The Role of Mitochondrial Fat Oxidation in Cancer Cell Proliferation and Survival
Abstract
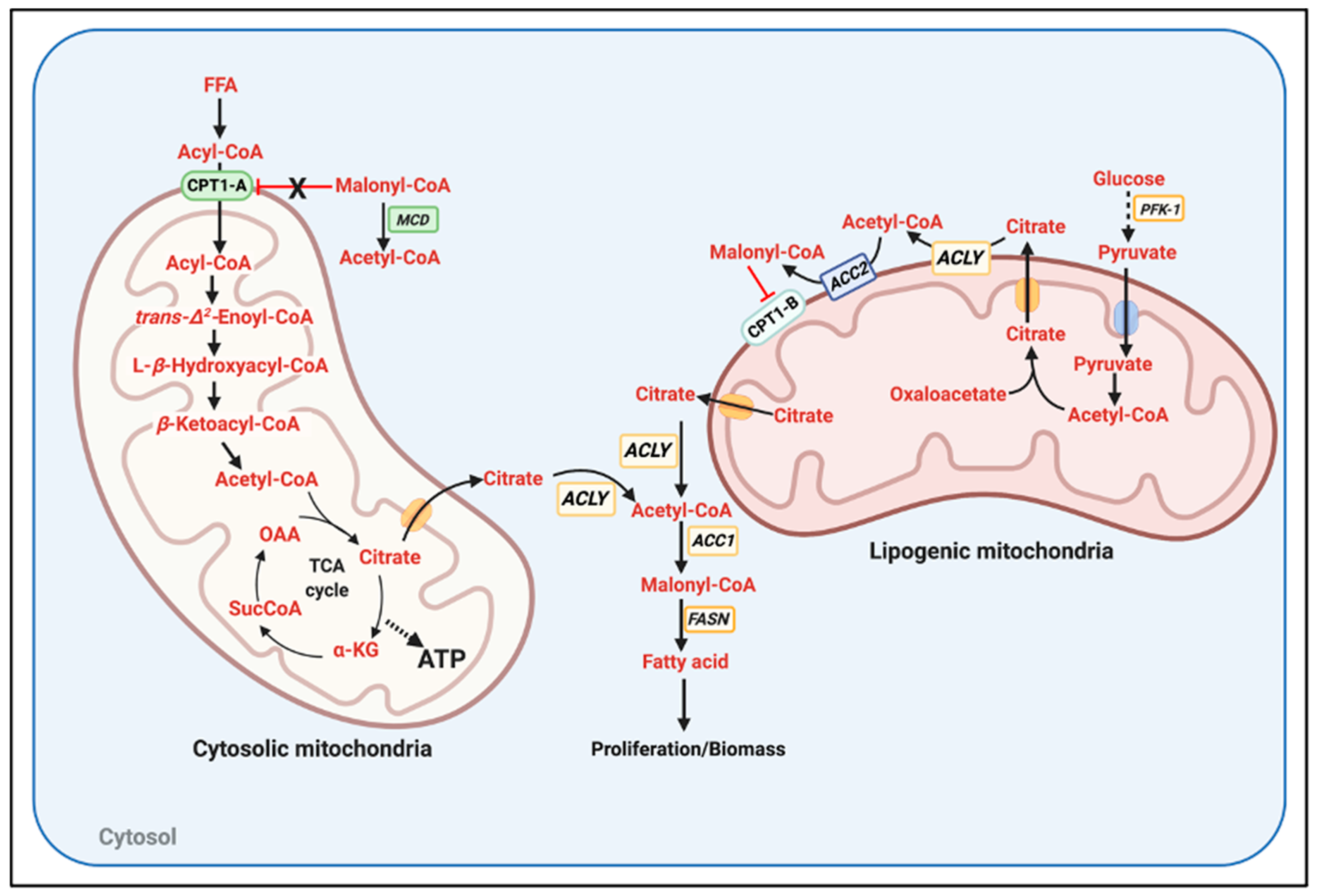
1. The Randle Cycle: A Hormone Independent Mechanism Linking Nutrient Availability to Anabolism and Catabolism
- (1)
- The mechanisms regulating ACLY, ACC1, and ACC2 activity and their subcellular distribution. Particularly: (a) How ACC2 association to the mitochondria is regulated; (b) whether ACLY could be located in close apposition to the mitochondria; (c) whether ACC1 can be kept far from mitochondria; and (d) how increased FAO blocks the activity of ACLY, ACC1, and ACC2 even in the presence of high glucose and independently of transcriptional changes. One can hypothesize that if citrate is not broken down close to ACC2 and mitochondria, this citrate might be preferentially routed toward FA synthesis.
