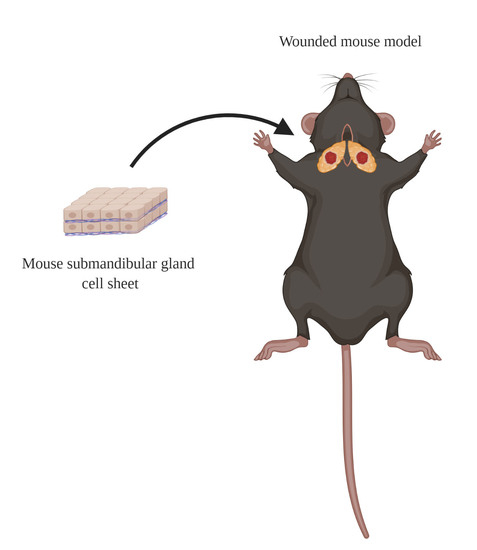
Cell Sheets Restore Secretory Function in Wounded Mouse Submandibular Glands
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
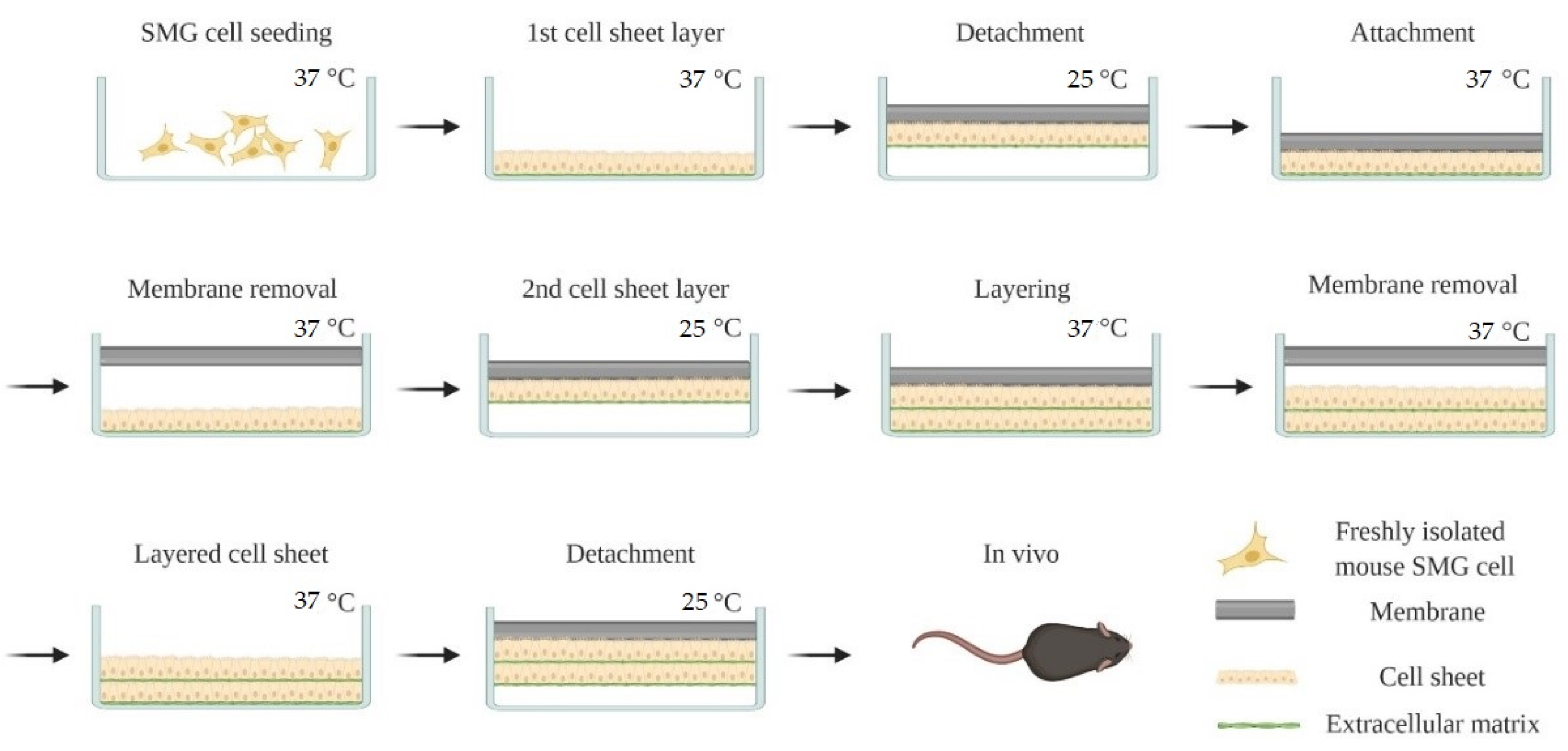
2.3. Cell Sheet Preparation
2.4. Wounded SMG Mouse Model
2.5. Histological Studies
