; Fassler, R. Beta1 integrin is not essential for hematopoiesis but is necessary for the T cell-dependent IgM antibody response. Immunity 2002, 16, 465–477. [Google Scholar] [PubMed]
- Ziegler, P.; Boettcher, S.; Takizawa, H.; Manz, M.G.; Brummendorf, T.H. LPS-stimulated human bone marrow stroma cells support myeloid cell development and progenitor cell maintenance. Ann. Hematol. 2016, 95, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, S.; Ziegler, P.; Schmid, M.A.; Takizawa, H.; van Rooijen, N.; Kopf, M.; Heikenwalder, M.; Manz, M.G. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing nonhematopoietic cells. J. Immunol. 2012, 188, 5824–5828. [Google Scholar] [CrossRef]
- Boettcher, S.; Gerosa, R.C.; Radpour, R.; Bauer, J.; Ampenberger, F.; Heikenwalder, M.; Kopf, M.; Manz, M.G. Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. Blood 2014, 124, 1393–1403. [Google Scholar] [CrossRef]
- Schuettpelz, L.G.; Borgerding, J.N.; Christopher, M.J.; Gopalan, P.K.; Romine, M.P.; Herman, A.C.; Woloszynek, J.R.; Greenbaum, A.M.; Link, D.C. G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling. Leukemia 2014, 28, 1851–1860. [Google Scholar] [CrossRef]
- Malara, A.; Gruppi, C.; Abbonante, V.; Cattaneo, D.; De Marco, L.; Massa, M.; Iurlo, A.; Gianelli, U.; Balduini, C.L.; Tira, M.E.; et al. EDA fibronectin-TLR4 axis sustains megakaryocyte expansion and inflammation in bone marrow fibrosis. J. Exp. Med. 2019, 216, 587–604. [Google Scholar] [CrossRef]
- Pietras, E.M.; Mirantes-Barbeito, C.; Fong, S.; Loeffler, D.; Kovtonyuk, L.V.; Zhang, S.; Lakshminarasimhan, R.; Chin, C.P.; Techner, J.M.; Will, B.; et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 2016, 18, 607–618. [Google Scholar] [CrossRef]
- Bhatia, R.; Munthe, H.A.; Verfaillie, C.M. Role of abnormal integrin-cytoskeletal interactions in impaired beta1 integrin function in chronic myelogenous leukemia hematopoietic progenitors. Exp. Hematol. 1999, 27, 1384–1396. [Google Scholar] [CrossRef]
- Arroyo, A.G.; Taverna, D.; Whittaker, C.A.; Strauch, U.G.; Bader, B.L.; Rayburn, H.; Crowley, D.; Parker, C.M.; Hynes, R.O. In vivo roles of integrins during leukocyte development and traffic: Insights from the analysis of mice chimeric for alpha 5, alpha v, and alpha 4 integrins. J. Immunol. 2000, 165, 4667–4675. [Google Scholar] [CrossRef]
- Zhao, J.L.; Ma, C.; O’Connell, R.M.; Mehta, A.; DiLoreto, R.; Heath, J.R.; Baltimore, D. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell 2014, 14, 445–459. [Google Scholar] [CrossRef]
- Hernandez, G.; Mills, T.S.; Rabe, J.L.; Chavez, J.S.; Kuldanek, S.; Kirkpatrick, G.; Noetzli, L.; Jubair, W.K.; Zanche, M.; Myers, J.R.; et al. Pro-inflammatory cytokine blockade attenuates myeloid expansion in a murine model of rheumatoid arthritis. Haematologica 2020, 105, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.; Cooper, R.; Zhang, L.; Williams, D.A. Cross-talk between alpha(4)beta(1)/alpha(5)beta(1) and c-Kit results in opposing effect on growth and survival of hematopoietic cells via the activation of focal adhesion kinase, mitogen-activated protein kinase, and Akt signaling pathways. Blood 2001, 97, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Vogelezang, M.G.; Hynes, R.O.; Griffith, L.G.; Lodish, H.F. Alpha4beta1 integrin and erythropoietin mediate temporally distinct steps in erythropoiesis: Integrins in red cell development. J. Cell Biol. 2007, 177, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Ulyanova, T.; Padilla, S.M.; Papayannopoulou, T. Stage-specific functional roles of integrins in murine erythropoiesis. Exp. Hematol. 2014, 42, 404–409. [Google Scholar] [CrossRef]
