Modification of a Constitutive to Glucose-Responsive Liver-Specific Promoter Resulted in Increased Efficacy of Adeno-Associated Virus Serotype 8-Insulin Gene Therapy of Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid DNA Constructs
2.2. Primary Rat Hepatocyte Cultures
2.3. Transfection of Primary Rat Hepatocytes
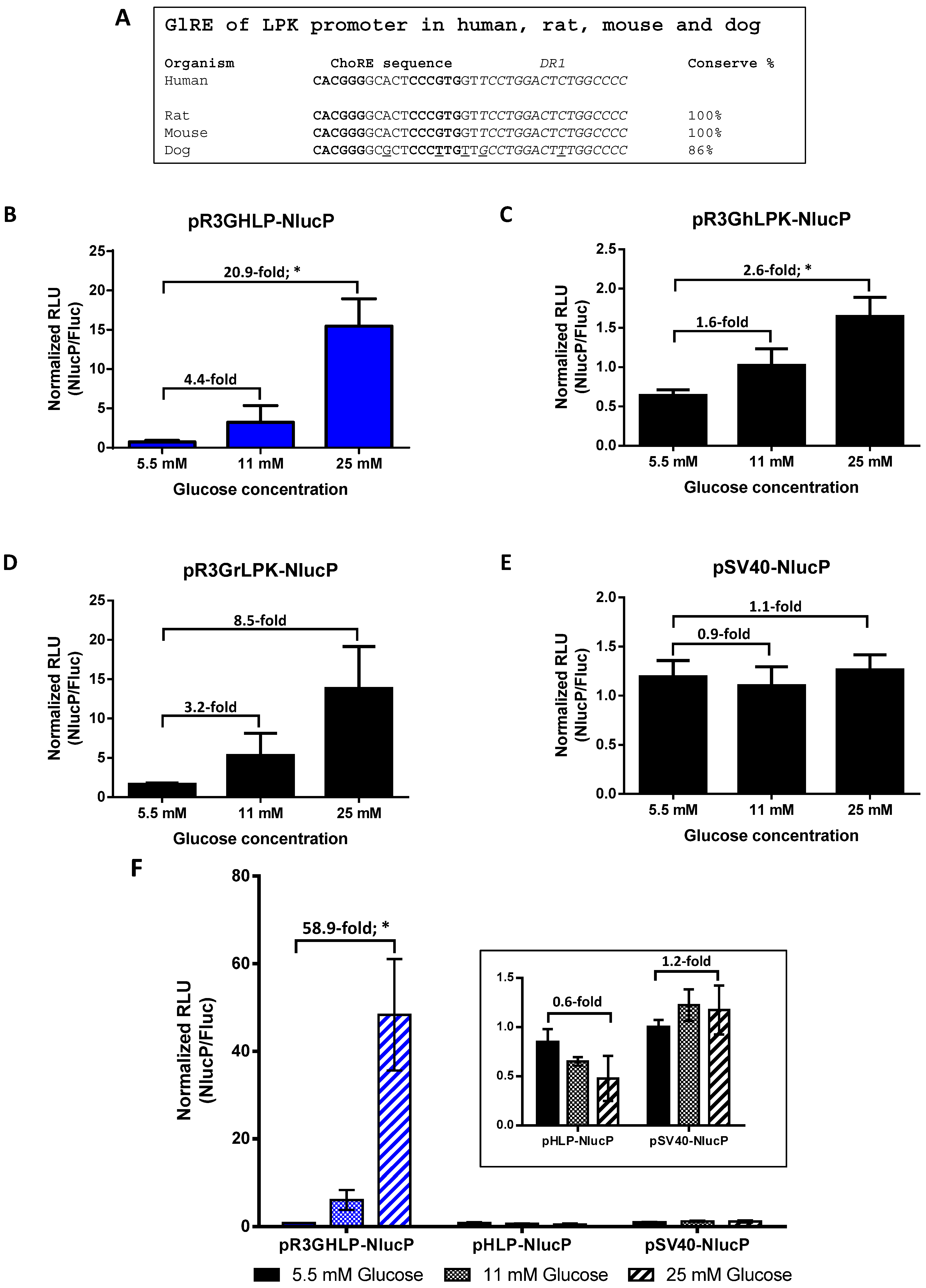
2.4. Luciferase Reporter Assay for Studying Glucose Responsive Promoter
2.5. Packaging, Purification and Titration of AAV8 Viral Vector
2.6. Animal Work
2.7. AAV Viral Genome Copies Quantification
2.8. C-Peptide ELISA
2.9. Fasting and Intraperitoneal Glucose Tolerant Test (IPGTT)
2.10. Statistical Analyses
3. Results
3.1. Generation of Novel Glucose Responsive Promoter from Constitutive Hybrid Liver-Specific Promoter for Diabetic Gene Therapy
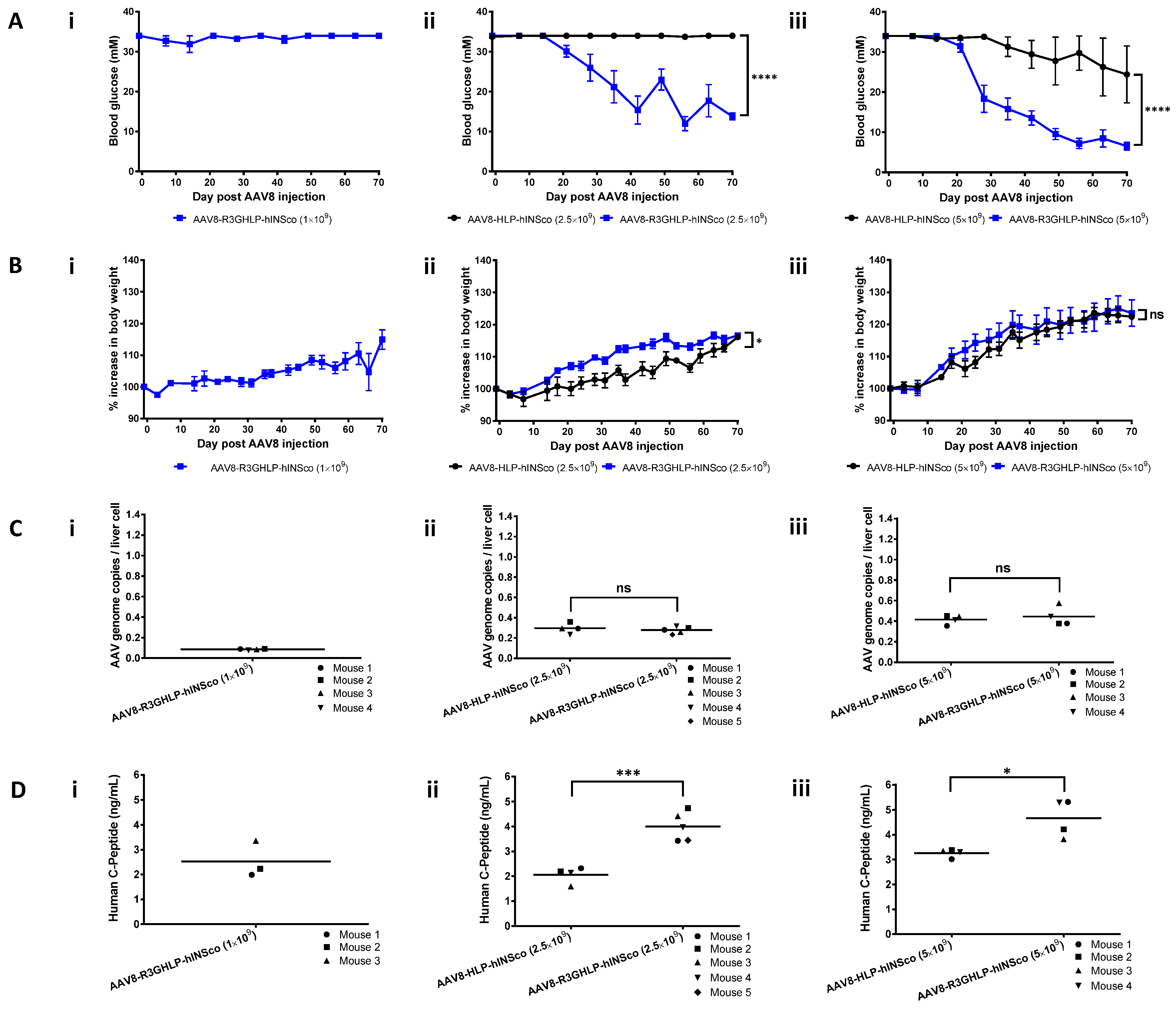
3.2. Treatment Efficacy of Diabetic Mouse with Constitutive and Glucose Responsive Promoter Driving the Expression of Human Insulin
3.3. Comparison Between Different Promoters for Fasting Effect and IPGTT
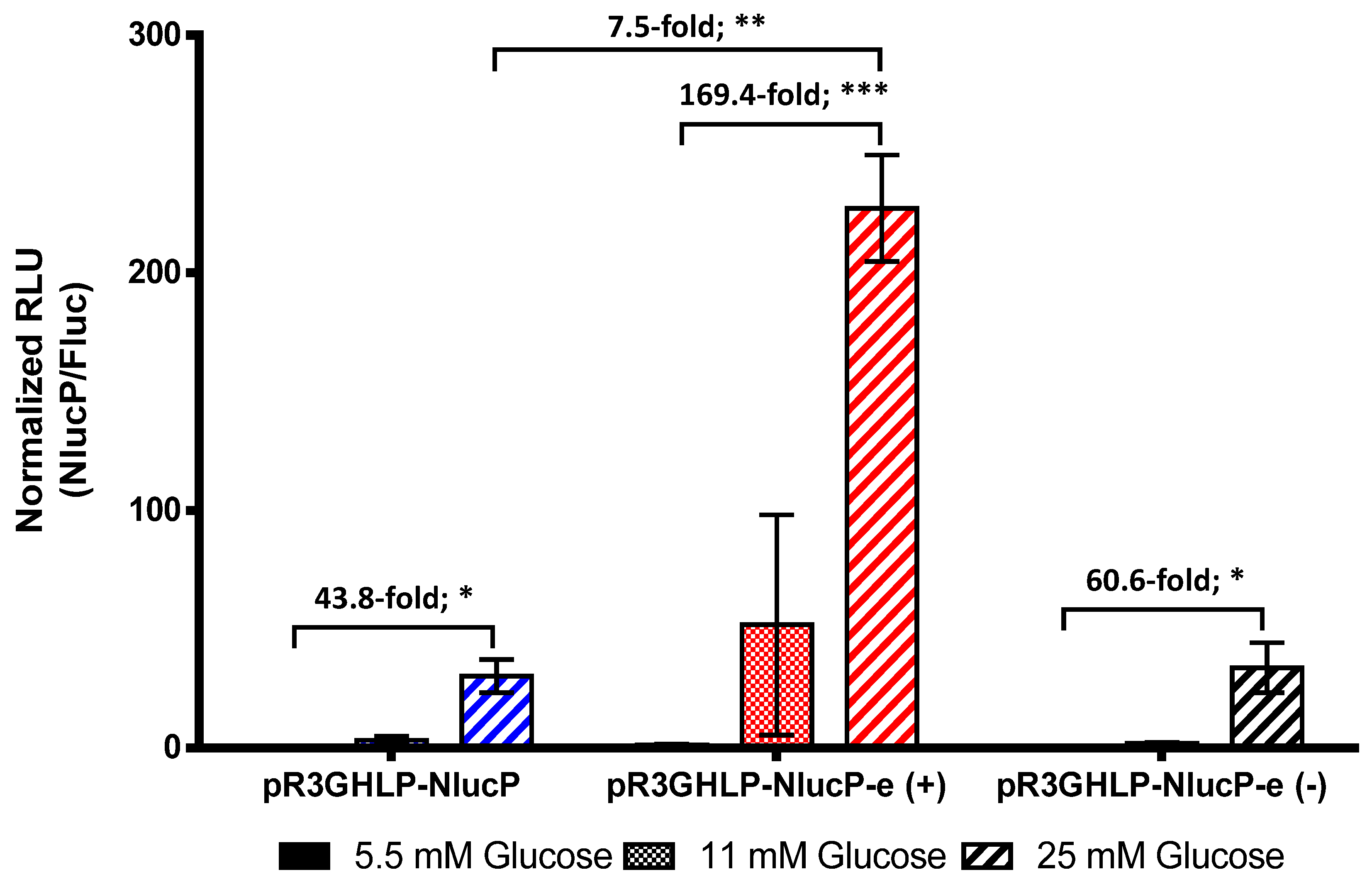
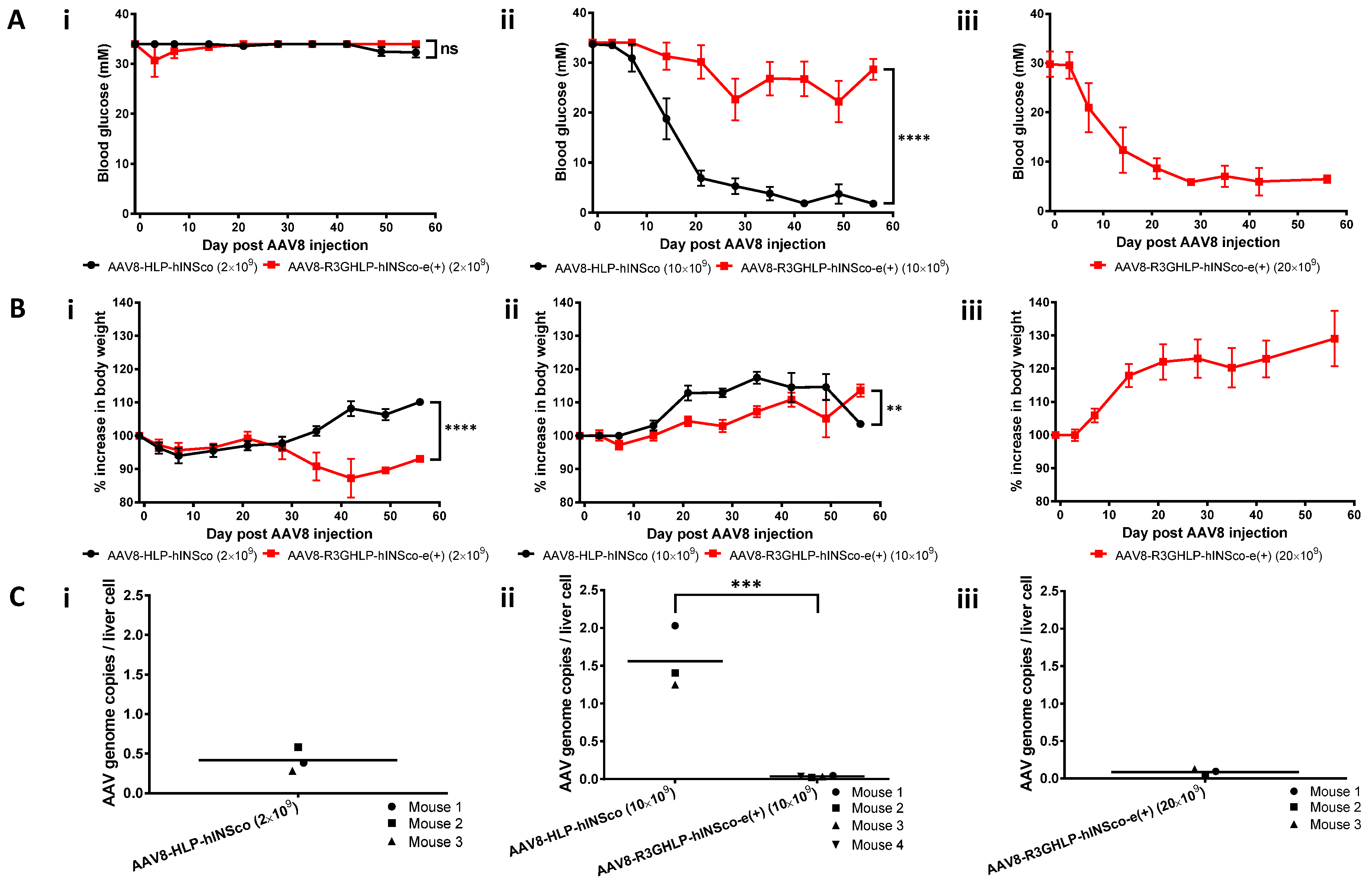
3.4. Enhanced R3GHLP Promoter Activity in Glucose Dependent Manner with 3′iALB Enhancer
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| AAV8 | AAV serotype 8 |
| HLP | hybrid liver-specific promoter |
| 3′UTR | 3′ untranslated region |
| 3′iALB | 3′UTR of albumin gene with intron 14 |
| GlRE | glucose responsive element |
| R3G | 3 copies of GlRE in reverse orientation |
| bp | base pair |
| STZ | streptozotocin |
| ChoRE | carbohydrate response element |
| ChREBP | carbohydrate response element binding protein |
| LPK | liver pyruvate kinase |
| hINSco | codon optimized furin cleavable human proinsulin |
| rHeps | primary rat hepatocytes |
