Human Tissue-Resident Memory T Cells in the Maternal–Fetal Interface. Lost Soldiers or Special Forces?
Abstract
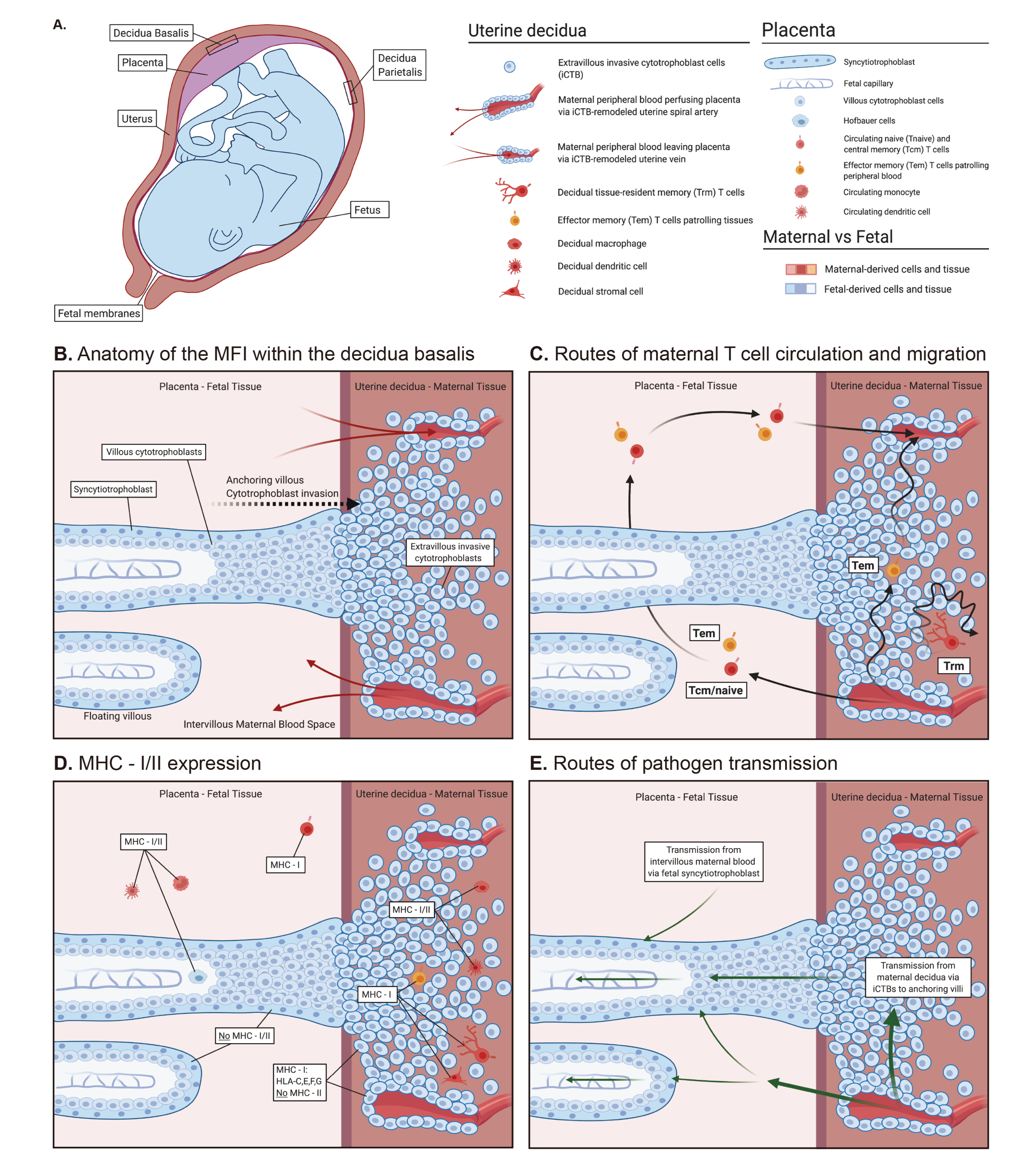
1. Human Pregnancy and the Maternal–Fetal Interface
2. Introduction to Memory T Cell Subsets Circulating in Human Blood and Tissues
3. The Memory T Cell Compartment Changes during the Course of Human Pregnancy in the Maternal–Fetal Interface
3.1. Tissue Residency: Preimplantation
3.2. Tissue Residency: Postimplantation
4. T-Cell-Mediated Protection at the Maternal–Fetal Interface
5. Open Research Questions Relating to Trm Cells in Pregnancy
Author Contributions
Funding
Conflicts of Interest
References
- Moffett, A.; Loke, C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 2006, 6, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Englund, J.A. Maternal immunization. Birth Defects Res. 2017, 109, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Abrahams, V.M. Immunology of the Placenta. Obstet. Gynecol. Clin. N. Am. 2020, 47, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef]
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Pathology of the Human Placenta, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-23940-3. [Google Scholar]
- Flynn, L.; Byrne, B.; Carton, J.; Kelehan, P.; O’Herlihy, C.; O’Farrelly, C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am. J. Reprod. Immunol. 2000, 43, 209–217. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, C.J.; Kim, D.-J.; Kang, J. Immune Cells in the Female Reproductive Tract. Immune Netw. 2015, 15, 16. [Google Scholar] [CrossRef]
- Manaster, I.; Mizrahi, S.; Goldman-Wohl, D.; Sela, H.Y.; Stern-Ginossar, N.; Lankry, D.; Gruda, R.; Hurwitz, A.; Bdolah, Y.; Haimov-Kochman, R.; et al. Endometrial NK Cells Are Special Immature Cells That Await Pregnancy. J. Immunol. 2008, 181, 1869–1876. [Google Scholar] [CrossRef]
- Tilburgs, T.; Claas, F.H.J.; Scherjon, S.A. Elsevier Trophoblast Research Award Lecture: Unique Properties of Decidual T Cells and their Role in Immune Regulation during Human Pregnancy. Placenta 2010, 31, S82–S86. [Google Scholar] [CrossRef]
- Van der Molen, R.G.; Schutten, J.H.F.; van Cranenbroek, B.; ter Meer, M.; Donckers, J.; Scholten, R.R.; van der Heijden, O.W.H.; Spaanderman, M.E.A.; Joosten, I. Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood. Hum. Reprod. 2014, 29, 303–314. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the Maternal-Fetal Interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Strominger, J.L. CD8+ Effector T Cells at the Fetal-Maternal Interface, Balancing Fetal Tolerance and Antiviral Immunity. Am. J. Reprod. Immunol. 2013, 69, 395–407. [Google Scholar] [CrossRef]
- Tilburgs, T.; Schonkeren, D.; Eikmans, M.; Nagtzaam, N.M.; Datema, G.; Swings, G.M.; Prins, F.; van Lith, J.M.; van der Mast, B.J.; Roelen, D.L.; et al. Human Decidual Tissue Contains Differentiated CD8 + Effector-Memory T Cells with Unique Properties. J. Immunol. 2010, 185, 4470–4477. [Google Scholar] [CrossRef] [PubMed]