- Matsuura, S.; Thompson, C.R.; Ng, S.K.; Ward, C.M.; Karagianni, A.; Mazzeo, C.; Malara, A.; Balduini, A.; Ravid, K. Adhesion to fibronectin via alpha5beta1 integrin supports expansion of the megakaryocyte lineage in primary myelofibrosis. Blood 2020, 135, 2286–2291. [Google Scholar] [CrossRef]
- Fogelgren, B.; Polgar, N.; Szauter, K.M.; Ujfaludi, Z.; Laczko, R.; Fong, K.S.; Csiszar, K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J. Biol. Chem. 2005, 280, 24690–24697. [Google Scholar]
- Zahr, A.A.; Salama, M.E.; Carreau, N.; Tremblay, D.; Verstovsek, S.; Mesa, R.; Hoffman, R.; Mascarenhas, J. Bone marrow fibrosis in myelofibrosis: Pathogenesis, prognosis and targeted strategies. Haematologica 2016, 101, 660–671. [Google Scholar] [CrossRef]
- Kimura, A.; Katoh, O.; Hyodo, H.; Kuramoto, A. Transforming growth factor-beta regulates growth as well as collagen and fibronectin synthesis of human marrow fibroblasts. Br. J. Haematol. 1989, 72, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K. The molecular mechanisms that control thrombopoiesis. J. Clin. Investig. 2005, 115, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.G.; Yang, J.T.; Rayburn, H.; Hynes, R.O. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell 1996, 85, 997–1008. [Google Scholar] [CrossRef]
- Welner, R.S.; Pelayo, R.; Nagai, Y.; Garrett, K.P.; Wuest, T.R.; Carr, D.J.; Borghesi, L.A.; Farrar, M.A.; Kincade, P.W. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood 2008, 112, 3753–3761. [Google Scholar] [CrossRef]
- Saito, Y.; Owaki, T.; Matsunaga, T.; Saze, M.; Miura, S.; Maeda, M.; Eguchi, M.; Tanaka, R.; Taira, J.; Kodama, H.; et al. Apoptotic death of hematopoietic tumor cells through potentiated and sustained adhesion to fibronectin via VLA-4. J. Biol. Chem. 2010, 285, 7006–7015. [Google Scholar] [CrossRef]
- Fox, N.E.; Kaushansky, K. Engagement of integrin alpha4beta1 enhances thrombopoietin-induced megakaryopoiesis. Exp. Hematol. 2005, 33, 94–99. [Google Scholar] [CrossRef]
- Tefferi, A. Primary myelofibrosis: 2019 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2018, 93, 1551–1560. [Google Scholar] [CrossRef]
- Manshouri, T.; Verstovsek, S.; Harris, D.M.; Veletic, I.; Zhang, X.; Post, S.M.; Bueso-Ramos, C.E.; Estrov, Z. Primary myelofibrosis marrow-derived CD14+/CD34− monocytes induce myelofibrosis-like phenotype in immunodeficient mice and give rise to megakaryocytes. PLoS ONE 2019, 14, e0222912. [Google Scholar] [CrossRef]
- Schneider, R.K.; Ziegler, S.; Leisten, I.; Ferreira, M.S.; Schumacher, A.; Rath, B.; Fahrenkamp, D.; Muller-Newen, G.; Crysandt, M.; Wilop, S.; et al. Activated fibronectin-secretory phenotype of mesenchymal stromal cells in pre-fibrotic myeloproliferative neoplasms. J. Hematol. Oncol. 2014, 7, 92. [Google Scholar] [CrossRef]
- Lim, K.H.; Staudt, L.M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar] [CrossRef]
- Starczynowski, D.T.; Kuchenbauer, F.; Wegrzyn, J.; Rouhi, A.; Petriv, O.; Hansen, C.L.; Humphries, R.K.; Karsan, A. MicroRNA-146a disrupts hematopoietic differentiation and survival. Exp. Hematol. 2011, 39, 167–178.e4. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Sun, T.; Yu, X.H.; Yang, Y.X.; Yeo, A.E. The effects of TLR activation on T-cell development and differentiation. Clin. Dev. Immunol. 2012, 2012, 836485. [Google Scholar] [CrossRef] [PubMed]
- San Roman, B.; De Andres, X.; Munoz, P.M.; Obregon, P.; Asensio, A.C.; Garrido, V.; Mansilla, C.; Arribillaga, L.; Lasarte, J.J.; De Andres, D.; et al. The extradomain A of fibronectin (EDA) combined with poly(I:C) enhances the immune response to HIV-1 p24 protein and the protection against recombinant Listeria monocytogenes-Gag infection in the mouse model. Vaccine 2012, 30, 2564–2569. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Malara, A.; Gruppi, C.; Celesti, G.; Romano, B.; Laghi, L.; De Marco, L.; Muro, A.F.; Balduini, A. Brief Report: Alternative Splicing of Extra Domain A (EIIIA) of Fibronectin Plays a Tissue-Specific Role in Hematopoietic Homeostasis. Stem Cells 2016, 34, 2263–2268. [Google Scholar] [CrossRef]