2.6. Confocal Analysis
2.7. Saliva Flow Rate
2.8. Saliva Protein Composition
3. Results
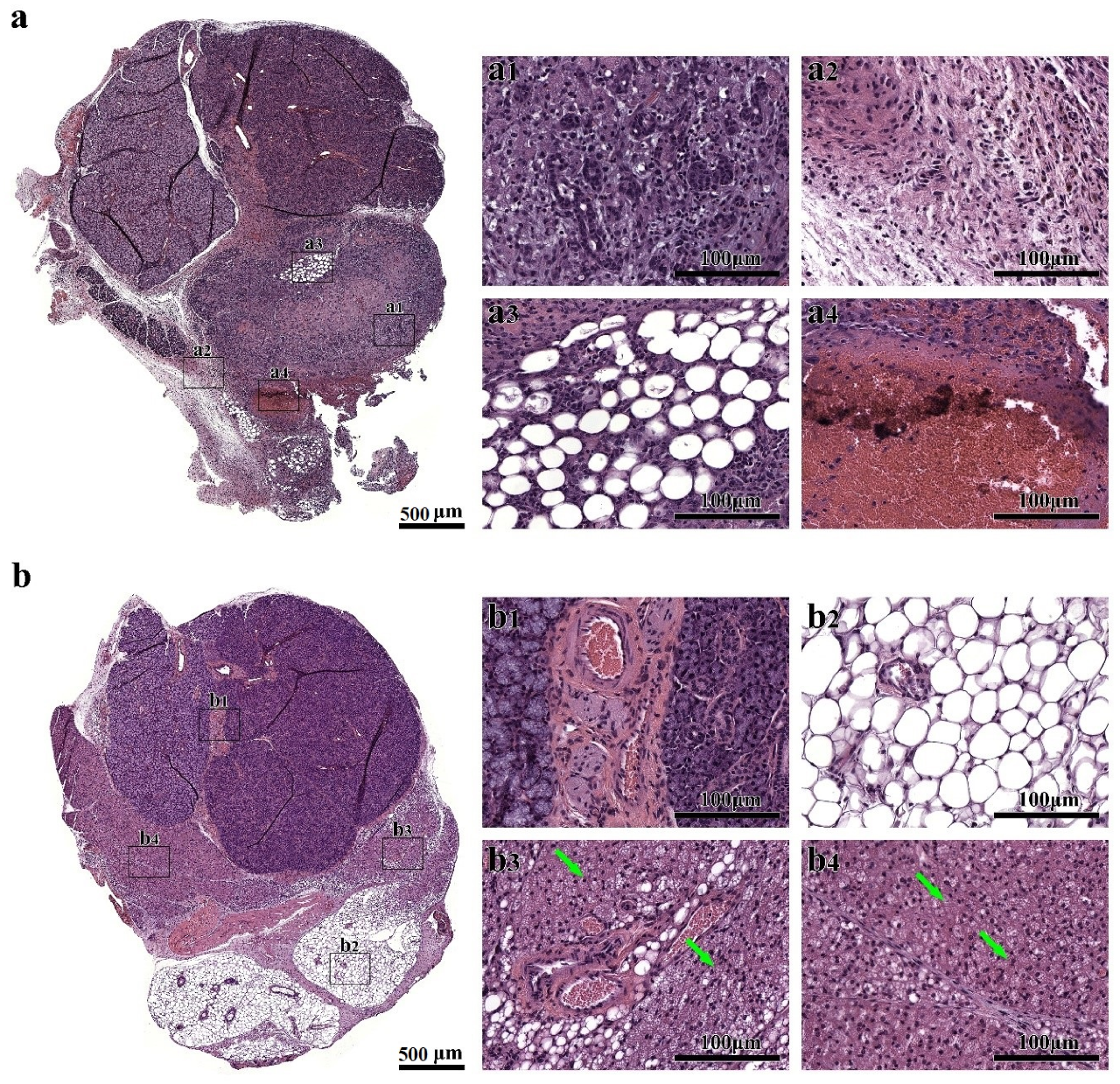
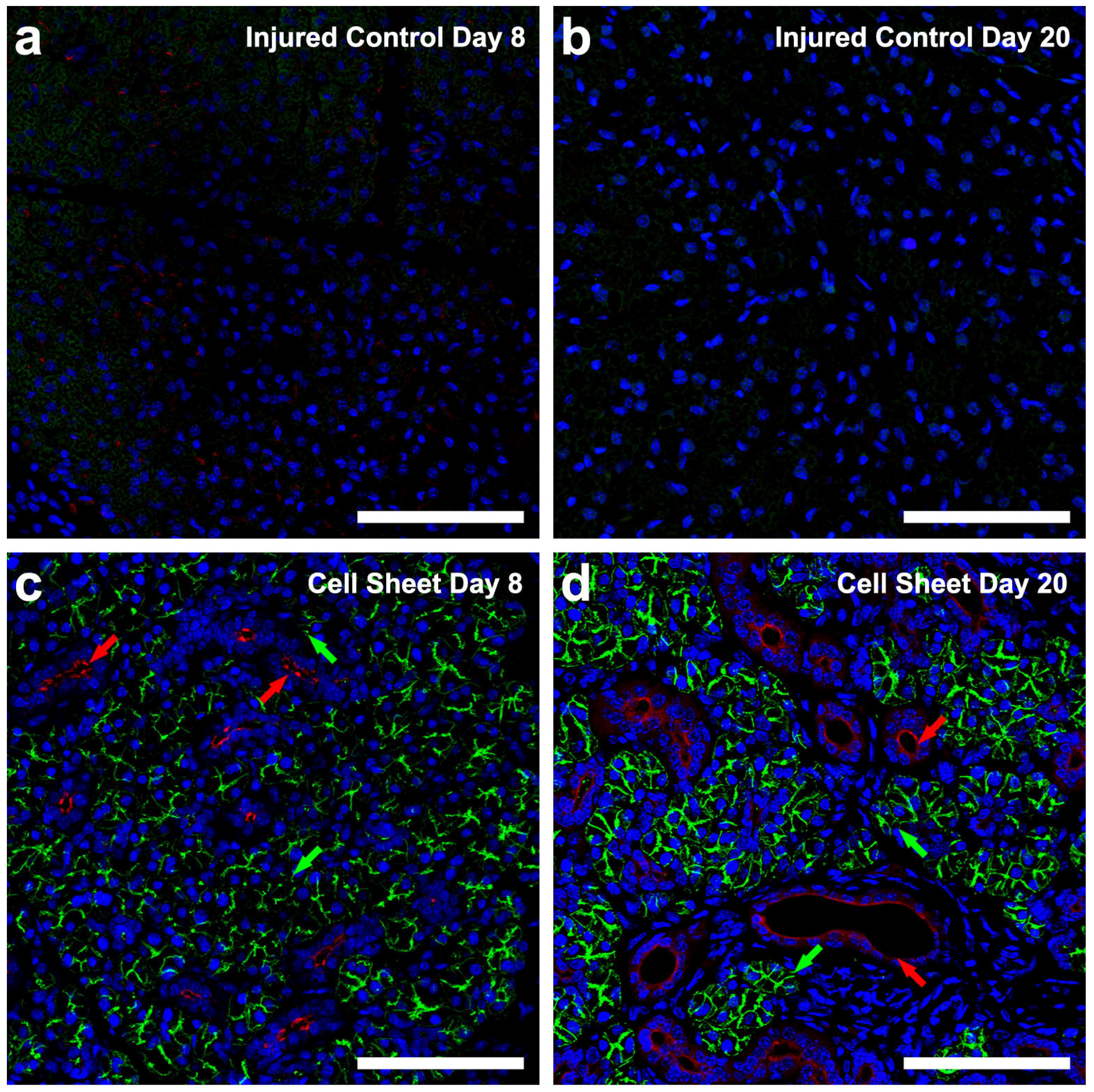
3.1. Treatment with Cell Sheets Promotes Tissue Organization
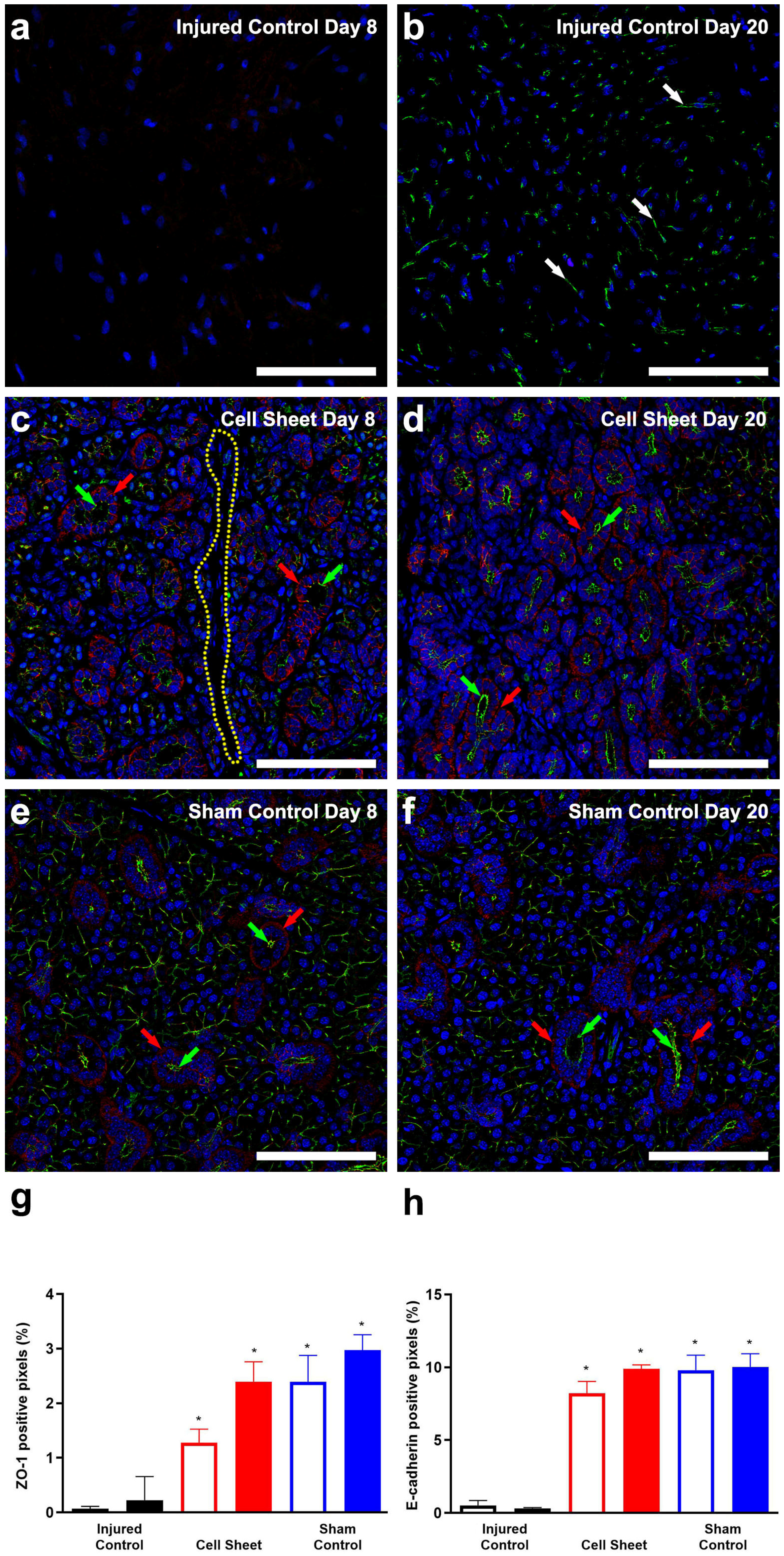
