Cytokines TNFα, IFNγ and IL-2 Are Responsible for Signal Transmission from the Innate Immunity Protein Tag7 (PGLYRP1) to Cytotoxic Effector Lymphocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Magnetic Bead-Based Cell Separation
2.3. Affinity Chromatography, Immunoadsorption and Western Blotting
2.4. Activating Agents
2.5. Cytotoxicity Assay
2.6. Antibodies and Inhibitors
2.7. ELISA
2.8. qPCR
2.9. Statistical Analysis
3. Results
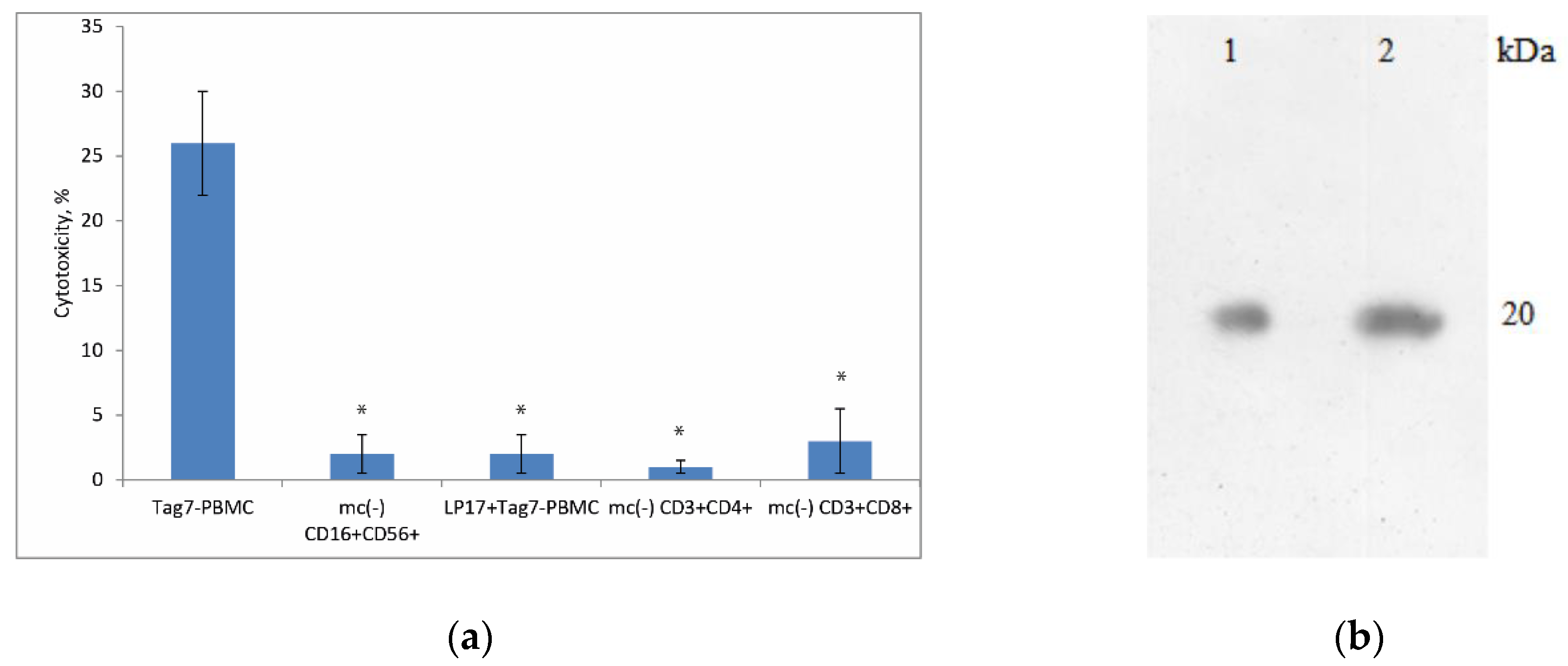
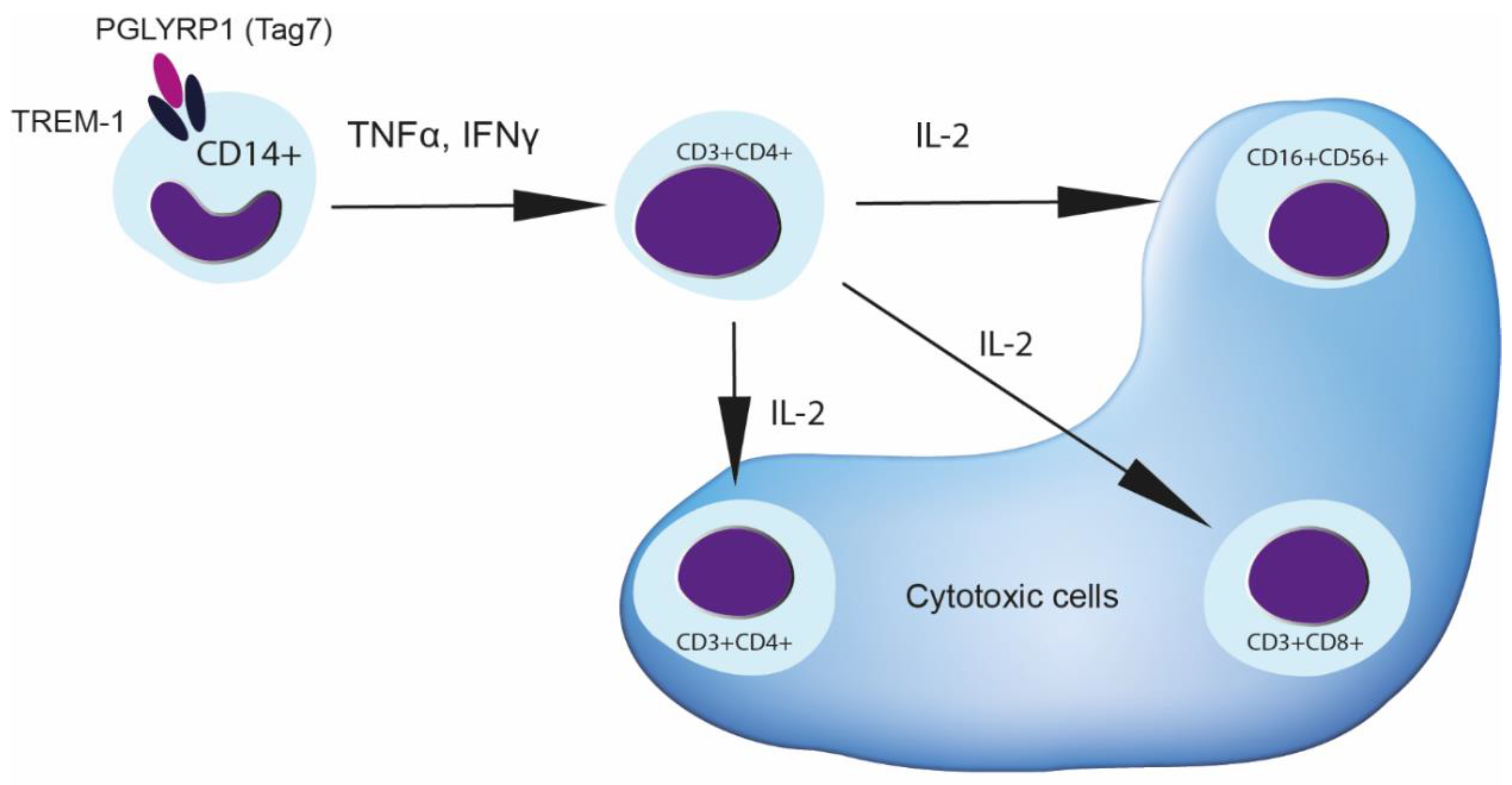
3.1. Treatment of PBMCs with Tag7 Results in the Formation of Cytotoxic Subpopulations of NK (CD16+CD56+), CD3+CD4+ and CD3+CD8+ Lymphocytes, But Only If the PBMC Pool Contains Monocytes
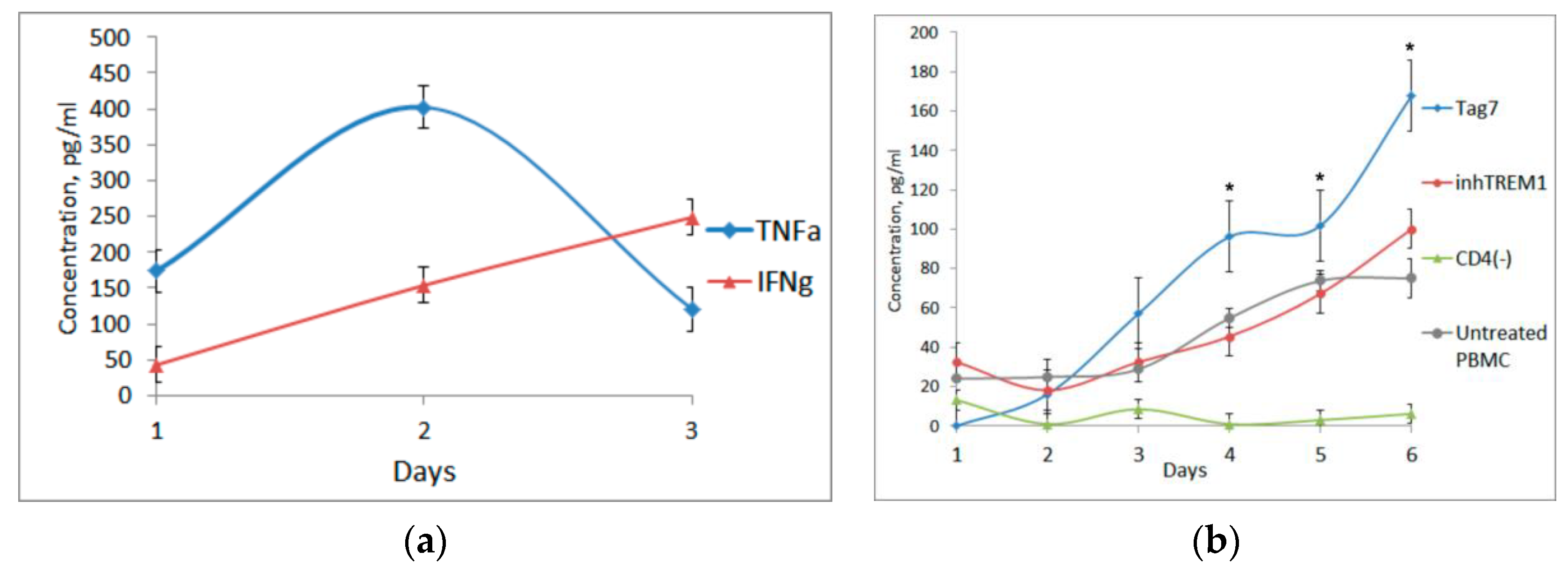
3.2. Tag7 Stimulates Secretion of Cytokines TNFα, IFNγ and IL-2
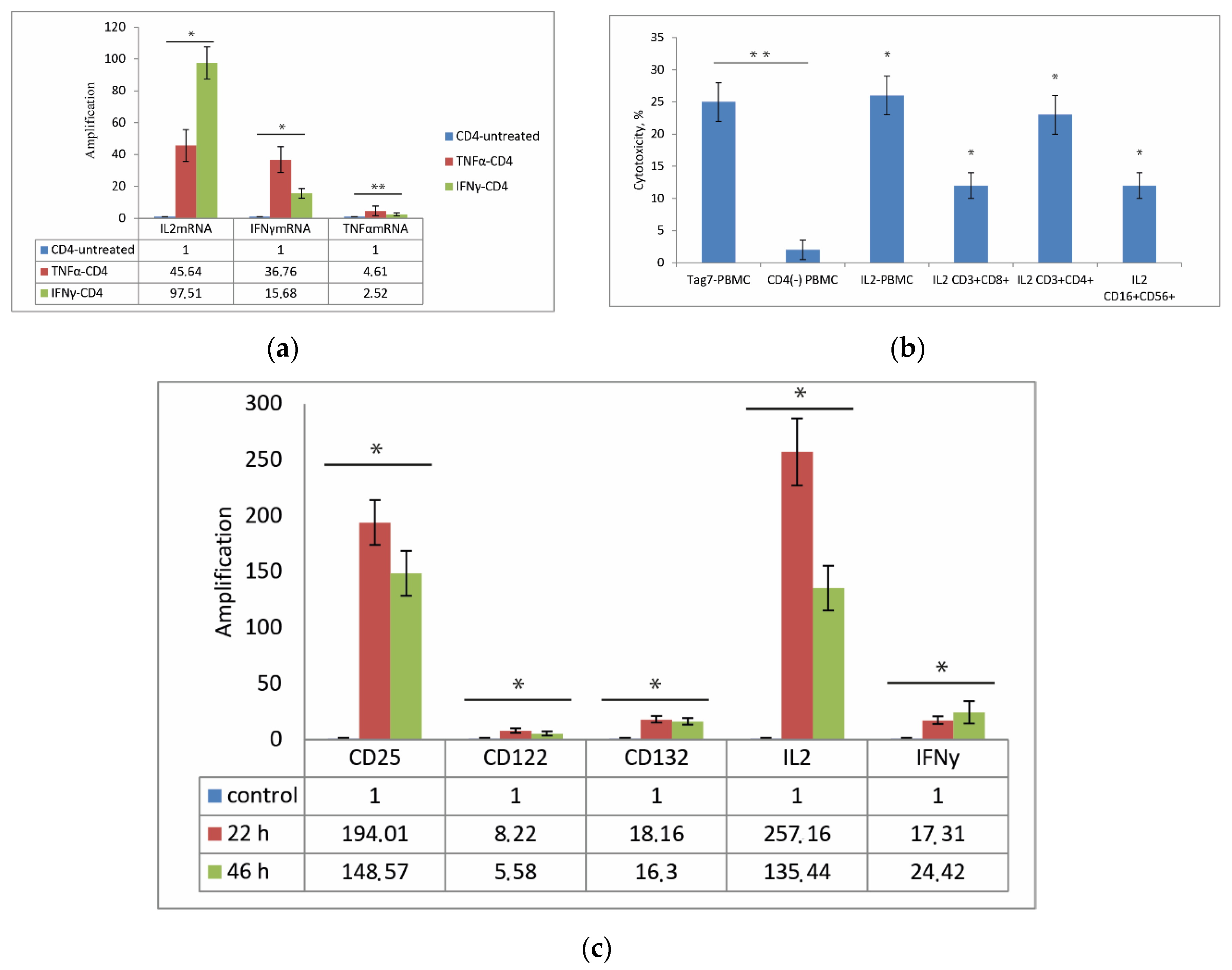
3.3. CD3+CD4+ Lymphocytes Are the Main Source of IL-2 and Are Necessary for the Formation of Each Cytotoxic Subpopulation
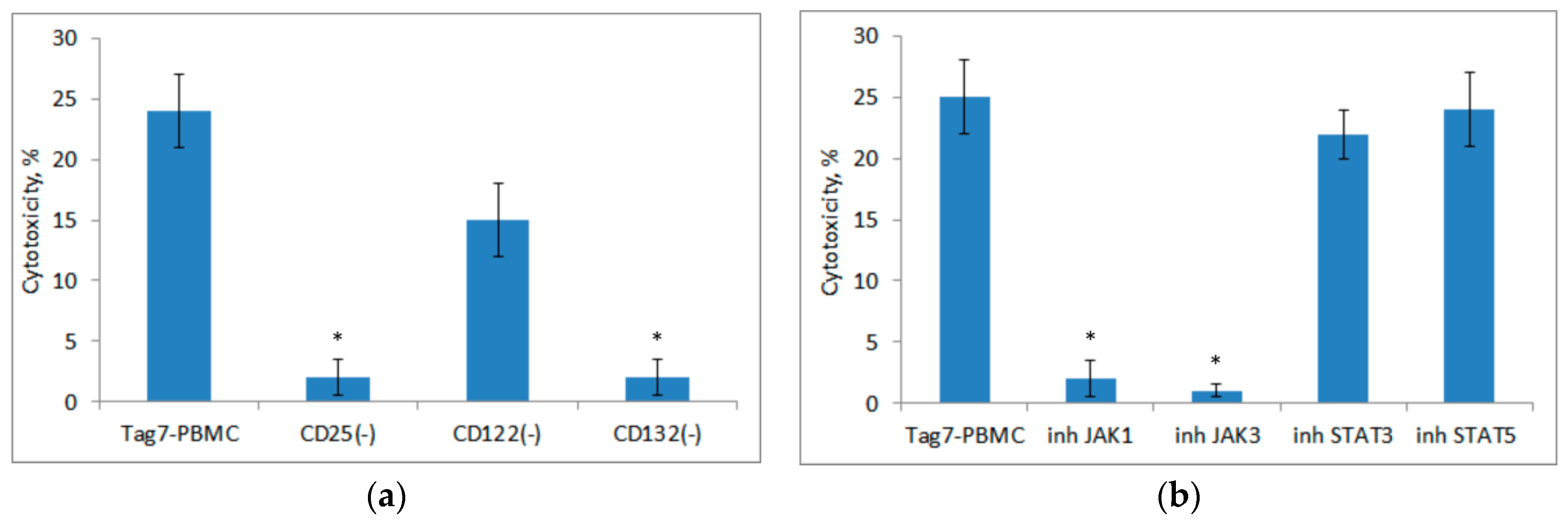
3.4. Role of IL-2 Receptor in the Induction of Cytotoxic Lymphocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kang, D.; Liu, G.; Lundström, A.; Gelius, E.; Steiner, H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. USA 1998, 95, 10078–10082. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Fraser, I.P.; Fukase, K.; Kusumoto, S.; Fujimoto, Y.; Stahl, G.L.; Ezekowitz, R.A.B. Human peptidoglycan recognition protein S is an effector of neutrophil-mediated innate immunity. Blood 2005, 106, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Z.; Gupta, D.; Dziarski, R. Peptidoglycan recognition proteins: A novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem. 2001, 276, 34686–34694. [Google Scholar] [CrossRef] [PubMed]
- Sashchenko, L.P.; Dukhanina, E.A.; Yashin, D.V.; Shatalov, Y.V.; Romanova, E.A.; Korobko, E.V.; Demin, A.V.; Lukyanova, T.I.; Kabanova, O.D.; Khaidukov, S.V.; et al. Peptidoglycan Recognition Protein Tag7 Forms a Cytotoxic Complex with Heat Shock Protein 70 in Solution and in Lymphocytes. J. Biol. Chem. 2004, 279, 2117–2124. [Google Scholar] [CrossRef]
- Yashin, D.V.; Ivanova, O.K.; Soshnikova, N.V.; Sheludchenkov, A.A.; Romanova, E.A.; Dukhanina, E.A.; Tonevitsky, A.G.; Gnuchev, N.V.; Gabibov, A.G.; Georgiev, G.P.; et al. Tag7 (PGLYRP1) in Complex with Hsp70 Induces Alternative Cytotoxic Processes in Tumor Cells via TNFR1 Receptor. J. Biol. Chem. 2015, 290, 21724–21731. [Google Scholar] [CrossRef]