| WEM | Williams’ E Medium |
| NSG | NOD.cg-PrkdcscidIl2rgtm1Wjl/SzJ |
| IPGTT | intraperitoneal glucose tolerant test |
| s.e.m. | standard error of mean |
| ns | not significant |
| NlucP | PEST-destabilized nanoLuc luciferase |
| vg | vector genome |
| Fluc | firefly luciferase |
| ITR | inverted terminal repeat |
References
- Danne, T.; Lupke, K.; Walte, K.; Von Schuetz, W.; Gall, M.A. Insulin detemir is characterized by a consistent pharmacokinetic profile across age-groups in children, adolescents, and adults with type 1 diabetes. Diabetes Care 2003, 26, 3087–3092. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Nosek, L.; Bottcher, S.G.; Hastrup, H.; Haahr, H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Altomonte, J.; Morral, N.; Meseck, M.; Thung, S.N.; Woo, S.L. Basal insulin gene expression significantly improves conventional insulin therapy in type 1 diabetic rats. Diabetes 2002, 51, 130–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tien, K.J.; Hung, Y.J.; Chen, J.F.; Chen, C.C.; Wang, C.Y.; Hwu, C.M.; Huang, Y.Y.; Hsiao, P.J.; Tu, S.T.; Wang, C.H.; et al. Basal insulin therapy: Unmet medical needs in Asia and the new insulin glargine in diabetes treatment. J. Diabetes Investig. 2019, 10, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.U.; Notaridou, M.; Fu, Z.Y.; Lee, K.O.; Sia, K.C.; Nathwani, A.C.; Della Peruta, M.; Calne, R.Y. Correction of Murine Diabetic Hyperglycaemia With A Single Systemic Administration of An AAV2/8 Vector Containing A Novel Codon Optimized Human Insulin Gene. Curr. Gene Ther. 2016, 16, 65–72. [Google Scholar]
- Recino, A.; Gan, S.U.; Sia, K.C.; Sawyer, Y.; Trendell, J.; Kay, R.; Gribble, F.M.; Reimann, F.; Foale, R.; Notaridou, M.; et al. Immunosuppression overcomes insulin- and vector-specific immune responses that limit efficacy of AAV2/8-mediated insulin gene therapy in NOD mice. Gene Ther. 2018. [Google Scholar] [CrossRef]