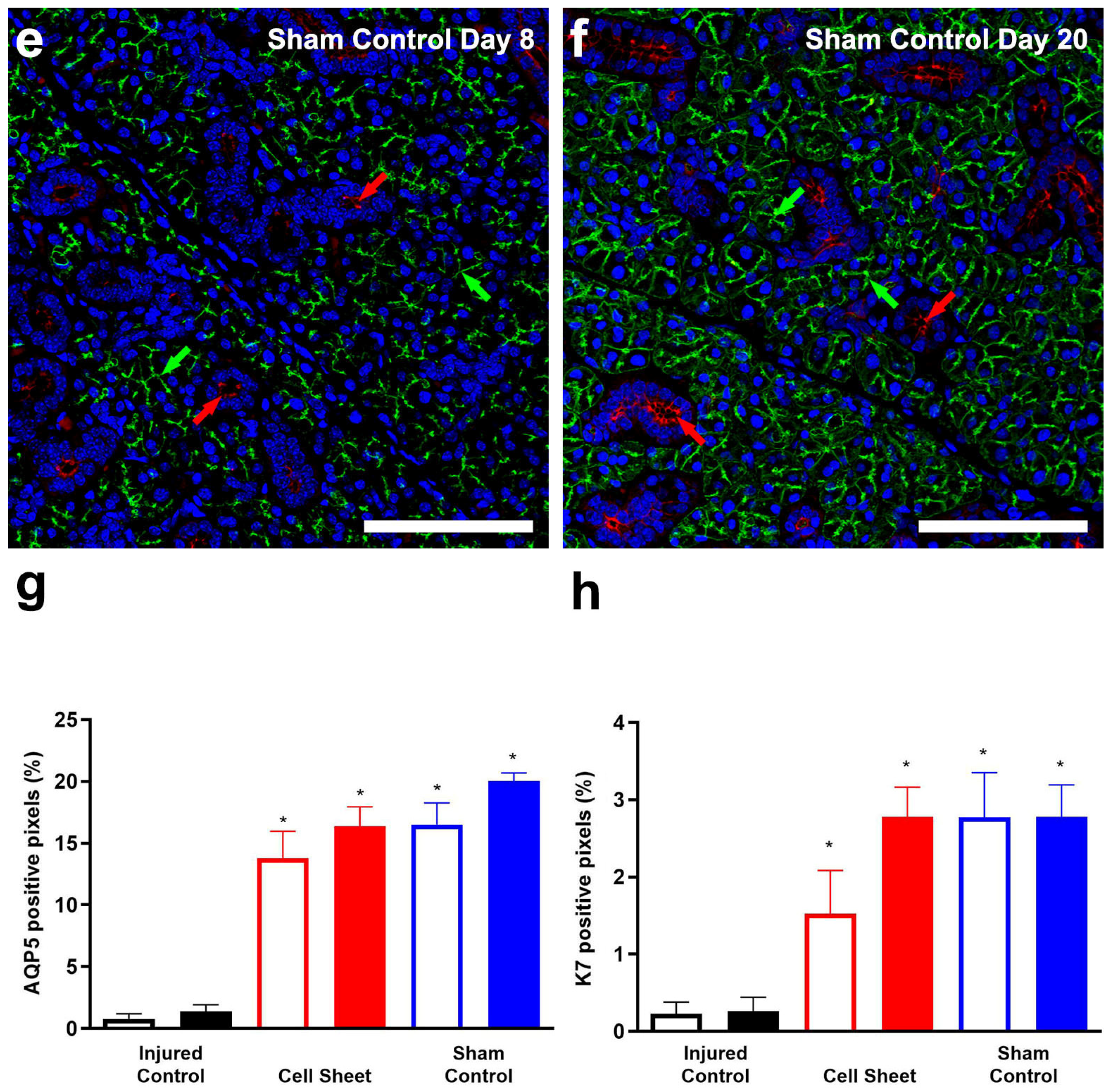
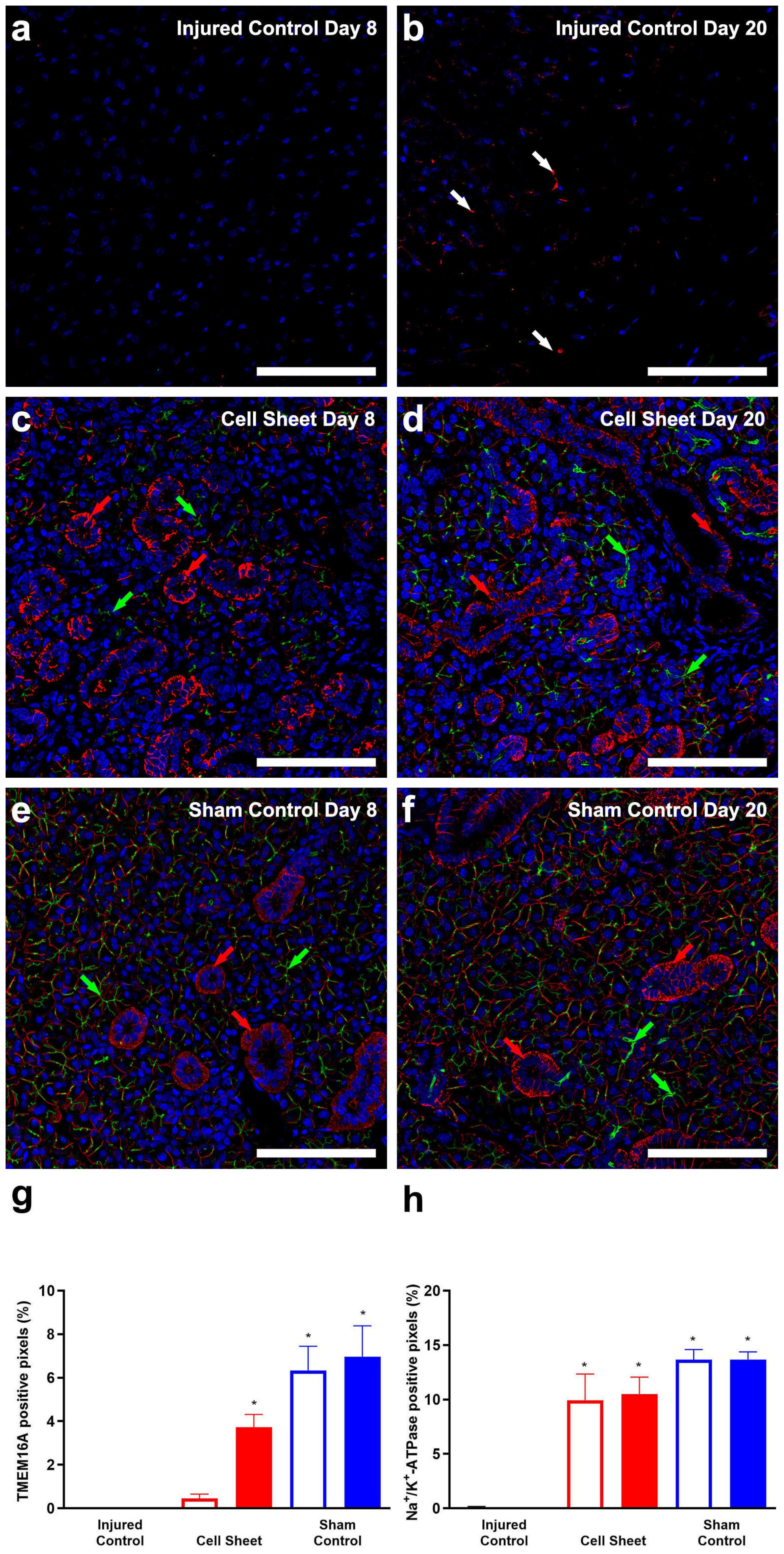
3.2. Treatment with Cell Sheets Promotes Epithelial Polarization
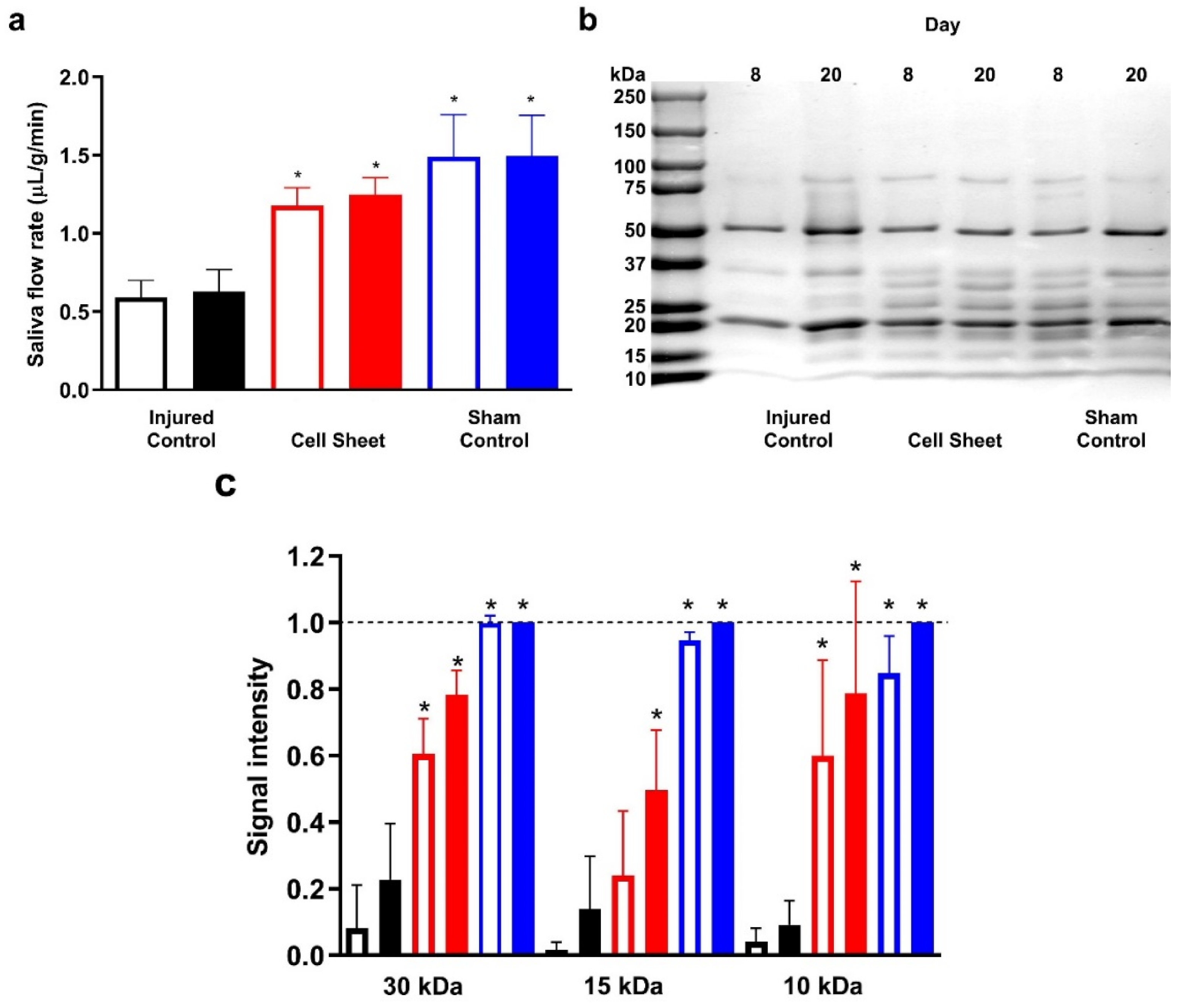
3.3. Treatment with Cell Sheets Promotes Secretory Function
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lawrence, H.P.; Thomson, W.M.; Broadbent, J.M.; Poulton, R. Oral health-related quality of life in a birth cohort of 32-year olds. Community Dent. Oral Epidemiol. 2008, 36, 305–316. [Google Scholar] [CrossRef]
- Chambers, M.S.; Garden, A.S.; Kies, M.S.; Martin, J.W. Radiation-induced xerostomia in patients with head and neck cancer: Pathogenesis, impact on quality of life, and management. Head Neck 2004, 26, 796–807. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts and Figures 2020. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf (accessed on 16 November 2020).