- (2)
- The mechanisms determining FAO sensitivity to malonyl-CoA-mediated inhibition. A major process determining this sensitivity could be the regulation of CPT1-A and CPT1-B protein ratio in each tissue. If malonyl-CoA does not reach concentrations in the 100 µM range (IC50 for CPT1-A) in a CPT1-A-expressing tissue, FAO could still proceed despite elevated glycolysis.
- (3)
- The activity and subcellular distribution of malonyl-CoA decarboxylase (MCD), particularly the potential regulation of MCD apposition to mitochondria. The enzymatic degradation of malonyl-CoA close to the mitochondrial outer membrane could explain how some tissues might concurrently execute FAO and FA synthesis.
- (4)
- A major mechanism by which glucose restriction activates FAO and decreases malonyl-CoA synthesis is through an inhibitory phosphorylation of ACC1 and 2 executed by AMPK, a kinase activated when ATP levels drop [7]. However, Randle and colleagues observed that high FA availability could increase FAO in hearts perfused with glucose without changing ATP levels [1]. Therefore, it is a possibility that FA entry into the cells or its oxidation can decrease ACLY and ACC activities by a still uncharacterized post-translational mechanism. Other possibilities could include that FA activated AMPK by other mechanisms not involving decreased ATP content (i.e., ROS production derived from FAO) or that high levels of FA outcompeted the malonyl-CoA produced in tissues expressing CPT1-A.
2. The Role of Upregulated Glucose and Aminoacids Oxidation to Support Anabolism in Tumor Proliferation: It Is Not about ATP, but Anaplerosis and FA Synthesis
3. The Role of Mitochondrial Fatty Acid Oxidation (FAO) Supporting Cancer Survival and Proliferation
| Tumor Type | Primary (Warburg Effect) | Metastasis/Environmental Stress | Reference | |
| Randle Cycle | Colorectal cancer | High glycolysis to lactate Dependent on glucose and glutamine to proliferate. Glucose and glutamine used for FA synthesis. | FAO is elevated as an acidic stress response. FAO is needed to survive detachment and the metastatic process. | [21,22,23,27] |
| Glioblastoma | High glycolysis to lactate Dependent on glucose and glutamine to proliferate. Glucose and glutamine used for FA synthesis. | FAO is recruited to survive stresses imposed by the microenvironment. FAO is recruited to protect from oxidative stress (elevated ROS). | [16,28,29,30] | |
| Tumor Type | Primary (Non-Solid Tumors) | Metastasis/Environmental Stress | Reference | |
| Non-Randle Cycle | Lymphomas/ Leukemias | High glycolysis to lactate Dependent on glucose and glutamine to proliferate. FAO needed to meet ATP demand and counteract high ROS production caused by growth in suspension. | The primary tumor already show a shift in nutrient preference similar to solid tumors when they are detaching and metastasizing. FAO is not a defining feature of all non-solid tumors, as a subset of B-cell lymphoma are Warburg-like and do not recruit FAO. | [25,26,31] |
- (1)
- What is the main molecular mechanism allowing FAO and FA synthesis to concurrently occur in this subset of B-cell lymphomas? Are the epigenetic changes mediated by acetyl-CoA decreasing ACC2 transcription responsible [22] or other post-translational mechanisms exist?
- (2)
- Is there a reason beyond the higher demand for ATP and NAD(P)H synthesis associated with being a non-solid tumor to choose FAO, as suggested by the high proliferation rates of the other subset of B-cell lymphomas that do not use FAO?
- (3)
- Is there any change in the glycolytic machinery of tumors that could make them insensitive to the inhibitory effects of FAO on glycolysis? On the contrary, is there a change in the mitochondrial machinery executing FAO to make them insensitive to glycolysis-mediated inhibition of FAO?
3.1. Glycolytic Reprogramming in Colorectal Cancers Allows a Randle Cycle-Abiding Behavior and Recruit FAO to Survive Redox Stress and Metastasis
3.2. Does Metastasis Mimic the Fasting State as the Randle Cycle Defined, Explaining the Shift to FAO?
3.3. How Do B-Cell Lymphomas and Leukemias Concurrently Perform FAO and FA Synthesis, Escaping from the Randle Cycle?
3.4. Can Mitochondrial Heterogeneity and Dynamics Explain How B-Cell Lymphoma and Leukemias Concurrently Perform FAO and FA Synthesis?
