Effect of SUV39H1 Histone Methyltransferase Knockout on Expression of Differentiation-Associated Genes in HaCaT Keratinocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. SUV39H1 Knockout Cells
2.3. Sample Preparation, Western Blot, and Dot Blot
2.4. Immunocytochemistry (ICCH)
2.5. Quantitative Reverse Transcription PCR (RT-qPCR)
2.6. Chromatin Immunoprecipitation (ChIP)
2.7. Cell Adhesion Assays
2.8. Statistical Analysis
3. Results
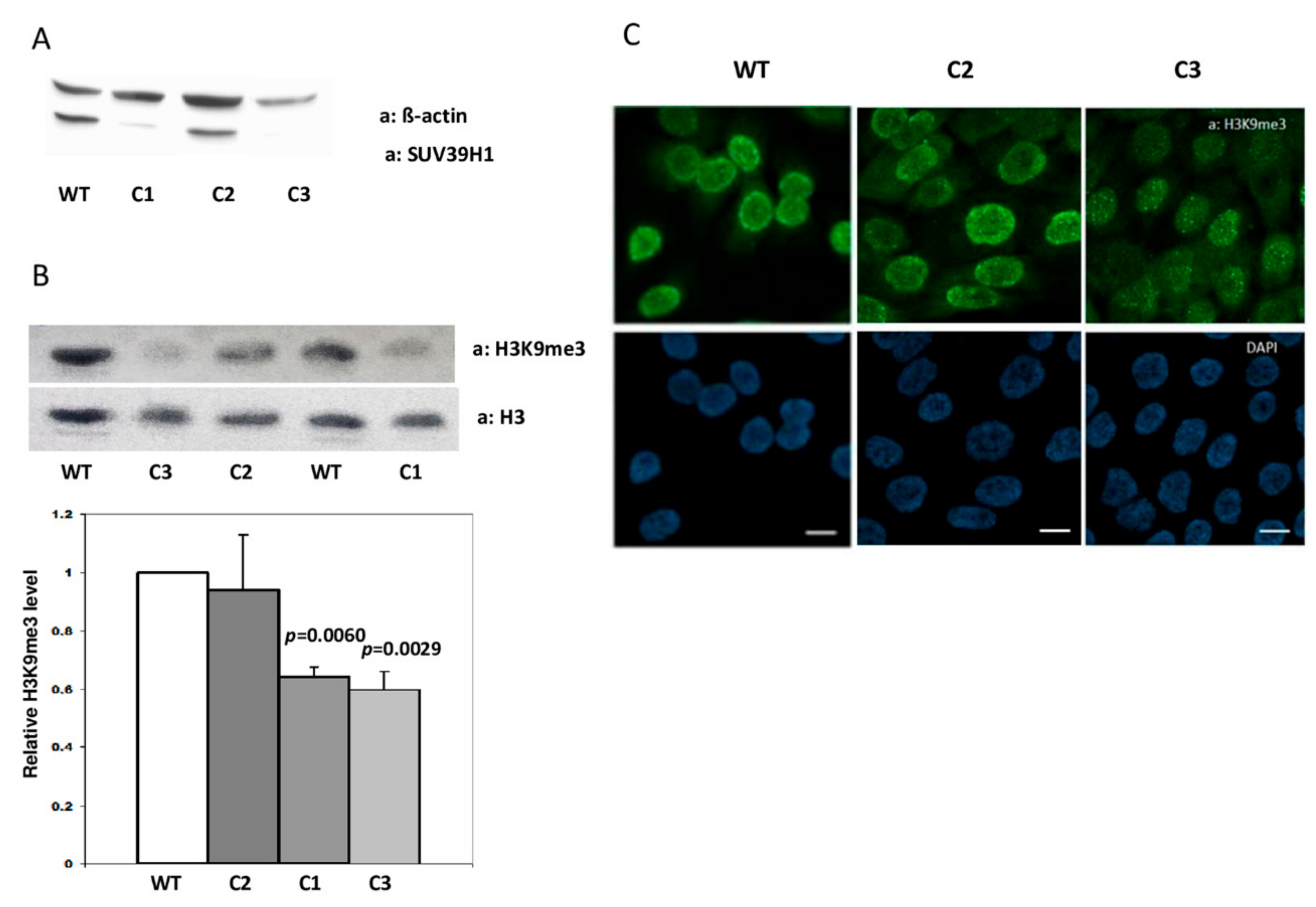
3.1. Generation of HaCaT Cells with Knockout of SUV39H1 Histone Methyltransferase Using the CRISPR/Cas9 Approach
3.2. Changes in Expression of Selected EDC Genes and Non-EDC-Encoded Keratinocyte Differentiation Markers in SUV39H1-KO HaCaT Cells
3.3. Changes in Histone Modifications at LCE1 Promoters during Keratinocyte Differentiation
3.4. Changes in Cell-to-Cell and Cell-to-Surface Adhesion of SUV39H1-KO HaCaT Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef]
- Bowden, P.E. The human type II keratin gene cluster on chromosome 12q13.13: Final count or hidden secrets? J. Investig. Dermatol. 2005, 124. [Google Scholar] [CrossRef] [PubMed]
- Mischke, D.; Korge, B.P.; Marenholz, I.; Volz, A.; Ziegler, A. Genes encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex “epidermal differentiation complex” on human chromosome 1q21. J. Investig. Dermatol. 1996, 106, 989–992. [Google Scholar] [CrossRef]
- Kypriotou, M.; Huber, M.; Hohl, D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the “fused genes” family. Exp. Dermatol. 2012, 2, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, W.; Graczyk-Jarzynka, A. The S100 proteins in epidermis: Topology and function. Biochim. Biophysica. Acta 2015, 1850, 2563–2572. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Takaishi, M.; Morohashi, M.; Huh, N. Hornerin, a novel profilaggrin-like protein and differentiation-specific marker isolated from mouse skin. J. Biol. Chem. 2001, 276, 47445–47452. [Google Scholar] [CrossRef]
- Hohl, D.; de Viragh, P.A.; Amiguet-Barras, F.; Gibbs, S.; Backendorf, C.; Huber, M. The small proline-rich proteins constitute a multigene family of differentially regulated cornified cell envelope precursor proteins. J. Investig. Dermatol. 1995, 104, 902–909. [Google Scholar] [CrossRef]
- Marshall, D.; Hardman, M.J.; Nield, K.M.; Byrne, C. Differentially expressed late constituents of the epidermal cornified envelope. Proc. Natl. Acad. Sci. USA 2001, 98, 13031–13036. [Google Scholar] [CrossRef]
- Zhang, X. Genome-wide association study of skin complex diseases. J. Dermatol. Sci. 2012, 66, 89–97. [Google Scholar] [CrossRef]
- Truong, A.B.; Kretz, M.; Ridky, T.W.; Kimmel, R.; Khavari, P.A. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006, 20, 3185–3197. [Google Scholar] [CrossRef]