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Clin. Risk Manag. 2015, 11, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Skrinjar, I.; Vucicevic Boras, V.; Bakale, I.; Andabak Rogulj, A.; Brailo, V.; Vidovic Juras, D.; Alajbeg, I.; Vrdoljak, D.V. Comparison between three different saliva substitutes in patients with hyposalivation. Clin. Oral Investig. 2015, 19, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Scully, C.; Hegarty, A.M. An update of the etiology and management of xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Beier Jensen, S. Management of hyposalivation and xerostomia: Criteria for treatment strategies. Compend. Contin. Educ. Dent. 2015, 36, 600–603. [Google Scholar] [PubMed]
- Barbieri, T.; da Costa, K.C.; de Freitas Cuba Guerra, L. Current alternatives in the prevention and treatment of xerostomia in cancer therapy. Rev. Gaúcha Odontol. 2020, 68, 1–12. [Google Scholar] [CrossRef]
- Ogawa, M.; Oshima, M.; Imamura, A.; Sekine, Y.; Ishida, K.; Yamashita, K.; Nakajima, K.; Hirayama, M.; Tachikawa, T.; Tsuji, T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Kagami, H.; Wang, S.; Hai, B. Restoring the function of salivary glands. Oral Dis. 2008, 14, 15–24. [Google Scholar] [CrossRef]
- Jeong, J.; Baek, H.; Kim, Y.-J.; Choi, Y.; Lee, H.; Lee, E.; Kim, E.S.; Hah, J.H.; Kwon, T.-K.; Choi, I.J.; et al. Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Exp. Mol. Med. 2013, 45, e58. [Google Scholar] [CrossRef]
- Pringle, S.; Van Os, R.; Coppes, R.P. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 2013, 31, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Faber, H.; Stokman, M.A.; Kok, T.; Visser, W.H.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE 2008, 3, e2063. [Google Scholar] [CrossRef]
- Xiao, N.; Lin, Y.; Cao, H.; Sirjani, D.; Giaccia, A.J.; Koong, A.C.; Kong, C.S.; Diehn, M.; Le, Q.-T. Neurotrophic factor GDNF promotes survival of salivary stem cells. J. Clin. Investig. 2014, 124, 3364–3377. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Dawson, P.; Zhang, Q.; Harris, Z.; Limesand, K.H. Administration of growth factors promotes salisphere formation from irradiated parotid salivary glands. PLoS ONE 2018, 13, e0193942. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Marrocco, I.; Yarden, Y. Roles for receptor tyrosine kinases in tumor progression and implications for cancer treatment. Adv. Cancer Res. 2020, 147, 1–57. [Google Scholar] [CrossRef]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for growth factors in cancer progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef]
- Aframian, D.J.; Cukierman, E.; Nikolovski, J.; Mooney, D.J.; Yamada, K.M.; Baum, B.J. The growth and morphological behavior of salivary epithelial cells on matrix protein-coated biodegradable substrata. Tissue Eng. 2000, 6, 209–216. [Google Scholar] [CrossRef]
- Cantara, S.I.; Soscia, D.A.; Sequeira, S.J.; Jean-Gilles, R.P.; Castracane, J.; Larsen, M. Selective functionalization of nanofiber scaffolds to regulate salivary gland epithelial cell proliferation and polarity. Biomaterials 2012, 33, 8372–8382. [Google Scholar] [CrossRef]
- Soscia, D.A.; Sequeira, S.J.; Schramm, R.A.; Jayarathanam, K.; Cantara, S.I.; Larsen, M.; Castracane, J. Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters. Biomaterials 2013, 34, 6773–6784. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Yang, T.L. Data supporting chitosan facilitates structure formation of the salivary gland by regulating the basement membrane components. Data Brief. 2015, 4, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.; Hsiao, Y.C. Chitosan facilitates structure formation of the salivary gland by regulating the basement membrane components. Biomaterials 2015, 66, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhu, J.; Yang, X.; Wang, S. Growth of miniature pig parotid cells on biomaterials in vitro. Arch. Oral Biol. 2006, 51, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Pradhan-Bhatt, S.; Harrington, D.A.; Duncan, R.L.; Farach-Carson, M.C.; Jia, X.; Witt, R.L. A novel in vivo model for evaluating functional restoration of a tissue-engineered salivary gland. Laryngoscope 2014, 124, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Zurina, I.M.; Presniakova, V.S.; Butnaru, D.V.; Svistunov, A.A.; Timashev, P.S.; Rochev, Y.A. Tissue engineering using a combined cell sheet technology and scaffolding approach. Acta Biomater. 2020, 113, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Okano, T. Thermally-triggered fabrication of cell sheets for tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2019, 138, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Tang, Z.; Okano, T. Recent development of temperature-responsive surfaces and their application for cell sheet engineering. Regen. Biomater. 2014, 1, 91–102. [Google Scholar] [CrossRef]
- Sato, M.; Ishihara, M.; Furukawa, K.; Kaneshiro, N.; Nagai, T.; Mitani, G.; Kutsuna, T.; Ohta, N.; Kokubo, M.; Kikuchi, T.; et al. Recent technological advancements related to articular cartilage regeneration. Med. Biol. Eng. Comput. 2008, 46, 735–743. [Google Scholar] [CrossRef]
- Nandkumar, M.A.; Yamato, M.; Kushida, A.; Konno, C.; Hirose, M.; Kikuchi, A.; Okano, T. Two-dimensional cell sheet manipulation of heterotypically co-cultured lung cells utilizing temperature-responsive culture dishes results in long-term maintenance of differentiated epithelial cell functions. Biomaterials 2002, 23, 1121–1130. [Google Scholar] [CrossRef]
- Matsuura, K.; Haraguchi, Y.; Shimizu, T.; Okano, T. Cell sheet transplantation for heart tissue repair. J. Control. Release 2013, 169, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Baimakhanov, Z.; Yamanouchi, K.; Sakai, Y.; Koike, M.; Soyama, A.; Hidaka, M.; Takatsuki, M.; Fujita, F.; Kanetaka, K.; Kuroki, T.; et al. Efficacy of multilayered hepatocyte sheet transplantation for radiation-induced liver damage and partial hepatectomy in a rat model. Cell Transpl. 2016, 25, 549–558. [Google Scholar] [CrossRef]