4. Evidence That Targeting Mitochondrial-Derived FA and FAO Synthesis Is an Anti-Tumor Strategy
4.1. Examples of Blocking FAO as a Broad Anti-Cancer Strategy
4.2. Examples of Blocking Lipid Synthesis as a Potential Therapeutic Strategy
4.3. Examples of Stimulating FAO as a Mechanism to Stop Cancer Growth
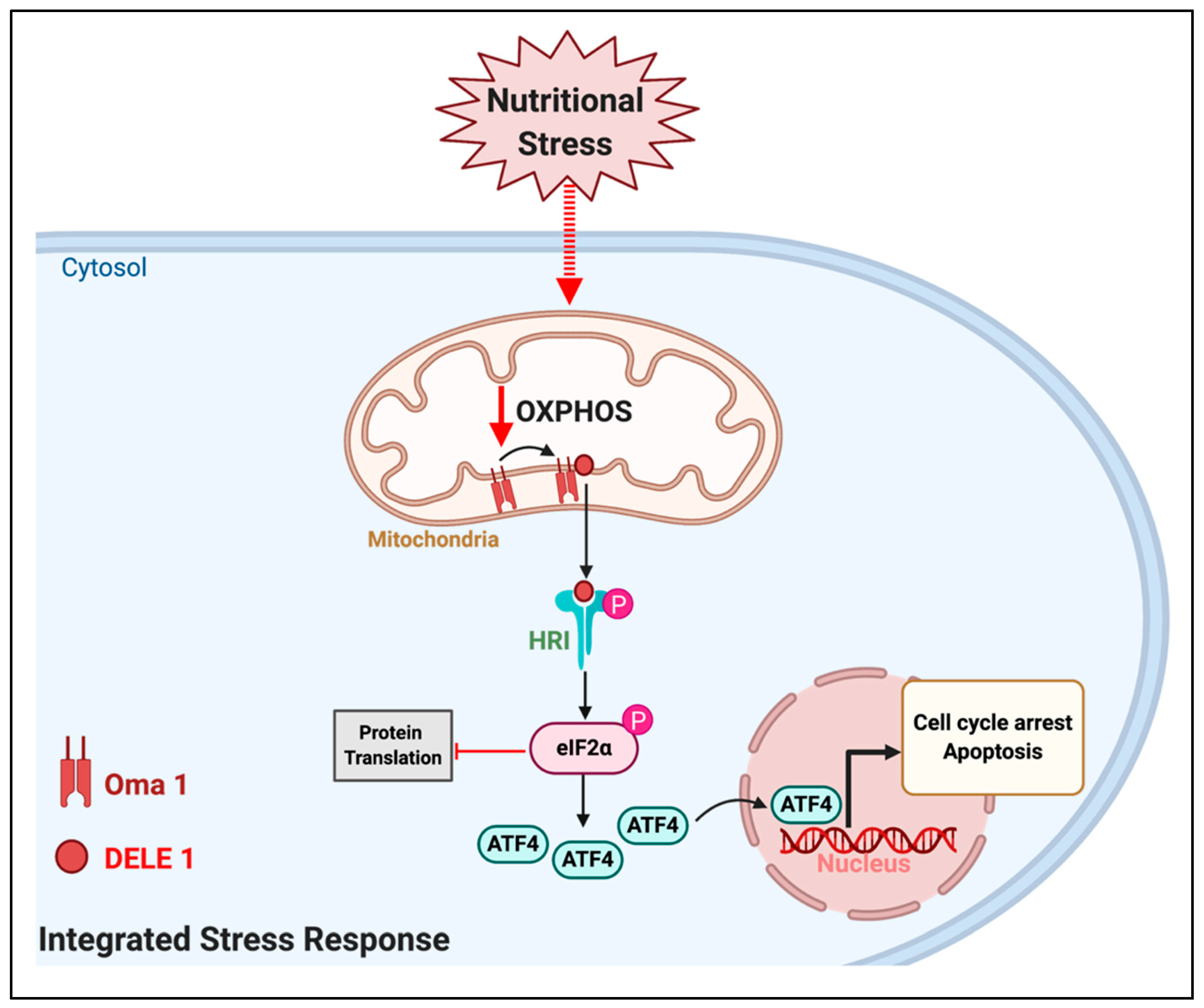
5. Could Forcing the Mitochondria to Use an Inadequate Fuel Induce the Integrated Stress Response (ISR) to Stop Tumor Growth?