- Astrof, S.; Crowley, D.; Hynes, R.O. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev. Biol. 2007, 311, 11–24. [Google Scholar] [CrossRef]
| Fibronectin Receptors Expressed on Hematopoietic Stem Cells | |
|---|---|
| Integrins | α5β1 [21] |
| α4β1 [22] | |
| α4β7 [23] | |
| α9β1 [24] | |
| αvβ3 [25] | |
| TLR-4 | [26,27] |
| CD44 | [28] |
| Function of Hematopoietic Stem Cell | Role of Fibronectin | Role of Fibronectin Receptors |
|---|---|---|
| Migration and homing | Fibronectin acts in vitro to maintain the ability of stem cells to engraft [50] | Migration diminishes if β1 is deleted in hematopoietic cells [47,51] |
| α4β1 and α5β1 are required for homing in adults [21,53,54,55] | ||
| CD44 supports homing [28,56] | ||
| Retention | Fibronectin supports attachment in vitro [57] | α4β1 enhances retention through actions on stromal cells [55,58] |
| αvβ3 maintains long-term repopulation capacity, requires thrombopoietin [25] | ||
| CD44 stimulates adhesion. Possible cooperation with α4β1 [38] | ||
| Proliferation | Fibronectin stimulates cell proliferation [9] | αvβ3 engagement suppresses proliferation [25,59] |
| TLR-4 activation with LPS increases proliferation [60] | ||
| CD44 inhibits proliferation [28] |
| Differentiation Step | Role of Fibronectin | Role of Fibronectin Receptors |
|---|---|---|
| Myelopoiesis | EDA fibronectin stimulates myelopoiesis and changes the immune response towards cancer [19] | CML is associated with abnormal adhesion to α4β1 and α5β1 [71] |
| α4 deletion leads to inefficient myelopoiesis [52,72] | ||
| α5, αv, β1, and β7 deletion do not affect myelopoiesis [23,72] | ||
| TLR-4 boosts myelopoiesis [66,70,73,74] | ||
| CD44 knockout increases monocytes in the bone marrow and circulation [28] | ||
| Erythropoiesis | Fibronectin stimulates erythropoiesis in vitro [75,76] | α5 deletion did not affect erythropoiesis [77] |
| α4 deletion or combined β1 and β7 deletion showed limited recovery after hemolysis [23,55] | ||
| Thrombopoiesis | In myelofibrosis, fibronectin contributes to the disease directly, by binding to α5β1 with the possible involvement of stored molecules [15,69,78,79,80,81] | α4, β1, and β7 deletion did not affect thrombopoiesis [23,52] |
| α4 deletion in hematopoietic and stromal cells simultaneously prevented the expected increase after hemolysis [55,82] | ||
| Lymphopoiesis | α4 is required for postnatal lymphopoiesis [83] | |
| β1 is required for T-cell dependent IgM response to immunization [64] | ||
| TLR-4 affects B- and T-cell maturation [84] | ||
| CD44 deletion increases B-lymphocytes [28] | ||
| Syndecan-4 modulates chemotaxis [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirth, F.; Lubosch, A.; Hamelmann, S.; Nakchbandi, I.A. Fibronectin and Its Receptors in Hematopoiesis. Cells 2020, 9, 2717. https://doi.org/10.3390/cells9122717
Wirth F, Lubosch A, Hamelmann S, Nakchbandi IA. Fibronectin and Its Receptors in Hematopoiesis. Cells. 2020; 9(12):2717. https://doi.org/10.3390/cells9122717
Chicago/Turabian StyleWirth, Franziska, Alexander Lubosch, Stefan Hamelmann, and Inaam A. Nakchbandi. 2020. "Fibronectin and Its Receptors in Hematopoiesis" Cells 9, no. 12: 2717. https://doi.org/10.3390/cells9122717
APA StyleWirth, F., Lubosch, A., Hamelmann, S., & Nakchbandi, I. A. (2020). Fibronectin and Its Receptors in Hematopoiesis. Cells, 9(12), 2717. https://doi.org/10.3390/cells9122717