- Mcintosh, J.; Lenting, P.J.; Rosales, C.; Lee, D.; Rabbanian, S.; Raj, D.; Patel, N.; Tuddenham, E.G.D.; Christophe, O.D.; Mcvey, J.H.; et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 2013, 121, 3335–3344. [Google Scholar] [CrossRef]
- Foale, R.; Recino, A.; Wallberg, M.; Gan, S.U.; Lee, K.O.; Nathwani, A.; Lever, A.; Calne, S.R. AAV-8 gene therapy in canine diabetes mellitus; a potential future treatment? In BSAVA Congress Proceedings 2017; British Small Animal Veterinary Association: Birmingham, UK, 2017. [Google Scholar]
- Diaz Guerra, M.J.; Bergot, M.O.; Martinez, A.; Cuif, M.H.; Kahn, A.; Raymondjean, M. Functional characterization of the L-type pyruvate kinase gene glucose response complex. Mol. Cell. Biol. 1993, 13, 7725–7733. [Google Scholar]
- Shih, H.M.; Liu, Z.; Towle, H.C. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J. Biol. Chem. 1995, 270, 21991–21997. [Google Scholar]
- Ma, L.; Robinson, L.N.; Towle, H.C. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J. Biol. Chem. 2006, 281, 28721–28730. [Google Scholar] [CrossRef]
- Adamson, A.W.; Suchankova, G.; Rufo, C.; Nakamura, M.T.; Teran-Garcia, M.; Clarke, S.D.; Gettys, T.W. Hepatocyte nuclear factor-4alpha contributes to carbohydrate-induced transcriptional activation of hepatic fatty acid synthase. Biochem. J. 2006, 399, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.J.; Collier, J.J.; Scott, D.K. cAMP opposes the glucose-mediated induction of the L-PK gene by preventing the recruitment of a complex containing ChREBP, HNF4α, and CBP. FASEB J. 2009, 23, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Christian, B.; Jump, D.B. Regulation of rat hepatic L-pyruvate kinase promoter composition and activity by glucose, n-3 polyunsaturated fatty acids, and peroxisome proliferator-activated receptor-alpha agonist. J. Biol. Chem. 2006, 281, 18351–18362. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.Q.; Tannour, M.; Selig, L.; Thomas, D.; Kahn, A.; Vasseur-Cognet, M. Chicken ovalbumin upstream promoter-transcription factor II, a new partner of the glucose response element of the L-type pyruvate kinase gene, acts as an inhibitor of the glucose response. J. Biol. Chem. 1999, 274, 28385–28394. [Google Scholar]
- Perilhou, A.; Tourrel-Cuzin, C.; Kharroubi, I.; Henique, C.; Fauveau, V.; Kitamura, T.; Magnan, C.; Postic, C.; Prip-Buus, C.; Vasseur-Cognet, M. The transcription factor COUP-TFII is negatively regulated by insulin and glucose via Foxo1- and ChREBP-controlled pathways. Mol. Cell. Biol. 2008, 28, 6568–6579. [Google Scholar] [CrossRef]
- Duran-Sandoval, D.; Cariou, B.; Percevault, F.; Hennuyer, N.; Grefhorst, A.; van Dijk, T.H.; Gonzalez, F.J.; Fruchart, J.-C.; Kuipers, F.; Staels, B. The Farnesoid X Receptor Modulates Hepatic Carbohydrate Metabolism during the Fasting-Refeeding Transition. J. Biol. Chem. 2005, 280, 29971–29979. [Google Scholar] [CrossRef]
- Poupeau, A.; Postic, C. Cross-regulation of hepatic glucose metabolism via ChREBP and nuclear receptors. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 995–1006. [Google Scholar] [CrossRef]
- Alam, T.; Wai, P.; Held, D.; Vakili, S.T.T.; Forsberg, E.; Sollinger, H. Correction of Diabetic Hyperglycemia and Amelioration of Metabolic Anomalies by Minicircle DNA Mediated Glucose-Dependent Hepatic Insulin Production. PLoS ONE 2013, 8, e67515. [Google Scholar] [CrossRef]