- Papúchová, H.; Meissner, T.B.; Li, Q.; Strominger, J.L.; Tilburgs, T. The Dual Role of HLA-C in Tolerance and Immunity at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Hammarström, S.; Hammarström, M.L. Human decidual leukocytes from early pregnancy contain high numbers of gamma delta+ cells and show selective down-regulation of alloreactivity. J. Immunol. 1992, 149, 2203–2211. [Google Scholar]
- Saito, S.; Nishikawa, K.; Morii, T.; Narita, N.; Enomoto, M.; Ito, A.; Ichijo, M. A study of CD45RO, CD45RA and CD29 antigen expression on human decidual T cells in an early stage of pregnancy. Immunol. Lett. 1994, 40, 193–197. [Google Scholar] [CrossRef]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef]
- Red-Horse, K.; Rivera, J.; Schanz, A.; Zhou, Y.; Winn, V.; Kapidzic, M.; Maltepe, E.; Okazaki, K.; Kochman, R.; Kim, C.V.; et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Investig. 2006, 116, 2643–2652. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Johnson, C.M.; Mescher, M.F. CD8 T Cell Clonal Expansion and Development of Effector Function Require Prolonged Exposure to Antigen, Costimulation, and Signal 3 Cytokine. J. Immunol. 2003, 171, 5165–5171. [Google Scholar] [CrossRef]
- Zhang, N.; Bevan, M.J. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gerner, M.Y.; Casey, K.A.; Kastenmuller, W.; Germain, R.N. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J. Exp. Med. 2017, 214, 3105–3122. [Google Scholar] [CrossRef] [PubMed]
- Gerner, M.Y.; Torabi-Parizi, P.; Germain, R.N. Strategically Localized Dendritic Cells Promote Rapid T Cell Responses to Lymph-Borne Particulate Antigens. Immunity 2015, 42, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Prlic, M.; Williams, M.A.; Bevan, M.J. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr. Opin. Immunol. 2007, 19, 315–319. [Google Scholar] [CrossRef]
- Stemberger, C.; Huster, K.M.; Koffler, M.; Anderl, F.; Schiemann, M.; Wagner, H.; Busch, D.H. A Single Naive CD8+ T Cell Precursor Can Develop into Diverse Effector and Memory Subsets. Immunity 2007, 27, 985–997. [Google Scholar] [CrossRef]
- Zehn, D.; Roepke, S.; Weakly, K.; Bevan, M.J.; Prlic, M. Inflammation and TCR Signal Strength Determine the Breadth of the T Cell Response in a Bim-Dependent Manner. J. Immunol. 2014, 192, 200–205. [Google Scholar] [CrossRef]
- Prlic, M.; Bevan, M.J. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc. Natl. Acad. Sci. USA 2008, 105, 16689–16694. [Google Scholar] [CrossRef]
- Jameson, S.C.; Masopust, D. Understanding Subset Diversity in T Cell Memory. Immunity 2018, 48, 214–226. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Clark, R.A.; Watanabe, R.; Teague, J.E.; Schlapbach, C.; Tawa, M.C.; Adams, N.; Dorosario, A.A.; Chaney, K.S.; Cutler, C.S.; LeBoeuf, N.R.; et al. Skin Effector Memory T Cells Do Not Recirculate and Provide Immune Protection in Alemtuzumab-Treated CTCL Patients. Sci. Transl. Med. 2012, 4, ra7–ra117. [Google Scholar] [CrossRef]