- Boglev, Y.; Wilanowski, T.; Caddy, J.; Parekh, V.; Auden, A.; Darido, C.; Hislop, N.R.; Cangkrama, M.; Ting, S.B.; Jane, S.M. The unique and cooperative roles of the Grainy head-like transcription factors in epidermal development reflect unexpected target gene specificity. Dev. Biol. 2011, 349, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Adhikary, G.; Christina, A.; Young, C.A.; Jans, R.; Crish, J.F.; Xu, W.; Ellen, A.; Rorke, E.A. AP1 transcription factors in epidermal differentiation and skin cancer. J. Ski. Cancer 2013, 537028. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Gdula, M.R.; Mardaryev, A.N.; Sharov, A.A.; Fessing, M.Y. Epigenetic regulation of gene expression in keratinocytes. J. Investig. Dermatol. 2012, 132, 2505–2521. [Google Scholar] [CrossRef]
- Sen, G.L.; Reuter, J.A.; Webster, D.E.; Zhu, L.; Khavari, P.A. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 2010, 463, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Sobiak, B.; Graczyk-Jarzynka, A.; Leśniak, W. Comparison of DNA methylation and expression pattern of S100 and other epidermal differentiation complex (EDC) genes in differentiating keratinocytes. J. Cell. Biochem. 2016, 117, 1092–1098. [Google Scholar] [CrossRef]
- Sobiak, B.; Leśniak, W. The effect of single CpG demethylation on the pattern of DNA- protein binding. Int. J. Mol. Sci. 2019, 20, 914. [Google Scholar] [CrossRef]
- Smits, J.P.H.; Dirks, R.A.M.; Qu, J.; Oortveld, M.A.W.; Brinkman, A.B.; Zeeuwen, P.L.J.M.; Schalkwijk, J.; Zhou, H.; Marks, H.; van den Bogaard, E.H. Terminal keratinocyte differentiation in vitro is associated with a stable DNA methylome. Exp. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, A.; Miccio, A.; Romano, O.; Petiti, L.; Malagoli Tagliazucchi, G.; Peano, C.; Severgnini, M.; Rizzi, E.; De Bellis, G.; Bicciato, S.; et al. Dynamic transcriptional and epigenetic regulation of human epidermal keratinocyte differentiation. Stem Cell Rep. 2016, 6, 618–632. [Google Scholar] [CrossRef]
- Ezhkova, E.; Pasolli, H.A.; Parker, J.S.; Stokes, N.; Su, I.H.; Hannon, G.; Tarakhovsky, A.; Fuchs, E. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 2009, 136, 1122–1135. [Google Scholar] [CrossRef]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]
- Graczyk, A.; Leśniak, W. S100A6 expression in keratinocytes and its impact on epidermal differentiation. Int. J. Biochem. Cell Biol. 2014, 57, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Graczyk-Jarzynka, A.; Sobiak, B.; Mlącki, M.; Wilanowski, T.; Leśniak, W. S100A6 activates EGFR and its downstream signaling in HaCaT keratinocytes. J. Cell. Physiol. 2019, 234, 17561–17569. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.S.; Varadaraj, K. Intact AQp0 performs cell-to-cell adhesion. Biochem. Biophys. Res. Commun. 2009, 390, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Green, H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 1980, 19, 1033–1042. [Google Scholar] [CrossRef]
