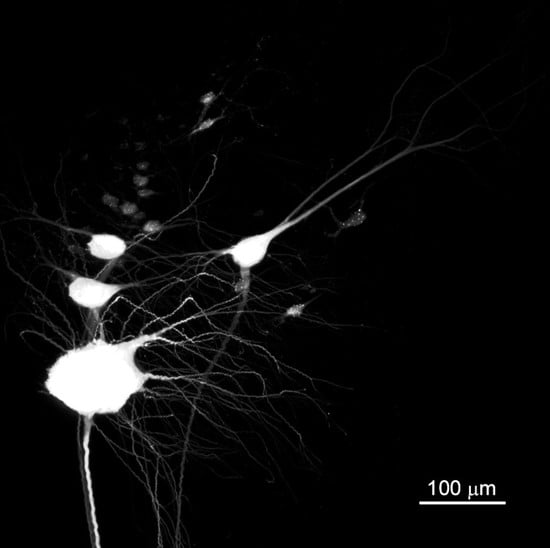
Source of Early Regenerating Axons in Lamprey Spinal Cord Revealed by Wholemount Optical Clearing with BABB
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Spinal Cord Transection
2.2. Immunohistochemistry
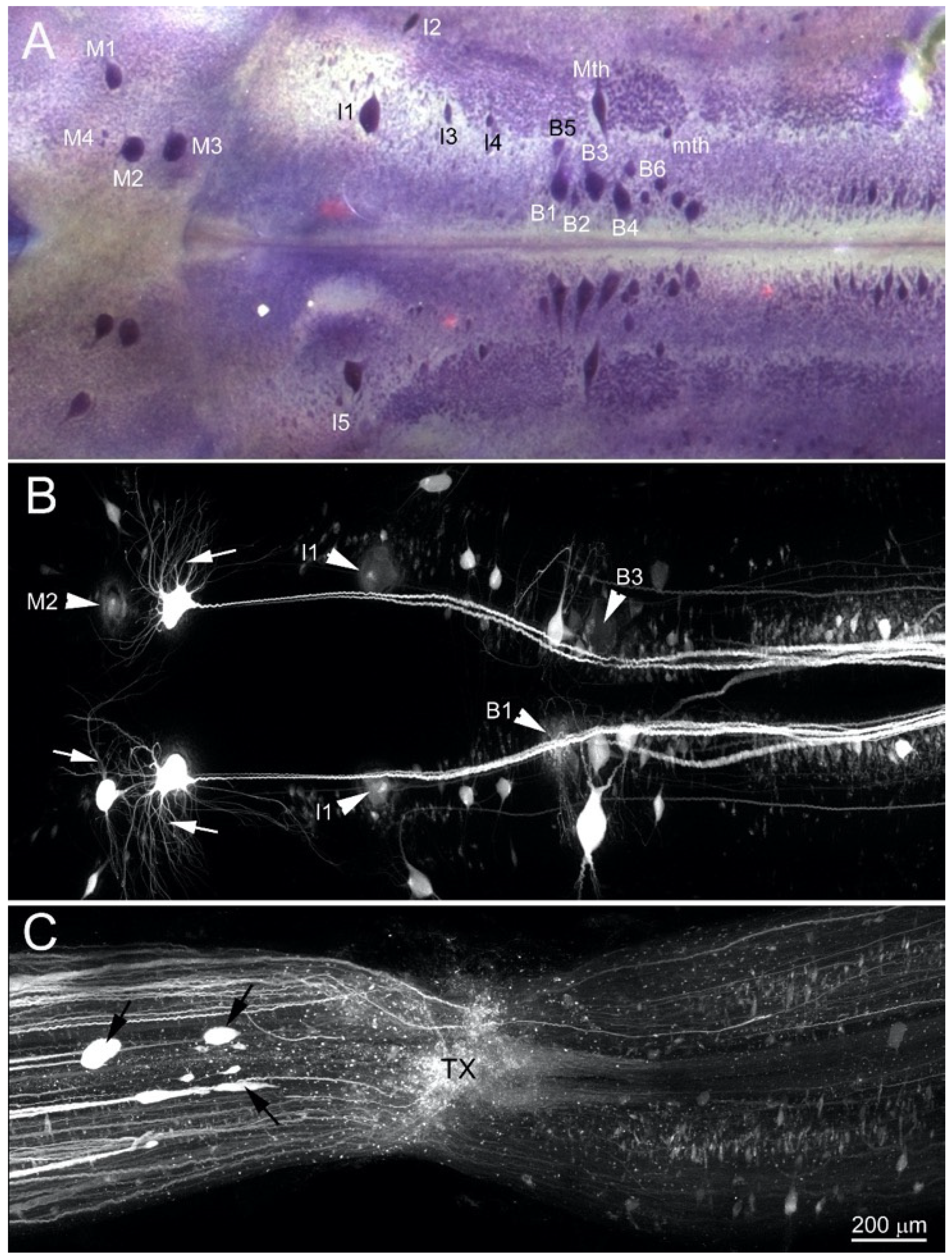
2.3. Retrograde Labeling of Reticulospinal Neurons
2.4. Anterograde Labeling of Degenerating