- Jiang, J.; Lau, L.L.; Shen, H. Selective Depletion of Nonspecific T Cells During the Early Stage of Immune Responses to Infection. J. Immunol. 2003, 171, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Müller, I.; Wolf, E.; Lipp, M. CCR7 Coordinates the Primary Immune Response by Establishing Functional Microenvironments in Secondary Lymphoid Organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef]
- Williams, M.A.; Bevan, M.J. Effector and Memory CTL Differentiation. Annu. Rev. Immunol. 2007, 25, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Park, C.O.; Kupper, T.S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 2015, 21, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Klonowski, K.D.; Williams, K.J.; Marzo, A.L.; Blair, D.A.; Lingenheld, E.G.; Lefrançois, L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 2004, 20, 551–562. [Google Scholar] [CrossRef]
- Wakim, L.M.; Waithman, J.; van Rooijen, N.; Heath, W.R.; Carbone, F.R. Dendritic Cell-Induced Memory T Cell Activation in Nonlymphoid Tissues. Science 2008, 319, 198–202. [Google Scholar] [CrossRef]
- Bartolomé-Casado, R.; Landsverk, O.J.B.; Chauhan, S.K.; Richter, L.; Phung, D.; Greiff, V.; Risnes, L.F.; Yao, Y.; Neumann, R.S.; Yaqub, S.; et al. Resident memory CD8 T cells persist for years in human small intestine. J. Exp. Med. 2019, 216, 2412–2426. [Google Scholar] [CrossRef]
- Snyder, M.E.; Finlayson, M.O.; Connors, T.J.; Dogra, P.; Senda, T.; Bush, E.; Carpenter, D.; Marboe, C.; Benvenuto, L.; Shah, L.; et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 2019, 4, eaav5581. [Google Scholar] [CrossRef]
- Klicznik, M.M.; Morawski, P.A.; Höllbacher, B.; Varkhande, S.R.; Motley, S.J.; Kuri-Cervantes, L.; Goodwin, E.; Rosenblum, M.D.; Alice Long, S.; Brachtl, G.; et al. Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol. 2019, 4, eaav8995. [Google Scholar] [CrossRef]
- Fonseca, R.; Beura, L.K.; Quarnstrom, C.F.; Ghoneim, H.E.; Fan, Y.; Zebley, C.C.; Scott, M.C.; Fares-Frederickson, N.J.; Wijeyesinghe, S.; Thompson, E.A.; et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 2020, 21, 412–421. [Google Scholar] [CrossRef]
- Paik, D.H.; Farber, D.L. Anti-viral protective capacity of tissue resident memory T cells. Curr. Opin. Virol. 2020, 46, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.E.; Farber, D.L.; Yates, A.J. Tissue-Resident Memory T Cells in Mice and Humans: Towards a Quantitative Ecology. J. Immunol. 2019, 203, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, D.; King, C.G. CD4+ memory T cells at home in the tissue: Mechanisms for health and disease. Front. Immunol. 2018, 9, 2394. [Google Scholar] [CrossRef] [PubMed]