- Park, G.T.; Lim, S.E.; Morasso, M.I. Suprabasin, a novel epidermal differentiation marker and potential cornified envelope precursor. J. Biol. Chem. 2002, 277, 45195–45202. [Google Scholar] [CrossRef] [PubMed]
- Amagai, M.; Stanley, J.R. Desmoglein as a target in skin disease and beyond. J. Investig. Dermatol. 2012, 132, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Walko, G.; Castanon, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 370, 529–544. [Google Scholar] [CrossRef]
- Peters, A.H.; O’Carroll, D.; Scherthan, H.; Mechtler, K.; Sauer, S.; Schöfer, C.; Weipoltshammer, K.; Pagani, M.; Lachner, M.; Kohlmaier, A.; et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 2001, 107, 323–337. [Google Scholar] [CrossRef]
- Bulut-Karslioglu, A.; De La Rosa-Velazquez, I.A.; Ramirez, F.; Barenboim, M.; Onishi-Seebacher, M.; Arand, J.; Galan, C.; Winter, G.E.; Engist, B.; Gerle, B.; et al. Suv39h-dependent H3K9me3 marks intact retrotransposons and silences LINE elements in mouse embryonic stem cells. Mol. Cell 2014, 55, 277–290. [Google Scholar] [CrossRef]
- García-Cao, M.; O’Sullivan, R.; Peters, A.H.F.M.; Jenuwein, T.; Blasco, M.A. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat. Genet. 2004, 36, 94–99. [Google Scholar] [CrossRef]
- Petti, E.; Jordi, F.; Buemi, V.; Dinami, R.; Benetti, R.; Blasco, M.A.; Schoeftner, S. Altered telomere homeostasis and resistance to skin carcinogenesis in Suv39h1 transgenic mice. Cell Cycle 2014, 14, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, S.; Kagey, M.H.; Frampton, G.M.; Rahl, P.B.; Young, R.A. SetDB1 contributes to repression of genes encoding developmental regulators and maintenance of ES cell state. Genes Dev. 2009, 23, 2484–2489. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.D.; Hon, G.C.; Lee, L.K.; Ngo, Q.; Lister, R.; Pelizzola, M.; Edsall, L.E.; Kuan, S.; Luu, Y.; Klugman, S.; et al. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell 2010, 6, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Ait-Si-Ali, S.; Guasconi, V.; Fritsch, L.; Yahi, H.; Sekhri, R.; Naguibneva, I.; Robin, P.; Cabon, F.; Polesskaya, A.; Harel-Bellan, A. A Suv39h-dependent mechanism for silencing S-phase genes in differentiating but not in cycling cells. EMBO J. 2004, 23, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Mardaryev, A.N. Epigenetic regulation in skin wound healing. In Epigenetic Regulation of Skin Development and Regeneration. Stem Cell Biology and Regenerative Medicine; Botchkarev, V., Millar, S., Eds.; Humana Press: Cham, Switzerland, 2014; pp. 293–314. [Google Scholar]
- Martin, M.J.; Estravis, M.; Garcia-Sanchez, A.; Davila, I.; Isidoro-Garcia, M.; Sanz, C. Genetics and epigenetics of atopic dermatitis: An updated systematic review. Genes 2010, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.; Fisher, A.G.; Watt, F.M. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS ONE 2007, 2, e763. [Google Scholar] [CrossRef] [PubMed]