- Wooddell, C.I.; Reppen, T.; Wolff, J.A.; Herweijer, H. Sustained liver-specific transgene expression from the albumin promoter in mice following hydrodynamic plasmid DNA delivery. J. Gene Med. 2008, 10, 551–563. [Google Scholar] [CrossRef]
- Matoulkova, E.; Michalova, E.; Vojtesek, B.; Hrstka, R. The role of the 3′ untranslated region in post-transcriptional regulation of protein expression in mammalian cells. RNA Biol. 2012, 9, 563–576. [Google Scholar] [CrossRef]
- Della Peruta, M.; Badar, A.; Rosales, C.; Chokshi, S.; Kia, A.; Nathwani, D.; Galante, E.; Yan, R.; Arstad, E.; Davidoff, A.M.; et al. Preferential targeting of disseminated liver tumors using a recombinant adeno-associated viral vector. Hum. Gene Ther. 2015, 26, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zolotukhin, S.; Byrne, B.J.; Mason, E.; Zolotukhin, I.; Potter, M.; Chesnut, K.; Summerford, C.; Samulski, R.J.; Muzyczka, N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999, 6, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Wright, J.F.; Nathwani, A.C.; Nienhuis, A.W.; Davidoff, A.M.; Gray, J.T. Systemic errors in quantitative polymerase chain reaction titration of self-complementary adeno-associated viral vectors and improved alternative methods. Hum. Gene Ther. Methods 2012, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sham, Y.Y.; Walters, K.J.; Towle, H.C. A critical role for the loop region of the basic helix-loop-helix/leucine zipper protein Mlx in DNA binding and glucose-regulated transcription. Nucleic Acids Res. 2007, 35, 35–44. [Google Scholar] [CrossRef]
- Alam, T.; Sollinger, H.W. Glucose-regulated insulin production in hepatocytes. Transplantation 2002, 74, 1781–1787. [Google Scholar] [CrossRef]
- Thulé, P.M.; Liu, J.; Phillips, L.S. Glucose regulated production of human insulin in rat hepatocytes. Gene Ther. 2000, 7, 205–214. [Google Scholar] [CrossRef]