- Woodward Davis, A.S.; Roozen, H.N.; Dufort, M.J.; DeBerg, H.A.; Delaney, M.A.; Mair, F.; Erickson, J.R.; Slichter, C.K.; Berkson, J.D.; Klock, A.M.; et al. The human tissue-resident CCR5+ T cell compartment maintains protective and functional properties during inflammation. Sci. Transl. Med. 2019, 11, eaaw8718. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.L.; Khoruts, A.; Merica, R.; Zell, T.; Jenkins, M.K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 2001, 410, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Masopust, D.; Vezys, V.; Marzo, A.L.; Lefrançois, L. Preferential Localization of Effector Memory Cells in Nonlymphoid Tissue. Science 2001, 291, 2413–2417. [Google Scholar] [CrossRef]
- Khan, T.N.; Mooster, J.L.; Kilgore, A.M.; Osborn, J.F.; Nolz, J.C. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med. 2016, 213, 951–966. [Google Scholar] [CrossRef]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef]
- Szabo, P.A.; Miron, M.; Farber, D.L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 2019, 4, eaas9673. [Google Scholar] [CrossRef]
- Geginat, J.; Geginat, J.; Lanzavecchia, A.; Lanzavecchia, A.; Sallusto, F.; Sallusto, F. Proliferation and differentiation potential of human CD8. Blood 2003, 101, 4260–4266. [Google Scholar] [CrossRef]
- Masopust, D.; Soerens, A.G. Tissue-Resident T Cells and Other Resident Leukocytes. Annu. Rev. Immunol. 2019, 37, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Southcombe, J.H.; Mounce, G.; Mcgee, K.; Elghajiji, A.; Brosens, J.; Quenby, S.; Child, T.; Granne, I. An altered endometrial CD8 tissue resident memory T cell population in recurrent miscarriage. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Shanmugasundaram, U.; Critchfield, J.W.; Pannell, J.; Perry, J.; Giudice, L.C.; Smith-Mccune, K.; Greenblatt, R.M.; Shacklett, B.L. Phenotype and Functionality of CD4+ and CD8+ T Cells in the Upper Reproductive Tract of Healthy Premenopausal Women. Am. J. Reprod. Immunol. 2014, 71, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadou, N.; Bulmer, J.N. Quantitative analysis of T lymphocyte subsets in pregnant and nonpregnant human endometrium. Biol. Reprod. 1996, 55, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Pattacini, L.; Woodward Davis, A.; Czartoski, J.; Mair, F.; Presnell, S.; Hughes, S.M.; Hyrien, O.; Lentz, G.M.; Kirby, A.C.; Fialkow, M.F.; et al. A pro-inflammatory CD8+ T-cell subset patrols the cervicovaginal tract. Mucosal Immunol. 2019, 12, 1118–1129. [Google Scholar] [CrossRef]
- Sathaliyawala, T.; Kubota, M.; Yudanin, N.; Turner, D.; Camp, P.; Thome, J.J.C.; Bickham, K.L.; Lerner, H.; Goldstein, M.; Sykes, M.; et al. Distribution and Compartmentalization of Human Circulating and Tissue-Resident Memory T Cell Subsets. Immunity 2013, 38, 187–197. [Google Scholar] [CrossRef]