and Regenerating Axons
2.5. Tissue Clearing and Imaging
2.6. FLICA Labeling of Apoptotic Neurons
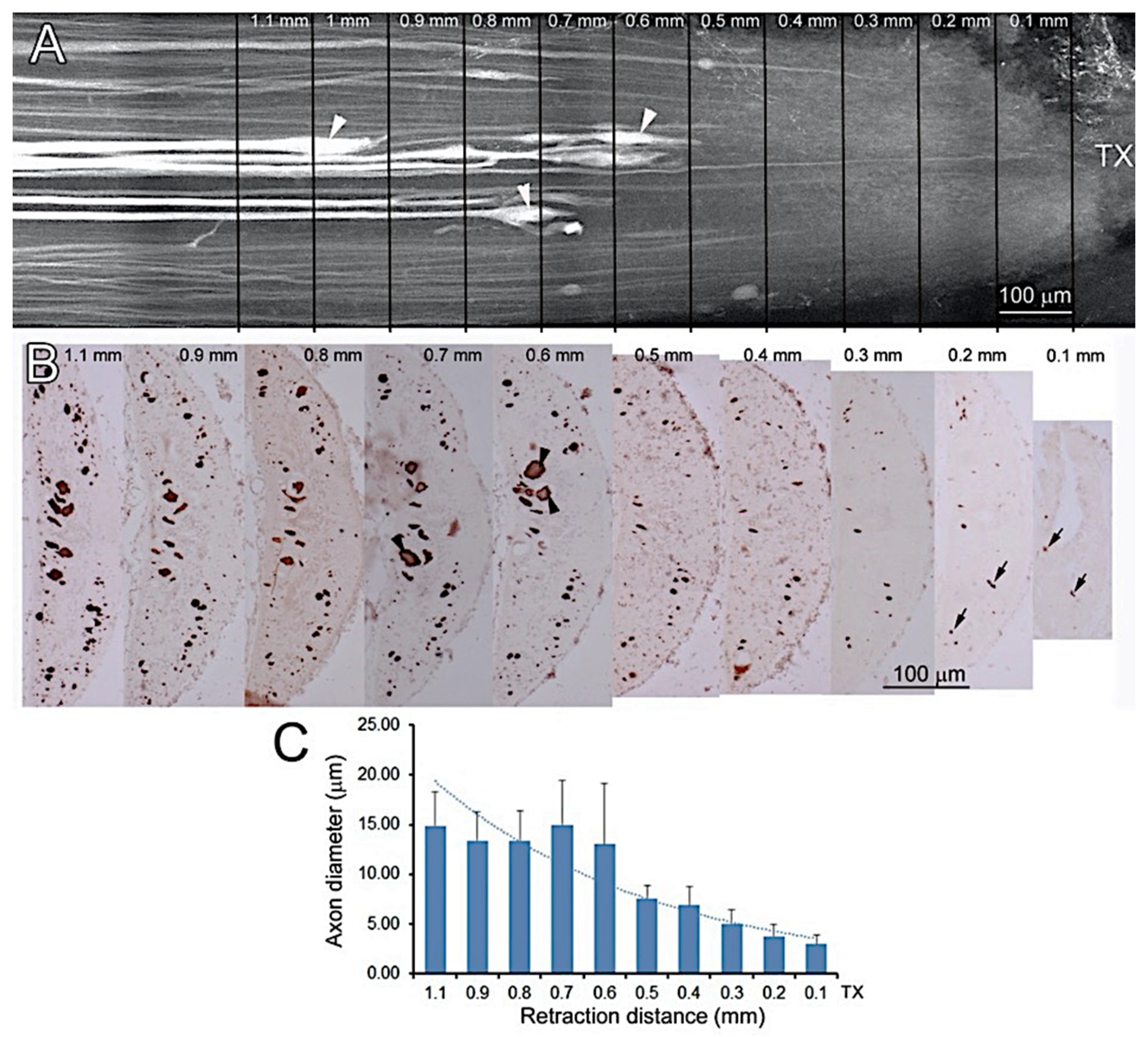