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 281, 785–789. [Google Scholar] [CrossRef]
- Randle, P.J. Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 1998, 14, 263–283. [Google Scholar] [CrossRef]
- Garland, P.B.; Shepherd, D.; Nicholls, D.G.; Ontko, J. Energy-dependent control of the tricarboxylic acid cycle by fatty acid oxidation in rat liver mitochondria. Adv. Enzym. Regul. 1968, 6, 3–30. [Google Scholar] [CrossRef]
- McGarry, J.D.; Mannaerts, G.P.; Foster, D.W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Investig. 1977, 60, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Munday, M.R. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 2002, 30, 1059–1064. [Google Scholar] [CrossRef]
- Zammit, V.A. Carnitine palmitoyltransferase 1: Central to cell function. IUBMB Life 2008, 60, 347–354. [Google Scholar] [CrossRef]
- Carling, D.; Fryer, L.G.D.; Woods, A.; Daniel, T.; Jarvie, S.L.C.; Whitrow, H. Bypassing the glucose/fatty acid cycle: AMP-activated protein kinase. Biochem. Soc. Trans. 2003, 31, 1157–1160. [Google Scholar] [CrossRef]
- Warburg, O. The metabolism of carcinoma cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Locasale, J.W.; Swanson, K.D.; Sharfi, H.; Heffron, G.J.; Amador-Noguez, D.; Christofk, H.R.; Wagner, G.; Rabinowitz, J.D.; Asara, J.M.; et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 2010, 329, 1492–1499. [Google Scholar] [CrossRef]
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.; Sethumadhavan, S.; Woo, H.-K.; Jang, H.G.; Jha, A.K.; et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 2011, 476, 346–350. [Google Scholar] [CrossRef]
- Feron, O. Pyruvate into lactate and back: From the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 2009, 92, 329–333. [Google Scholar] [CrossRef]
- Yang, H.-C.; Wu, Y.-H.; Yen, W.-C.; Liu, H.-Y.; Hwang, T.-L.; Stern, A.; Chiu, D.T.-Y. The redox role of G6PD in cell growth, cell death, and cancer. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, F.; Ciminale, V. Escaping death: Mitochondrial redox homeostasis in cancer cells. Front. Oncol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Moss, T.; Mangala, L.S.; Marini, J.; Zhao, H.; Wahlig, S.; Armaiz-Pena, G.; Jiang, D.; Achreja, A.; Win, J.; et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol. Syst. Biol. 2014, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bermudez, J.; Baudrier, L.; La, K.; Zhu, X.G.; Fidelin, J.; Sviderskiy, V.O.; Papagiannakopoulos, T.; Molina, H.; Snuderl, M.; Lewis, C.A.; et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat. Cell Biol. 2018, 20, 775–781. [Google Scholar] [CrossRef]
- Alkan, H.F.; Walter, K.E.; Luengo, A.; Madreiter-Sokolowski, C.T.; Stryeck, S.; Lau, A.N.; Al-Zoughbi, W.; Lewis, C.A.; Thomas, C.J.; Hoefler, G.; et al. Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metab. 2018, 28, 706–720.e6. [Google Scholar] [CrossRef]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef]
- Reckzeh, E.S.; Karageorgis, G.; Schwalfenberg, M.; Ceballos, J.; Nowacki, J.; Stroet, M.C.M.; Binici, A.; Knauer, L.; Brand, S.; Choidas, A.; et al. Inhibition of glucose transporters and glutaminase synergistically impairs tumor cell growth. Cell Chem. Biol. 2019, 26, 1214–1228.e25. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Pinto, A.; Martherus, R.; Santiago de Jesus, J.P.; Polet, F.; Feron, O. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 2016, 24, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, P.C.; Hughes, M.C.; Perry, C.G.R. The fatty acid derivative palmitoylcarnitine abrogates colorectal cancer cell survival by depleting glutathione. Am. J. Physiol. Cell Physiol. 2019, 317, C1278–C1288. [Google Scholar] [CrossRef] [PubMed]