- Wang, W.; Vilella, F.; Alama, P.; Moreno, I.; Mignardi, M.; Isakova, A.; Pan, W.; Simon, C.; Quake, S.R. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020, 26, 1644–1653. [Google Scholar] [CrossRef]
- Loetscher, P.; Uguccioni, M.; Bordoli, L.; Baggiolini, M.; Moser, B.; Chizzolini, C.; Dayer, J.-M. CCR5 is characteristic of Th1 lymphocytes. Nature 1998, 391, 344–345. [Google Scholar] [CrossRef]
- Sindram-Trujillo, A.; Scherjon, S.; Kanhai, H.; Roelen, D.; Claas, F. Increased T-Cell Activation in Decidua Parietalis Compared to Decidua Basalis in Uncomplicated Human Term Pregnancy. Am. J. Reprod. Immunol. 2003, 49, 261–268. [Google Scholar] [CrossRef]
- Saito, S.; Nishikawa, K.; Morii, T.; Narita, N.; Enomoto, M.; Ichijo, M. Expression of activation antigens CD69, HLA-DR, interleukin-2 receptor-alpha (IL-2R alpha) and IL-2R beta on T cells of human decidua at an early stage of pregnancy. Immunology 1992, 75, 710–712. [Google Scholar] [PubMed]
- Tilburgs, T.; Roelen, D.L.; van der Mast, B.J.; de Groot-Swings, G.M.; Kleijburg, C.; Scherjon, S.A.; Claas, F.H. Evidence for a Selective Migration of Fetus-Specific CD4 + CD25 bright Regulatory T Cells from the Peripheral Blood to the Decidua in Human Pregnancy. J. Immunol. 2008, 180, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, A.; Bi, K.; Norwitz, E.R.; Crespo, Â.C.; Claas, F.H.J.; Strominger, J.L.; Tilburgs, T. Mixed signature of activation and dysfunction allows human decidual CD8 + T cells to provide both tolerance and immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Sabbaj, S.; Hel, Z.; Richter, H.E.; Mestecky, J.; Goepfert, P.A. Menstrual blood as a potential source of endometrial derived CD3+ T cells. PLoS ONE 2011, 6, e28894. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Xu, C.; Peng, X.; Li, D.; Wang, L.; Du, M. Tissue resident CD8 + T cells with unique properties are present in human decidua during early pregnancy. Am. J. Reprod. Immunol. 2020, 84, e13254. [Google Scholar] [CrossRef]
- Zhang, N.; Bevan, M.J. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 2013, 39, 687–696. [Google Scholar] [CrossRef]
- Liu, L.; Huang, X.; Xu, C.; Chen, C.; Zhao, W.; Li, D.; Li, L.; Wang, L.; Du, M. Decidual CD8+T cells exhibit both residency and tolerance signatures modulated by decidual stromal cells. J. Transl. Med. 2020, 18, 1–14. [Google Scholar] [CrossRef]
- Slutsky, R.; Romero, R.; Xu, Y.; Galaz, J.; Miller, D.; Done, B.; Tarca, A.L.; Gregor, S.; Hassan, S.S.; Leng, Y.; et al. Exhausted and senescent T cells at the maternal-fetal interface in preterm and term labor. J. Immunol. Res. 2019, 2019, 3128010. [Google Scholar] [CrossRef]