- Sashchenko, L.P.; Dukhanina, E.A.; Shatalov, Y.V.; Yashin, D.V.; Lukyanova, T.I.; Kabanova, O.D.; Romanova, E.A.; Khaidukov, S.V.; Galkin, A.V.; Gnuchev, N.V.; et al. Cytotoxic T lymphocytes carrying a pattern recognition protein Tag7 can detect evasive, HLA-negative but Hsp70-exposing tumor cells, thereby ensuring FasL/Fas-mediated contact killing. Blood 2007, 110, 1997–2004. [Google Scholar] [CrossRef]
- Sharapova, T.N.; Ivanova, O.K.; Soshnikova, N.V.; Romanova, E.A.; Sashchenko, L.P.; Yashin, D.V. Innate Immunity Protein Tag7 Induces 3 Distinct Populations of Cytotoxic Cells That Use Different Mechanisms to Exhibit Their Antitumor Activity on Human Leukocyte Antigen-Deficient Cancer Cells. J. Innate Immun. 2017, 9, 598–608. [Google Scholar] [CrossRef]
- Gibot, S.; Cravoisy, A.; Kolopp-Sarda, M.-N.; Béné, M.-C.; Faure, G.; Bollaert, P.-E.; Levy, B. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit. Care Med. 2005, 33, 792–796. [Google Scholar] [CrossRef]
- Dower, K.; Ellis, D.K.; Saraf, K.; Jelinsky, S.A.; Lin, L.-L. Innate Immune Responses to TREM-1 Activation: Overlap, Divergence, and Positive and Negative Cross-Talk with Bacterial Lipopolysaccharide. J. Immunol. 2008, 180, 3520–3534. [Google Scholar] [CrossRef]