2.7. Correlating Retraction Distances of Axons with Their Diameters
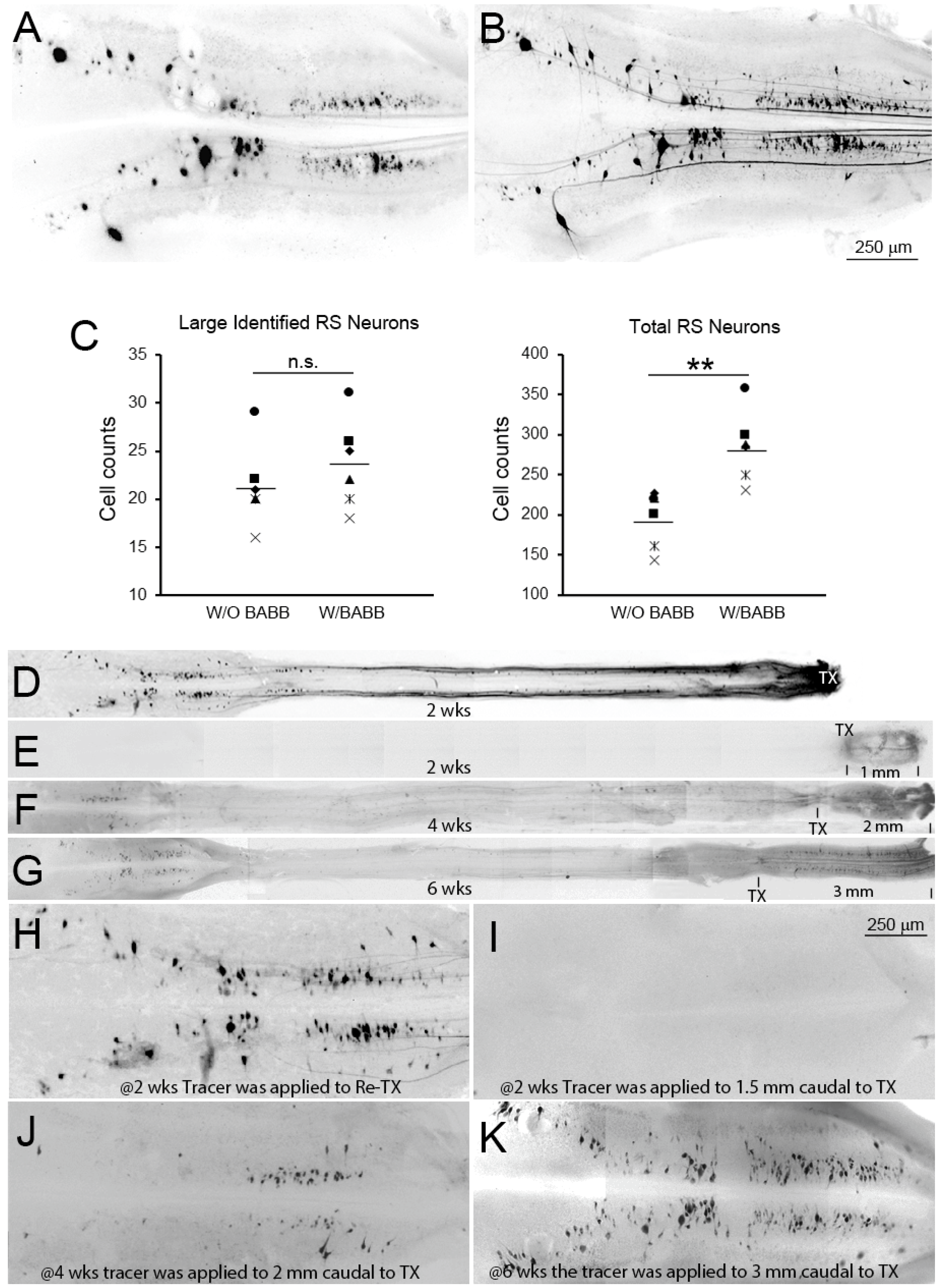