- Kuramoto, G.; Shimizu, T.; Takagi, S.; Ishitani, K.; Matsui, H.; Okano, T. Endometrial regeneration using cell sheet transplantation techniques in rats facilitates successful fertilization and pregnancy. Fertil. Steril. 2018, 110, 172–181.e174. [Google Scholar] [CrossRef] [PubMed]
- Maruya, Y.; Kanai, N.; Kobayashi, S.; Koshino, K.; Okano, T.; Eguchi, S.; Yamato, M. Autologous adipose-derived stem cell sheets enhance the strength of intestinal anastomosis. Regen. Ther. 2017, 7, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Ma, X.; Zhao, J.; Wen, Q.; Hu, X.; Yu, H.; Shi, W. Transplantation of tissue-engineered human corneal endothelium in cat models. Mol. Vis. 2013, 19, 400–407. [Google Scholar] [PubMed]
- Zhang, H.; Liu, S.; Zhu, B.; Xu, Q.; Ding, Y.; Jin, Y. Composite cell sheet for periodontal regeneration: Crosstalk between different types of MSCs in cell sheet facilitates complex periodontal-like tissue regeneration. Stem Cell Res. Ther. 2016, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, G.; Sato, M.; Yamato, M.; Mitani, G.; Kutsuna, T.; Nagai, T.; Ito, S.; Ukai, T.; Kobayashi, M.; Kokubo, M.; et al. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials 2012, 33, 3846–3851. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Washio, K.; Ando, T.; Okano, T.; Ishikawa, I. Cell sheets for periodontal tissue engineering. Curr. Oral Health Rep. 2015, 2, 252–256. [Google Scholar] [CrossRef][Green Version]
- Iwata, T.; Yamato, M.; Washio, K.; Yoshida, T.; Tsumanuma, Y.; Yamada, A.; Onizuka, S.; Izumi, Y.; Ando, T.; Okano, T.; et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets–A safety and efficacy study in ten patients. Regen. Ther. 2018, 9, 38–44. [Google Scholar] [CrossRef]
- Kanzaki, M.; Takagi, R.; Washio, K.; Kokubo, M.; Yamato, M. Bio-artificial pleura using an autologous dermal fibroblast sheet. NPJ Regen. Med. 2017, 2, 26. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y.; et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Yamato, M.; Ota, M.; Takagi, R.; Murakami, D.; Kondo, M.; Sasaki, R.; Namiki, H.; Okano, T.; Yamamoto, M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012, 143, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Sawa, Y.; Miyagawa, S.; Sakaguchi, T.; Fujita, T.; Matsuyama, A.; Saito, A.; Shimizu, T.; Okano, T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: Report of a case. Surg. Today 2012, 42, 181–184. [Google Scholar] [CrossRef]
- Sawa, Y.; Yoshikawa, Y.; Toda, K.; Fukushima, S.; Yamazaki, K.; Ono, M.; Sakata, Y.; Hagiwara, N.; Kinugawa, K.; Miyagawa, S. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ. J. 2015, 79, 991–999. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Isomoto, H.; Kobayashi, S.; Kanai, N.; Kanetaka, K.; Sakai, Y.; Kasai, Y.; Takagi, R.; Ohki, T.; Fukuda, H.; et al. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci. Rep. 2017, 7, 17460. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamato, M.; Morino, T.; Sugiyama, H.; Takagi, R.; Yaguchi, Y.; Okano, T.; Kojima, H. Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen. Med. 2017, 2, 6. [Google Scholar] [CrossRef]
- Nam, K.; Kim, K.; Dean, S.M.; Brown, C.T.; Davis, R.S.; Okano, T.; Baker, O.J. Using cell sheets to regenerate mouse submandibular glands. NPJ Regen. Med. 2019, 4, 16. [Google Scholar] [CrossRef]
- Nam, K.; Dean, S.M.; Brown, C.T.; Smith, R.J., Jr.; Lei, P.; Andreadis, S.T.; Baker, O.J. Synergistic effects of laminin-1 peptides, VEGF and FGF9 on salivary gland regeneration. Acta Biomater. 2019, 91, 186–194. [Google Scholar] [CrossRef]
- Nam, K.; Maruyama, C.L.; Wang, C.S.; Trump, B.G.; Lei, P.; Andreadis, S.T.; Baker, O.J. Laminin-111-derived peptide conjugated fibrin hydrogel restores salivary gland function. PLoS ONE 2017, 12, e0187069. [Google Scholar] [CrossRef]
- Nam, K.; Wang, C.S.; Maruyama, C.L.M.; Lei, P.; Andreadis, S.T.; Baker, O.J. L1 peptide-conjugated fibrin hydrogels promote salivary gland regeneration. J. Dent. Res. 2017, 96, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.A.; Manhardt, C.; Kamath, V.; Sui, Y.; Santamaria-Pang, A.; Can, A.; Bello, M.; Corwin, A.; Dinn, S.R.; Lazare, M.; et al. Quantitative single cell analysis of cell population dynamics during submandibular salivary gland development and differentiation. Biol. Open 2013, 2, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Oh, U.; Jung, J. Cellular functions of TMEM16/anoctamin. Pflug. Arch. 2016, 468, 443–453. [Google Scholar] [CrossRef]
- Lee, M.G.; Ohana, E.; Park, H.W.; Yang, D.; Muallem, S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3− secretion. Physiol. Rev. 2012, 92, 39–74. [Google Scholar] [CrossRef]
- Moschouris, K.; Firoozi, N.; Kang, Y. The application of cell sheet engineering in the vascularization of tissue regeneration. Regen. Med. 2016, 11, 559–570. [Google Scholar] [CrossRef]
- Burillon, C.; Huot, L.; Justin, V.; Nataf, S.; Chapuis, F.; Decullier, E.; Damour, O. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.R.; Pedroni, A.C.F.; Cury, D.P.; Moreira, M.S.; Rosin, F.; Sarra, G.; Marques, M.M. Effects of photobiomodulation therapy on the extracellular matrix of human dental pulp cell sheets. J. Photochem. Photobiol. B 2019, 194, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 130. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, B.; Gao, Z.; Xu, J.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Regeneration characteristics of different dental derived stem cell sheets. J. Oral Rehabil. 2019, 1, 66–72. [Google Scholar] [CrossRef]
- Fan, Z.; Liao, X.; Tian, Y.; Xie, X.; Nie, Y. A prevascularized nerve conduit based on a stem cell sheet effectively promotes the repair of transected spinal cord injury. Acta Biomater. 2020, 101, 304–313. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, C.; Liu, F.; Xie, S.; Qu, J.; Li, M.; Li, Z.; Li, X.; Shi, Q.; Li, S.; et al. Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials 2020, 241, 119837. [Google Scholar] [CrossRef]