- Han, J.; McLane, B.; Kim, E.H.; Yoon, J.W.; Jun, H.S. Remission of diabetes by insulin gene therapy using a hepatocyte-specific and glucose-responsive synthetic promoter. Mol. Ther. 2011, 19, 470–478. [Google Scholar] [CrossRef]
- Lan, M.S.; Wang, H.W.; Chong, J.; Breslin, M.B. Coupling of glucose response element from L-type pyruvate kinase and G6Pase promoter enhances glucose responsive activity in hepatoma cells. Mol. Cell. Biochem. 2007, 300, 191–196. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, H.H. Glucose-regulated insulin production in the liver improves glycemic control in type 1 diabetic mice. Mol. Metab. 2015, 4, 70–76. [Google Scholar] [CrossRef]
- Thulé, P.M.; Liu, J.-M. Regulated hepatic insulin gene therapy of STZ-diabetic rats. Gene Ther. 2000, 7, 1744–1752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, M.S.; Hawthorne, W.J.; Manolios, N. Gene therapy in diabetes. Self/Nonself—Immune Recognit. Signal. 2010, 1, 165–175. [Google Scholar] [CrossRef]
- Berns, K.I.; Muzyczka, N. AAV: An Overview of Unanswered Questions. Hum. Gene Ther. 2017, 28, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.U.; Fu, Z.; Sia, K.C.; Kon, O.L.; Calne, R.; Lee, K.O. Development of a liver-specific Tet-Off AAV8 vector for improved safety of insulin gene therapy for diabetes. J. Gene Med. 2018, e3067. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.; Amiel, S.A.; Choudhary, P. Optimal prandial timing of bolus insulin in diabetes management: A review. Diabet. Med. 2018, 35, 306–316. [Google Scholar] [CrossRef]
- Eliaschewitz, F.G.; Barreto, T. Concepts and clinical use of ultra-long basal insulin. Diabetol. Metab. Syndr. 2016, 8, 2. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Jia, W.; Wanke, I.E.; Muruve, D.A.; Xiao, H.P.; Wong, N.C. Glucose regulates secretion of exogenously expressed insulin from HepG2 cells in vitro and in a mouse model of diabetes mellitus in vivo. J. Mol. Endocrinol. 2013, 50, 337–346. [Google Scholar] [CrossRef]