- van der Zwan, A.; van Unen, V.; Beyrend, G.; Laban, S.; van der Keur, C.; Kapsenberg, H.J.M.; Höllt, T.; Chuva de Sousa Lopes, S.M.; van der Hoorn, M.-L.P.; Koning, F.; et al. Visualizing Dynamic Changes at the Maternal-Fetal Interface Throughout Human Pregnancy by Mass Cytometry. Front. Immunol. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- De Simone, M.; Arrigoni, A.; Rossetti, G.; Gruarin, P.; Ranzani, V.; Politano, C.; Bonnal, R.J.P.; Provasi, E.; Sarnicola, M.L.; Panzeri, I.; et al. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity 2016, 45, 1135–1147. [Google Scholar] [CrossRef]
- Wienke, J.; Brouwers, L.; van der Burg, L.M.; Mokry, M.; Scholman, R.C.; Nikkels, P.G.J.; van Rijn, B.B.; van Wijk, F. Human Tregs at the materno-fetal interface show site-specific adaptation reminiscent of tumor Tregs. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Salvany-Celades, M.; van der Zwan, A.; Benner, M.; Setrajcic-Dragos, V.; Bougleux Gomes, H.A.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface. Cell Rep. 2019, 27, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Aghaeepour, N.; Ganio, E.A.; Mcilwain, D.; Tsai, A.S.; Tingle, M.; Van Gassen, S.; Gaudilliere, D.K.; Baca, Q.; McNeil, L.; Okada, R.; et al. An immune clock of human pregnancy. Sci. Immunol. 2017, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Wilming, L.; Rand, V.; Lovering, R.C.; Bruford, E.A.; Khodiyar, V.K.; Lush, M.J.; Povey, S.; Talbot, C.C.; Wright, M.W.; et al. Gene map of the extended human MHC. Nat. Rev. Genet. 2004, 5, 889–899. [Google Scholar] [CrossRef]
- Apps, R.; Murphy, S.P.; Fernando, R.; Gardner, L.; Ahad, T.; Moffett, A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 2009, 127, 26–39. [Google Scholar] [CrossRef]
- Kovats, S.; Main, E.; Librach, C.; Stubblebine, M.; Fisher, S.; DeMars, R. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef]
- Juch, H.; Blaschitz, A.; Dohr, G.; Hutter, H. HLA class I expression in the human placenta. Wiener Med. Wochenschr. 2012, 162, 196–200. [Google Scholar] [CrossRef]
- Robbins, J.R.; Bakardjiev, A.I. Pathogens and the placental fortress. Curr. Opin. Microbiol. 2012, 15, 36–43. [Google Scholar] [CrossRef]
- Piper, K.P.; McLarnon, A.; Arrazi, J.; Horlock, C.; Ainsworth, J.; Kilby, M.D.; Martin, W.L.; Moss, P.A. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol. Reprod. 2007, 76, 96–101. [Google Scholar] [CrossRef]
- Tilburgs, T.; Scherjon, S.A.; van der Mast, B.J.; Haasnoot, G.W.; Versteeg-v.d.Voort-Maarschalk, M.; Roelen, D.L.; van Rood, J.J.; Claas, F.H.J. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J. Reprod. Immunol. 2009, 82, 148–157. [Google Scholar] [CrossRef]
- Van Egmond, A.; van der Keur, C.; Swings, G.M.J.S.; Scherjon, S.A.; Claas, F.H.J. The possible role of virus-specific CD8+ memory T cells in decidual tissue. J. Reprod. Immunol. 2016, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Crespo, Â.C.; van der Zwan, A.; Ramalho-Santos, J.; Strominger, J.L.; Tilburgs, T. Cytotoxic potential of decidual NK cells and CD8+ T cells awakened by infections. J. Reprod. Immunol. 2017, 119, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019, 4, eaat6114. [Google Scholar] [CrossRef] [PubMed]