- Chajès, V.; Cambot, M.; Moreau, K.; Lenoir, G.M.; Joulin, V. Acetyl-CoA carboxylase α is essential to breast cancer cell survival. Cancer Res. 2006, 66, 5287–5294. [Google Scholar] [CrossRef] [PubMed]
- Caro, P.; Kishan, A.U.; Norberg, E.; Stanley, I.; Chapuy, B.; Ficarro, S.B.; Polak, K.; Tondera, D.; Gounarides, J.; Yin, H.; et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B-cell lymphoma. Cancer Cell 2012, 22, 547–560. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Jacobs, S.R.; Freemerman, A.J.; Makowski, L.; Rathmell, J.C.; Dittmer, D.P.; Damania, B. Dysregulation of fatty acid synthesis and glycolysis in non-Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, 11818–11823. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef]
- Lin, H.; Patel, S.; Affleck, V.S.; Wilson, I.; Turnbull, D.M.; Joshi, A.R.; Maxwell, R.; Stoll, E.A. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro Oncol. 2017, 19, 43–54. [Google Scholar] [CrossRef]
- Kant, S.; Kesarwani, P.; Prabhu, A.; Graham, S.F.; Buelow, K.L.; Nakano, I.; Chinnaiyan, P. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Pike, L.S.; Smift, A.L.; Croteau, N.J.; Ferrick, D.A.; Wu, M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta BBA Bioenerg. 2011, 1807, 726–734. [Google Scholar] [CrossRef]
- German, N.J.; Yoon, H.; Yusuf, R.Z.; Murphy, J.P.; Finley, L.W.S.; Laurent, G.; Haas, W.; Satterstrom, F.K.; Guarnerio, J.; Zaganjor, E.; et al. PHD3 loss in cancer enables metabolic reliance on fatty acid oxidation via deactivation of ACC2. Mol. Cell 2016, 63, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Wu, N.; Asara, J.M.; Cantley, L.C. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 2008, 452, 181–186. [Google Scholar] [CrossRef]
- Morgan, H.P.; O’Reilly, F.J.; Wear, M.A.; O’Neill, J.R.; Fothergill-Gilmore, L.A.; Hupp, T.; Walkinshaw, M.D. M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc. Natl. Acad. Sci. USA 2013, 110, 5881–5886. [Google Scholar] [CrossRef] [PubMed]
- Parlo, R.A.; Coleman, P.S. Continuous pyruvate carbon flux to newly synthesized cholesterol and the suppressed evolution of pyruvate-generated CO2 in tumors: Further evidence for a persistent truncated Krebs cycle in hepatomas. Biochim. Biophys. Acta BBA Mol. Cell Res. 1986, 886, 169–176. [Google Scholar] [CrossRef]
- Jeon, S.-M.; Chandel, N.S.; Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012, 485, 661–665. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Ju, H.-Q.; Liu, Z.-X.; Chen, D.-L.; Wang, Y.; Zhao, Q.; Wu, Q.-N.; Zeng, Z.; Qiu, H.-B.; Hu, P.-S.; et al. ME1 regulates NADPH homeostasis to promote gastric cancer growth and metastasis. Cancer Res. 2018, 78, 1972–1985. [Google Scholar] [CrossRef]
- Zhang, X.; Gibhardt, C.S.; Will, T.; Stanisz, H.; Körbel, C.; Mitkovski, M.; Stejerean, I.; Cappello, S.; Pacheu-Grau, D.; Dudek, J.; et al. Redox signals at the ER–mitochondria interface control melanoma progression. EMBO J. 2019, 38, e100871. [Google Scholar] [CrossRef]
- Holla, V.R.; Wu, H.; Shi, Q.; Menter, D.G.; DuBois, R.N. Nuclear orphan receptor NR4A2 modulates fatty acid oxidation pathways in colorectal cancer. J. Biol. Chem. 2011, 286, 30003–30009. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wu, S.; Zhou, Z.; Ding, X.; Shi, R.; Thorne, R.F.; Zhang, X.D.; Hu, W.; Wu, M. CircACC1 regulates assembly and activation of AMPK complex under metabolic stress. Cell Metab. 2019, 30, 157–173.e7. [Google Scholar] [CrossRef]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; de Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Commander, R.; Wei, C.; Sharma, A.; Mouw, J.K.; Burton, L.J.; Summerbell, E.; Mahboubi, D.; Peterson, R.J.; Konen, J.; Zhou, W.; et al. Subpopulation targeting of pyruvate dehydrogenase and GLUT1 decouples metabolic heterogeneity during collective cancer cell invasion. Nat. Commun. 