2.8. Cell Counts and Statistical Analysis
2.9. Assessment of the Length of Regenerating Axons
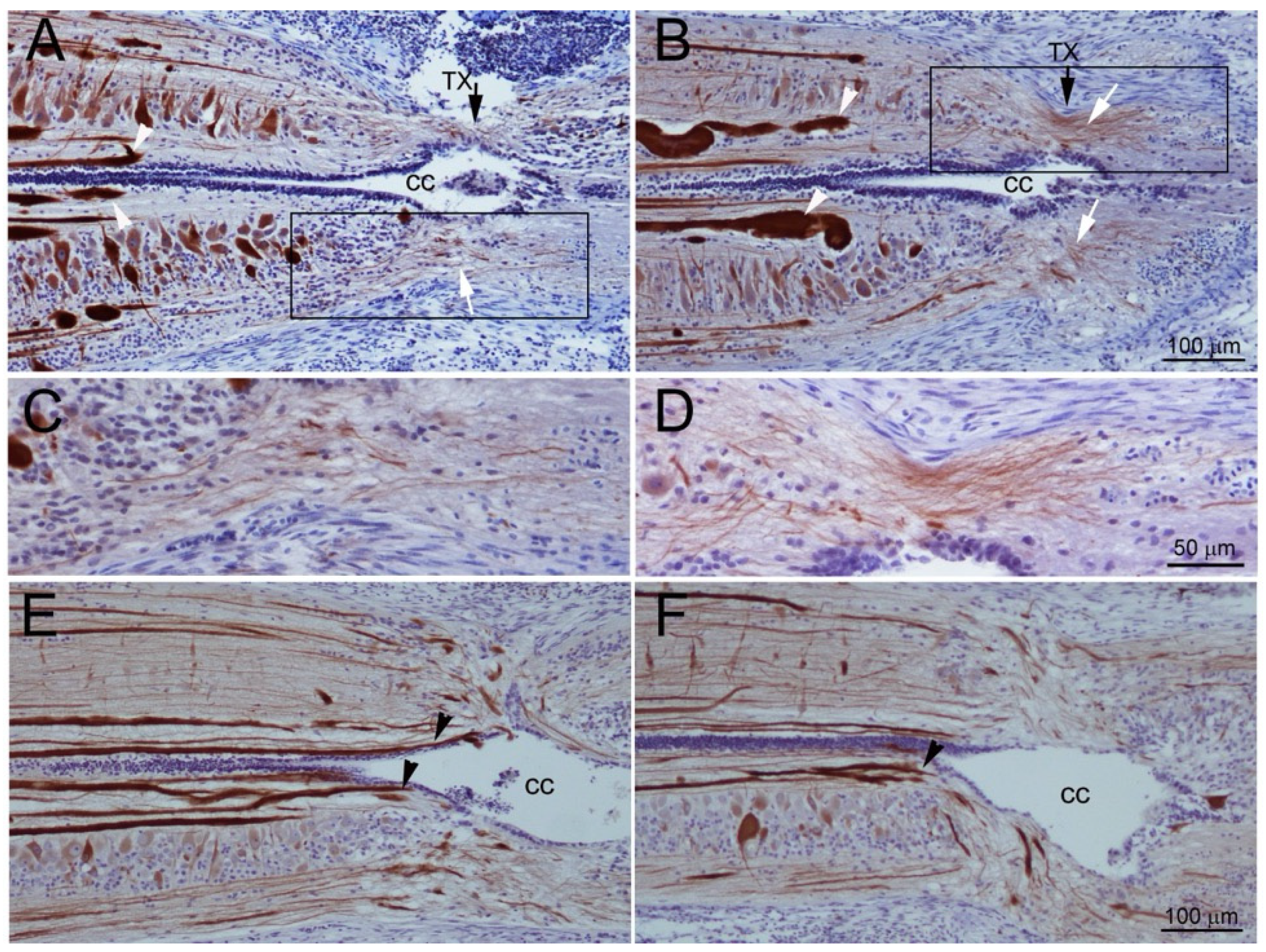