- Anders, A.P.; Gaddy, J.A.; Doster, R.S.; Aronoff, D.M. Current concepts in maternal-fetal immunology: Recognition and response to microbial pathogens by decidual stromal cells. Am. J. Reprod. Immunol. 2017, 77, e12623. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Gonçalves, L.F.; Kusanovic, J.P.; Friel, L.; Hassan, S. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 2007, 25, 21–39. [Google Scholar] [CrossRef]
- Solders, M.; Gorchs, L.; Gidlöf, S.; Tiblad, E.; Lundell, A.C.; Kaipe, H. Maternal Adaptive Immune Cells in Decidua Parietalis Display a More Activated and Coinhibitory Phenotype Compared to Decidua Basalis. Stem Cells Int. 2017, 2017, 8010961. [Google Scholar] [CrossRef]
- Chu, T.; Tyznik, A.J.; Roepke, S.; Berkley, A.M.; Woodward-Davis, A.; Pattacini, L.; Bevan, M.J.; Zehn, D.; Prlic, M. Bystander-Activated Memory CD8 T Cells Control Early Pathogen Load in an Innate-like, NKG2D-Dependent Manner. Cell Rep. 2013, 3, 701–708. [Google Scholar] [CrossRef]
- Ge, C.; Monk, I.R.; Pizzolla, A.; Wang, N.; Bedford, J.G.; Stinear, T.P.; Westall, G.P.; Wakim, L.M. Bystander Activation of Pulmonary Trm Cells Attenuates the Severity of Bacterial Pneumonia by Enhancing Neutrophil Recruitment. Cell Rep. 2019, 29, 4236–4244. [Google Scholar] [CrossRef]
- Maurice, N.J.; McElrath, M.J.; Andersen-Nissen, E.; Frahm, N.; Prlic, M. CXCR3 enables recruitment and site-specific bystander activation of memory CD8+ T cells. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, D.Y.; Lee, H.W.; Lee, H.; Kim, J.H.; Sung, P.S.; Kim, K.H.; Hong, S.H.; Kang, W.; Lee, J.; et al. Innate-like Cytotoxic Function of Bystander-Activated CD8 + T Cells Is Associated with Liver Injury in Acute Hepatitis A. Immunity 2018, 48, 161–173. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeJong, C.S.; Maurice, N.J.; McCartney, S.A.; Prlic, M. Human Tissue-Resident Memory T Cells in the Maternal–Fetal Interface. Lost Soldiers or Special Forces? Cells 2020, 9, 2699. https://doi.org/10.3390/cells9122699
DeJong CS, Maurice NJ, McCartney SA, Prlic M. Human Tissue-Resident Memory T Cells in the Maternal–Fetal Interface. Lost Soldiers or Special Forces? Cells. 2020; 9(12):2699. https://doi.org/10.3390/cells9122699
Chicago/Turabian StyleDeJong, Caitlin S., Nicholas J. Maurice, Stephen A. McCartney, and Martin Prlic. 2020. "Human Tissue-Resident Memory T Cells in the Maternal–Fetal Interface. Lost Soldiers or Special Forces?" Cells 9, no. 12: 2699. https://doi.org/10.3390/cells9122699
APA StyleDeJong, C. S., Maurice, N. J., McCartney, S. A., & Prlic, M. (2020). Human Tissue-Resident Memory T Cells in the Maternal–Fetal Interface. Lost Soldiers or Special Forces? Cells, 9(12), 2699. https://doi.org/10.3390/cells9122699