- Chu, W.-M. Tumor necrosis factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF–TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Stetson, D.B.; Mohrs, M.; Reinhardt, R.L.; Baron, J.L.; Wang, Z.-E.; Gapin, L.; Kronenberg, M.; Locksley, R.M. Constitutive Cytokine mRNAs Mark Natural Killer (NK) and NK T Cells Poised for Rapid Effector Function. J. Exp. Med. 2003, 198, 1069–1076. [Google Scholar] [CrossRef]
- Cote-Sierra, J.; Foucras, G.; Guo, L.; Chiodetti, L.; Young, H.A.; Hu-Li, J.; Zhu, J.; Paul, W.E. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 3880–3885. [Google Scholar] [CrossRef]
- Reem, G.H.; Yeh, N.H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science 1984, 225, 429–430. [Google Scholar] [CrossRef]
- Romanova, E.A.; Dukhanina, E.A.; Sharapova, T.N.; Sashchenko, L.P.; Gnuchev, N.V.; Yashin, D.V. Lymphocytes incubated in the presence of IL-2 lose the capacity for chemotaxis but acquire antitumor activity. Dokl. Biol. Sci. 2017, 472, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Read, C.B.; Kuijper, J.L.; Hjorth, S.A.; Heipel, M.D.; Tang, X.; Fleetwood, A.J.; Dantzler, J.L.; Grell, S.N.; Kastrup, J.; Wang, C.; et al. Cutting Edge: Identification of Neutrophil PGLYRP1 as a Ligand for TREM-1. J. Immunol. 