2020, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Payen, V.L.; Pérez-Escuredo, J.; De Saedeleer, C.J.; Danhier, P.; Copetti, T.; Dhup, S.; Tardy, M.; Vazeille, T.; Bouzin, C.; et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014, 8, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Caneba, C.A.; Bellance, N.; Yang, L.; Pabst, L.; Nagrath, D. Pyruvate uptake is increased in highly invasive ovarian cancer cells under anoikis conditions for anaplerosis, mitochondrial function, and migration. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1036–E1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jeong, S.; Jang, C.; Bae, H.; Kim, Y.H.; Park, I.; Kim, S.K.; Koh, G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 2019, 363, 644–649. [Google Scholar] [CrossRef]
- Wen, Y.-A.; Xing, X.; Harris, J.W.; Zaytseva, Y.Y.; Mitov, M.I.; Napier, D.L.; Weiss, H.L.; Mark Evers, B.; Gao, T. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017, 8, e2593. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Zeng, Z.-L.; Lu, J.; Wang, Y.; Liu, Z.-X.; He, M.-M.; Zhao, Q.; Wang, Z.-X.; Li, T.; Lu, Y.-X.; et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene 2018, 37, 6025–6040. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Guan, L.; Chen, Y.; Chen, P.; Sun, J.; Gonzalez, F.J.; Huang, M.; Bi, H. Lipidomics reveals carnitine palmitoyltransferase 1C protects cancer cells from lipotoxicity and senescence. J. Pharm. Anal. 2020. [Google Scholar] [CrossRef]
- Wright, G.; Higgin, J.J.; Raines, R.T.; Steenbergen, C.; Murphy, E. Activation of the prolyl hydroxylase oxygen-sensor results in induction of GLUT1, heme oxygenase-1, and nitric-oxide synthase proteins and confers protection from metabolic inhibition to cardiomyocytes. J. Biol. Chem. 2003, 278, 20235–20239. [Google Scholar] [CrossRef]
- Wikstrom, J.D.; Katzman, S.M.; Mohamed, H.; Twig, G.; Graf, S.A.; Heart, E.; Molina, A.J.A.; Corkey, B.E.; de Vargas, L.M.; Danial, N.N.; et al. beta-Cell mitochondria exhibit membrane potential heterogeneity that can be altered by stimulatory or toxic fuel levels. Diabetes 2007, 56, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Ferree, A.W.; Trudeau, K.; Zik, E.; Benador, I.Y.; Twig, G.; Gottlieb, R.A.; Shirihai, O.S. MitoTimer probe reveals the impact of autophagy, fusion, and motility on subcellular distribution of young and old mitochondrial protein and on relative mitochondrial protein age. Autophagy 2013, 9, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Benador, I.Y.; Veliova, M.; Mahdaviani, K.; Petcherski, A.; Wikstrom, J.D.; Assali, E.A.; Acín-Pérez, R.; Shum, M.; Oliveira, M.F.; Cinti, S.; et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 2018, 27, 869–885.e6. [Google Scholar] [CrossRef]
- Shi, J.; Fu, H.; Jia, Z.; He, K.; Fu, L.; Wang, W. High expression of CPT1A predicts adverse outcomes: A potential therapeutic target for acute myeloid leukemia. EBioMedicine 2016, 14, 55–64. [Google Scholar] [CrossRef]
- Wright, H.J.; Hou, J.; Xu, B.; Cortez, M.; Potma, E.O.; Tromberg, B.J.; Razorenova, O.V. CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc. Natl. Acad. Sci. USA 2017, 114, E6556–E6565. [Google Scholar] [CrossRef]
- Chen, C.-L.; Uthaya Kumar, D.B.; Punj, V.; Xu, J.; Sher, L.; Tahara, S.M.; Hess, S.; Machida, K. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016, 23, 206–219. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, G.; Wang, Y.; Cai, L.; Qian, K.; Ju, L.; Liu, X.; Xiao, Y.; Wang, X. Fatty acid oxidation inhibitor etomoxir suppresses tumor progression and induces cell cycle arrest via PPARγ-mediated pathway in bladder cancer. Clin. Sci. 2019, 133, 1745–1758. [Google Scholar] [CrossRef]