- Gannon, O.M.; Merida de Long, L.; Saunders, N.A. DUX4 is derepressed in late-differentiating keratinocytes in conjunction with loss of H3K9me3 epigenetic repression. J. Investig. Dermatol. 2016, 136, 1299–1302. [Google Scholar] [CrossRef]
- Jagannathan, V.; Bannoehr, J.; Plattet, P.; Hauswirth, R.; Drögemüller, C.; Drögemüller, M.; Wiener, D.J.; Doherr, M.; Owczarek-Lipska, M.; Galichet, A.; et al. A mutation in the SUV39H2 gene in Labrador Retrievers with hereditary nasal parakeratosis (HNPK) provides insights into the epigenetics of keratinocyte differentiation. PLoS Genet 2013, 9, e1003848. [Google Scholar] [CrossRef]
- Bannoehr, J.; Balmer, P.; Stoffel, M.H.; Jagannathan, V.; Gaschen, V.; Kuhni, K.; Sayar, B.; Drogemuller, M.; Howald, D.; Wieber, D.J.; et al. Abnormal keratinocyte differentiation in the nasal planum of labrador retrievers with hereditary nasal parakeratosis (HNPK). PLoS ONE 2020, 15, e0225901. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Huebner, A.J.; Rice, R.H.; Koch, P.J.; Speransky, V.V.; Steven, A.C.; Roop, D.R. Lce1 Family Members Are Nrf2-Target Genes that Are Induced to Compensate for the Loss of Loricrin. J. Investig. Dermatol. 2016, 136, 1656–1663. [Google Scholar] [CrossRef]
- Jackson, B.; Brown, S.J.; Avilion, A.A.; O’Shaughnessy, R.F.L.; Sully, K.; Akinduro, O.; Murphy, M.; Cleary, M.L.; Byrne, C. TALE homeodomain proteins regulate site-specific terminal differentiation, LCE genes and epidermal barrier. J. Cell Sci. 2011, 124, 1681–1690. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, B.; Tilli, C.M.; Hardman, M.J.; Avilion, A.A.; MacLeod, M.C.; Ashcroft, G.S.; Byrne, C. Late cornified envelope family in differentiating epithelia—Response to calcium and ultraviolet irradiation. J. Investig. Dermatol. 2005, 124, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, C.; Voss, A.; Scholzen, T.E.; Averill, M.M.; Zänker, K.S.; Bornfeldt, K.E. Novel insights into the role of S100A8/A9 in skin biology. Exp. Dermatol. 2012, 21, 822–826. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobiak, B.; Leśniak, W. Effect of SUV39H1 Histone Methyltransferase Knockout on Expression of Differentiation-Associated Genes in HaCaT Keratinocytes. Cells 2020, 9, 2628. https://doi.org/10.3390/cells9122628
Sobiak B, Leśniak W. Effect of SUV39H1 Histone Methyltransferase Knockout on Expression of Differentiation-Associated Genes in HaCaT Keratinocytes. Cells. 2020; 9(12):2628. https://doi.org/10.3390/cells9122628
Chicago/Turabian StyleSobiak, Barbara, and Wiesława Leśniak. 2020. "Effect of SUV39H1 Histone Methyltransferase Knockout on Expression of Differentiation-Associated Genes in HaCaT Keratinocytes" Cells 9, no. 12: 2628. https://doi.org/10.3390/cells9122628
APA StyleSobiak, B., & Leśniak, W. (2020). Effect of SUV39H1 Histone Methyltransferase Knockout on Expression of Differentiation-Associated Genes in HaCaT Keratinocytes. Cells, 9(12), 2628. https://doi.org/10.3390/cells9122628