- Rivera, V.M.; Wang, X.; Wardwell, S.; Courage, N.L.; Volchuk, A.; Keenan, T.; Holt, D.A.; Gilman, M.; Orci, L.; Cerasoli, F., Jr.; et al. Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science (80-) 2000, 287, 826–830. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sia, K.C.; Fu, Z.Y.; Calne, R.Y.; Nathwani, A.C.; Lee, K.O.; Gan, S.U. Modification of a Constitutive to Glucose-Responsive Liver-Specific Promoter Resulted in Increased Efficacy of Adeno-Associated Virus Serotype 8-Insulin Gene Therapy of Diabetic Mice. Cells 2020, 9, 2474. https://doi.org/10.3390/cells9112474
Sia KC, Fu ZY, Calne RY, Nathwani AC, Lee KO, Gan SU. Modification of a Constitutive to Glucose-Responsive Liver-Specific Promoter Resulted in Increased Efficacy of Adeno-Associated Virus Serotype 8-Insulin Gene Therapy of Diabetic Mice. Cells. 2020; 9(11):2474. https://doi.org/10.3390/cells9112474
Chicago/Turabian StyleSia, Kian Chuan, Zhen Ying Fu, Roy Y. Calne, Amit C. Nathwani, Kok Onn Lee, and Shu Uin Gan. 2020. "Modification of a Constitutive to Glucose-Responsive Liver-Specific Promoter Resulted in Increased Efficacy of Adeno-Associated Virus Serotype 8-Insulin Gene Therapy of Diabetic Mice" Cells 9, no. 11: 2474. https://doi.org/10.3390/cells9112474
APA StyleSia, K. C., Fu, Z. Y., Calne, R. Y., Nathwani, A. C., Lee, K. O., & Gan, S. U. (2020). Modification of a Constitutive to Glucose-Responsive Liver-Specific Promoter Resulted in Increased Efficacy of Adeno-Associated Virus Serotype 8-Insulin Gene Therapy of Diabetic Mice. Cells, 9(11), 2474. https://doi.org/10.3390/cells9112474