- Camarda, R.; Zhou, A.Y.; Kohnz, R.A.; Balakrishnan, S.; Mahieu, C.; Anderton, B.; Eyob, H.; Kajimura, S.; Tward, A.; Krings, G.; et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016, 22, 427–432. [Google Scholar] [CrossRef]
- Aloia, A.; Müllhaupt, D.; Chabbert, C.D.; Eberhart, T.; Flückiger-Mangual, S.; Vukolic, A.; Eichhoff, O.; Irmisch, A.; Alexander, L.T.; Scibona, E.; et al. A Fatty acid oxidation-dependent metabolic shift regulates the adaptation of BRAF-mutated melanoma to MAPK inhibitors. Clin. Cancer Res. 2019, 25, 6852–6867. [Google Scholar] [CrossRef]
- Han, A.; Bennett, N.; Ahmed, B.; Whelan, J.; Donohoe, D.R. Butyrate decreases its own oxidation in colorectal cancer cells through inhibition of histone deacetylases. Oncotarget 2018, 9, 27280–27292. [Google Scholar] [CrossRef] [PubMed]
- Itkonen, H.M.; Poulose, N.; Walker, S.; Mills, I.G. CDK9 inhibition induces a metabolic switch that renders prostate cancer cells dependent on fatty acid oxidation. Neoplasia 2019, 21, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nguyen, T.T.T.; Shang, E.; Mela, A.; Humala, N.; Mahajan, A.; Zhao, J.; Shu, C.; Torrini, C.; Sanchez-Quintero, M.J.; et al. MET inhibition elicits PGC1α-dependent metabolic reprogramming in glioblastoma. Cancer Res. 2020, 80, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, G.; Song, L.; Cao, L.; Tan, Z.; Tang, M.; Li, Z.; Shi, D.; Zhang, S.; Li, J. NKX2-8 deletion-induced reprogramming of fatty acid metabolism confers chemoresistance in epithelial ovarian cancer. EBioMedicine 2019, 43, 238–252. [Google Scholar] [CrossRef]
- Kant, S.; Kumar, A.; Singh, S.M. Tumor growth retardation and chemosensitizing action of fatty acid synthase inhibitor orlistat on T cell lymphoma: Implication of reconstituted tumor microenvironment and multidrug resistance phenotype. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 294–302. [Google Scholar] [CrossRef]
- Amrutha Nisthul, A.; Retnakumari, A.P.; Shabna, A.; Anto, R.J.; Sadasivan, C. In silico screening for identification of fatty acid synthase inhibitors and evaluation of their antiproliferative activity using human cancer cell lines. J. Recept. Signal Transduct. 2018, 38, 335–341. [Google Scholar] [CrossRef]
- Zhou, W.; Simpson, P.J.; McFadden, J.M.; Townsend, C.A.; Medghalchi, S.M.; Vadlamudi, A.; Pinn, M.L.; Ronnett, G.V.; Kuhajda, F.P. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003, 63, 7330–7337. [Google Scholar]
- Nowinski, S.M.; Solmonson, A.; Rundhaug, J.E.; Rho, O.; Cho, J.; Lago, C.U.; Riley, C.L.; Lee, S.; Kohno, S.; Dao, C.K.; et al. Mitochondrial uncoupling links lipid catabolism to Akt inhibition and resistance to tumorigenesis. Nat. Commun. 2015, 6, 8137. [Google Scholar] [CrossRef]
- Ni, T.; He, Z.; Dai, Y.; Yao, J.; Guo, Q.; Wei, L. Oroxylin A suppresses the development and growth of colorectal cancer through reprogram of HIF1α-modulated fatty acid metabolism. Cell Death Dis. 2017, 8, e2865. [Google Scholar] [CrossRef]
- Bader, D.A.; Hartig, S.M.; Putluri, V.; Foley, C.; Hamilton, M.P.; Smith, E.A.; Saha, P.K.; Panigrahi, A.; Walker, C.; Zong, L.; et al. Mitochondrial pyruvate import is a metabolic vulnerability in androgen receptor-driven prostate cancer. Nat. Metab. 2019, 1, 70–85. [Google Scholar] [CrossRef]
- Rajanala, S.H.; Ringquist, R.; Cryns, V.L. Methionine restriction activates the integrated stress response in triple-negative breast cancer cells by a GCN2- and PERK-independent mechanism. Am. J. Cancer Res. 2019, 9, 1766–1775. [Google Scholar] [PubMed]