2015, 194, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Dayer, J.-M. The role of human T-lymphocyte-monocyte contact in inflammation and tissue destruction. Arthritis Res. 2002, 4 (Suppl. 3), S169–S176. [Google Scholar] [CrossRef] [PubMed]
- Sojka, R.K.; Bruniquel, D.; Schwartz, R.H.; Singh, N.J. IL-2 Secretion by CD4+ T Cells In Vivo Is Rapid, Transient, and Influenced by TCR-Specific Competition. J. Immunol. 2004, 172, 6136–6143. [Google Scholar] [CrossRef]
- Nelson, B.H.; Willerford, D.M. Biology of the Interleukin-2 Receptor. Adv. Immunol. 1998, 70, 1–81. [Google Scholar] [CrossRef]
- Johnston, J.A.; Bacon, C.M.; Riedy, M.; O’Shea, J.J. Signaling by IL-2 and related cytokines: JAKs, STATs, and relationship to immunodeficiency. J. Leukoc. Biol. 1996, 60, 441–452. [Google Scholar] [CrossRef]
- Taniguchi, T.; Miyazaki, T.; Minami, Y.; Kawahara, A.; Fujii, H.; Nakagawa, Y.; Hatakeyama, M.; Liu, Z.-J. IL-2 Signaling Involves Recruitment and Activation of Multiple Protein Tyrosine Kinases by the IL-2 Receptor. Ann. N. Y. Acad. Sci. 1995, 766, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.H. IL-2, Regulatory T Cells, and Tolerance. J. Immunol. 