- Alexandrushkina, N.; Nimiritsky, P.; Eremichev, R.; Popov, V.; Arbatskiy, M.; Danilova, N.; Malkov, P.; Akopyan, Z.; Tkachuk, V.; Makarevich, P. Cell sheets from adipose tissue MSC induce healing of pressure ulcer and prevent fibrosis via trigger effects on granulation tissue growth and vascularization. Int. J. Mol. Sci. 2020, 21, 5567. [Google Scholar] [CrossRef]
- Imafuku, A.; Oka, M.; Miyabe, Y.; Sekiya, S.; Nitta, K.; Shimizu, T. Rat mesenchymal stromal cell sheets suppress renal fibrosis via microvascular protection. Stem Cells Transl. Med. 2019, 8, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Itaba, N.; Kono, Y.; Watanabe, K.; Yokobata, T.; Oka, H.; Osaki, M.; Kakuta, H.; Morimoto, M.; Shiota, G. Reversal of established liver fibrosis by IC-2-engineered mesenchymal stem cell sheets. Sci. Rep. 2019, 9, 6841. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Yaji, N.; Yamato, M.; Yang, J.; Okano, T.; Hori, S. Transplantation of tissue-engineered retinal pigment epithelial cell sheets in a rabbit model. Biomaterials 2009, 30, 797–803. [Google Scholar] [CrossRef]
- Jia, Y.; Li, W.; Duan, H.; Li, Z.; Zhou, Q.; Shi, W. Mini-sheet injection for cultured corneal endothelial transplantation. Tissue Eng. Part C Methods 2018, 24, 474–479. [Google Scholar] [CrossRef]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating eye tissues to preserve and restore vision. Cell Stem Cell 2018, 22, 834–849. [Google Scholar] [CrossRef]
- Catalán, M.A.; Kondo, Y.; Peña-Munzenmayer, G.; Jaramillo, Y.; Liu, F.; Choi, S.; Crandall, E.; Borok, Z.; Flodby, P.; Shull, G.E.; et al. A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc. Natl. Acad. Sci. USA 2015, 112, 2263–2268. [Google Scholar] [CrossRef]
- Jørgensen, P.L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na++K+)-ATPase. Biochim. Biophys. Acta 1982, 694, 27–68. [Google Scholar] [CrossRef]
- Sumide, T.; Nishida, K.; Yamato, M.; Ide, T.; Hayashida, Y.; Watanabe, K.; Yang, J.; Kohno, C.; Kikuchi, A.; Maeda, N.; et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 2006, 20, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, J.U.N.; Gao, Y.; Yang, L.E.I. Cell sheet technology: A promising strategy in regenerative medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Akahane, M.; Shigematsu, H.; Tadokoro, M.; Morita, Y.; Ohgushi, H.; Dohi, Y.; Imamura, T.; Tanaka, Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone 2010, 46, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Akahane, M.; Nakamura, A.; Ohgushi, H.; Shigematsu, H.; Dohi, Y.; Takakura, Y. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J. Tissue Eng. Regen. Med. 2008, 2, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.P. Salivary (SD-Type) cystatins: Over one billion years in the making-but to what purpose? Crit. Rev. Oral Biol. Med. 2002, 13, 485–508. [Google Scholar] [CrossRef]
- Baker, O.J. Tight junctions in salivary epithelium. J. Biomed. Biotechnol. 2010, 2010, 278948. [Google Scholar] [CrossRef]
- Samiei, M.; Ahmadian, E.; Eftekhari, A.; Eghbal, M.A.; Rezaie, F.; Vinken, M. Cell junctions and oral health. EXCLI J. 2019, 18, 317–330. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Memon, I.A.; Sawa, Y.; Fukushima, N.; Matsumiya, G.; Miyagawa, S.; Taketani, S.; Sakakida, S.K.; Kondoh, H.; Aleshin, A.N.; Shimizu, T.; et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J. Thorac. Cardiovasc. 2005, 130, 1333–1341. [Google Scholar] [CrossRef]
- Narita, T.; Shintani, Y.; Ikebe, C.; Kaneko, M.; Campbell, N.G.; Coppen, S.R.; Uppal, R.; Sawa, Y.; Yashiro, K.; Suzuki, K. The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mol. Ther. 2013, 21, 860–867. [Google Scholar] [CrossRef]
- Jayasinghe, N.R.; Cope, G.H.; Jacob, S. Morphometric studies on the development and sexual dimorphism of the submandibular gland of the mouse. J. Anat. 1990, 172, 115–127. [Google Scholar] [PubMed]
- Denny, P.C.; Denny, P.A. Dynamics of parenchymal cell division, differentiation, and apoptosis in the young adult female mouse submandibular gland. Anat. Rec. 1999, 254, 408–417. [Google Scholar] [CrossRef]
- Pinkstaff, C.A. Salivary gland sexual dimorphism: A brief review. Eur. J. Morphol. 1998, 36, 31–34. [Google Scholar] [PubMed]
- Brown, C.T.; Nam, K.; Zhang, Y.; Qiu, Y.; Dean, S.M.; Dos Santos, H.T.; Lei, P.; Andreadis, S.T.; Baker, O.J. Sex-dependent regeneration patterns in mouse submandibular glands. J. Histochem. Cytochem. 2020, 68, 305–318. [Google Scholar] [CrossRef]
- Varghese, J.J.; Schmale, I.L.; Mickelsen, D.; Hansen, M.E.; Newlands, S.D.; Benoit, D.S.W.; Korshunov, V.A.; Ovitt, C.E. Localized delivery of amifostine enhances salivary gland radioprotection. J. Dent. Res. 2018, 97, 1252–1259. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, H.T.; Kim, K.; Okano, T.; Camden, J.M.; Weisman, G.A.; Baker, O.J.; Nam, K. Cell Sheets Restore Secretory Function in Wounded Mouse Submandibular Glands. Cells 2020, 9, 2645. https://doi.org/10.3390/cells9122645
dos Santos HT, Kim K, Okano T, Camden JM, Weisman GA, Baker OJ, Nam K. Cell Sheets Restore Secretory Function in Wounded Mouse Submandibular Glands. Cells. 2020; 9(12):2645. https://doi.org/10.3390/cells9122645
Chicago/Turabian Styledos Santos, Harim T., Kyungsook Kim, Teruo Okano, Jean M. Camden, Gary A. Weisman, Olga J. Baker, and Kihoon Nam. 2020. "Cell Sheets Restore Secretory Function in Wounded Mouse Submandibular Glands" Cells 9, no. 12: 2645. https://doi.org/10.3390/cells9122645
APA Styledos Santos, H. T., Kim, K., Okano, T., Camden, J. M., Weisman, G. A., Baker, O. J., & Nam, K. (2020). Cell Sheets Restore Secretory Function in Wounded Mouse Submandibular Glands. Cells, 9(12), 2645. https://doi.org/10.3390/cells9122645