- Linares, J.F.; Cordes, T.; Duran, A.; Reina-Campos, M.; Valencia, T.; Ahn, C.S.; Castilla, E.A.; Moscat, J.; Metallo, C.M.; Diaz-Meco, M.T. ATF4-induced metabolic reprograming is a synthetic vulnerability of the p62-deficient tumor stroma. Cell Metab. 2017, 26, 817–829.e6. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Sánchez-Aragó, M.; Cuezva, J.M. AMPK and GCN2–ATF4 signal the repression of mitochondria in colon cancer cells. Biochem. J. 2012, 444, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-C.; Lai, K.-H.; Su, J.-H.; Wu, Y.-J.; Sheu, J.-H. 7-acetylsinumaximol B induces apoptosis and autophagy in human gastric carcinoma cells through mitochondria dysfunction and activation of the PERK/eIF2α/ATF4/CHOP signaling pathway. Mar. Drugs 2018, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-F.; Chen, M.-S.; Chou, Y.-C.; Ueng, Y.-F.; Yin, P.-H.; Yeh, T.-S.; Lee, H.-C. Mitochondrial dysfunction enhances cisplatin resistance in human gastric cancer cells via the ROS-activated GCN2-eIF2α-ATF4-xCT pathway. Oncotarget 2016, 7, 74132–74151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Su, J.-H.; Tsao, C.-Y.; Hung, C.-T.; Chao, H.-H.; Lin, J.-J.; Liao, M.-H.; Yang, Z.-Y.; Huang, H.H.; Tsai, F.-J.; et al. Sinulariolide induced hepatocellular carcinoma apoptosis through activation of mitochondrial-related apoptotic and PERK/eIF2α/ATF4/CHOP pathway. Molecules 2013, 18, 10146–10161. [Google Scholar] [CrossRef] [PubMed]
- Ameri, K.; Lewis, C.E.; Raida, M.; Sowter, H.; Hai, T.; Harris, A.L. Anoxic induction of ATF-4 through HIF-1–independent pathways of protein stabilization in human cancer cells. Blood 2004, 103, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Aviles, G.; Liu, Y.; Tian, R.; Unger, B.A.; Lin, Y.-H.T.; Wiita, A.P.; Xu, K.; Correia, M.A.; Kampmann, M. Mitochondrial stress is relayed to the cytosol by an OMA1–DELE1–HRI pathway. Nature 2020, 579, 427–432. [Google Scholar] [CrossRef]
- Zhang, S.; Macias-Garcia, A.; Ulirsch, J.C.; Velazquez, J.; Butty, V.L.; Levine, S.S.; Sankaran, V.G.; Chen, J.-J. HRI coordinates translation necessary for protein homeostasis and mitochondrial function in erythropoiesis. eLife 2019, 8, e46976. [Google Scholar] [CrossRef]
- Veliova, M.; Ferreira, C.M.; Benador, I.Y.; Jones, A.E.; Desousa, B.R.; Mahdaviani, K.; Acín-Pérez, R.; Petcherski, A.; Divakaruni, A.S.; Prentki, M.; et al. Blocking mitochondrial pyruvate import causes energy wasting via futile lipid cycling in brown fat. bioRxiv 2019, 841551. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Zhao, M.; Han, Y.; Chen, P.; Mo, Q.; Wang, B.; Chen, G.; Fang, Y.; Tian, Y.; et al. Loss of the novel mitochondrial protein FAM210B promotes metastasis via PDK4-dependent metabolic reprogramming. Cell Death Dis. 2017, 8, e2870. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Oliveira, M.P.; Liesa, M. The Role of Mitochondrial Fat Oxidation in Cancer Cell Proliferation and Survival. Cells 2020, 9, 2600. https://doi.org/10.3390/cells9122600
De Oliveira MP, Liesa M. The Role of Mitochondrial Fat Oxidation in Cancer Cell Proliferation and Survival. Cells. 2020; 9(12):2600. https://doi.org/10.3390/cells9122600
Chicago/Turabian StyleDe Oliveira, Matheus Pinto, and Marc Liesa. 2020. "The Role of Mitochondrial Fat Oxidation in Cancer Cell Proliferation and Survival" Cells 9, no. 12: 2600. https://doi.org/10.3390/cells9122600
APA StyleDe Oliveira, M. P., & Liesa, M. (2020). The Role of Mitochondrial Fat Oxidation in Cancer Cell Proliferation and Survival. Cells, 9(12), 2600. https://doi.org/10.3390/cells9122600