2004, 172, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharapova, T.N.; Romanova, E.A.; Ivanova, O.K.; Sashchenko, L.P.; Yashin, D.V. Cytokines TNFα, IFNγ and IL-2 Are Responsible for Signal Transmission from the Innate Immunity Protein Tag7 (PGLYRP1) to Cytotoxic Effector Lymphocytes. Cells 2020, 9, 2602. https://doi.org/10.3390/cells9122602
Sharapova TN, Romanova EA, Ivanova OK, Sashchenko LP, Yashin DV. Cytokines TNFα, IFNγ and IL-2 Are Responsible for Signal Transmission from the Innate Immunity Protein Tag7 (PGLYRP1) to Cytotoxic Effector Lymphocytes. Cells. 2020; 9(12):2602. https://doi.org/10.3390/cells9122602
Chicago/Turabian StyleSharapova, Tatiana N., Elena A. Romanova, Olga K. Ivanova, Lidia P. Sashchenko, and Denis V. Yashin. 2020. "Cytokines TNFα, IFNγ and IL-2 Are Responsible for Signal Transmission from the Innate Immunity Protein Tag7 (PGLYRP1) to Cytotoxic Effector Lymphocytes" Cells 9, no. 12: 2602. https://doi.org/10.3390/cells9122602
APA StyleSharapova, T. N., Romanova, E. A., Ivanova, O. K., Sashchenko, L. P., & Yashin, D. V. (2020). Cytokines TNFα, IFNγ and IL-2 Are Responsible for Signal Transmission from the Innate Immunity Protein Tag7 (PGLYRP1) to Cytotoxic Effector Lymphocytes. Cells, 9(12), 2602. https://doi.org/10.3390/cells9122602