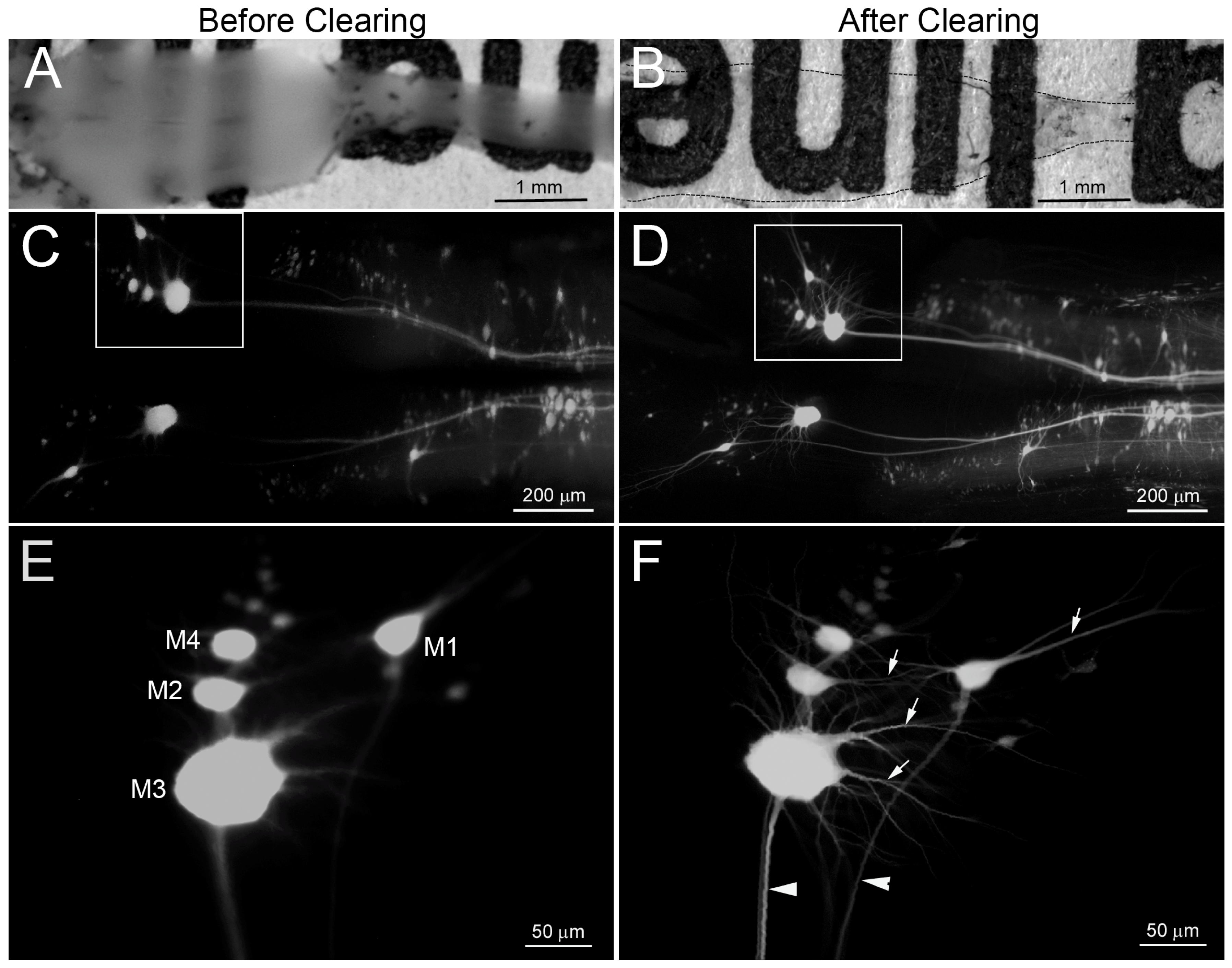
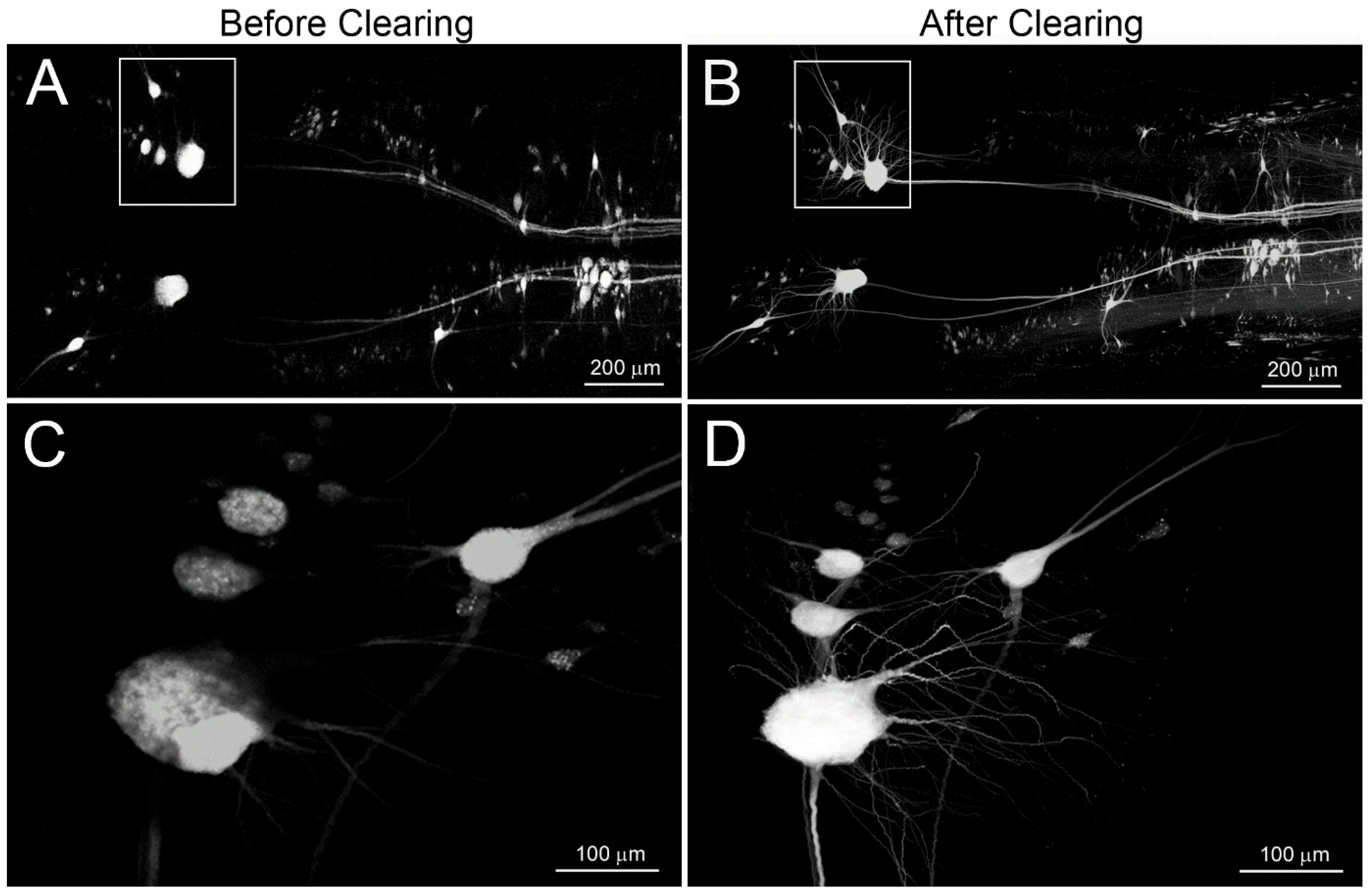
3. Results
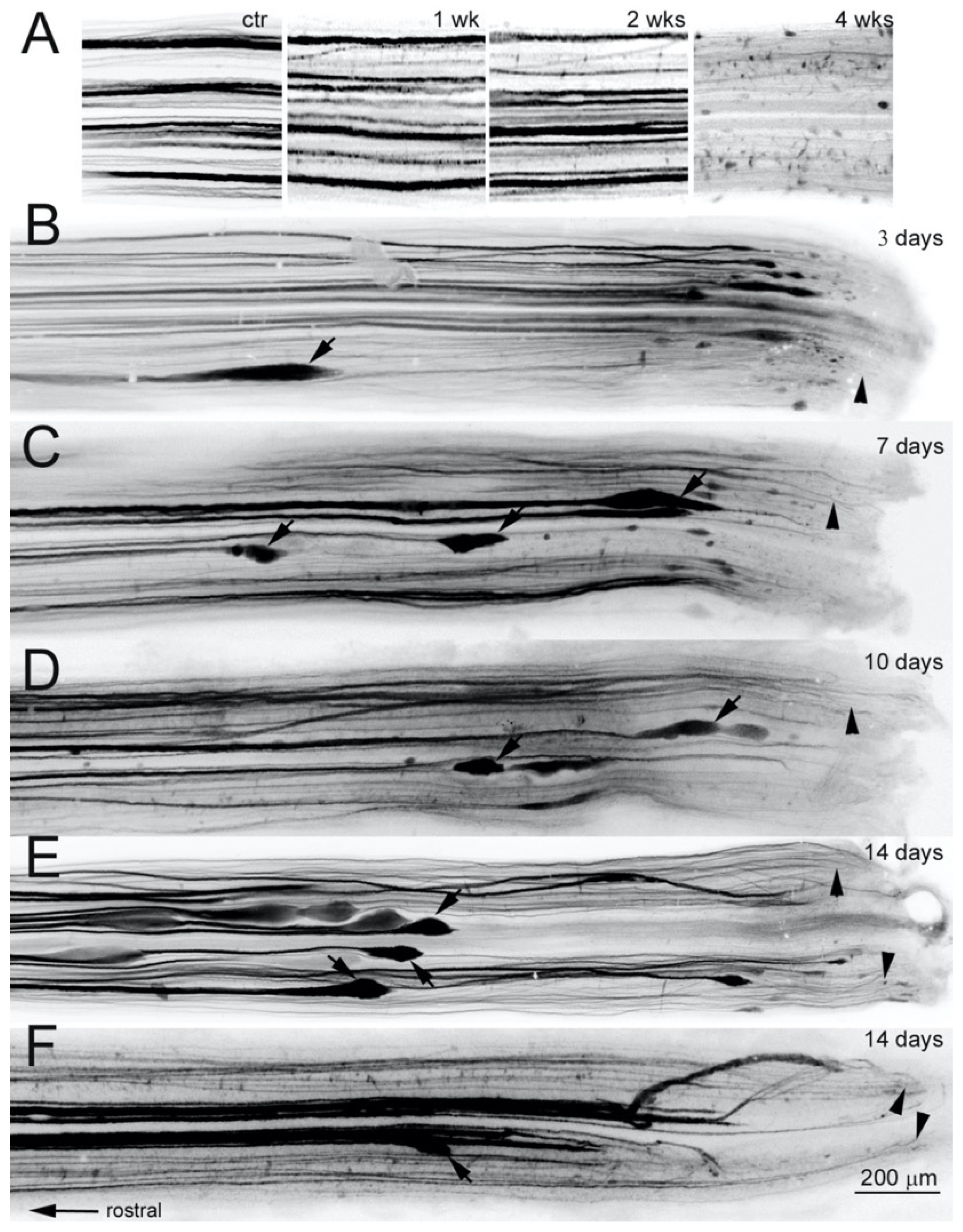
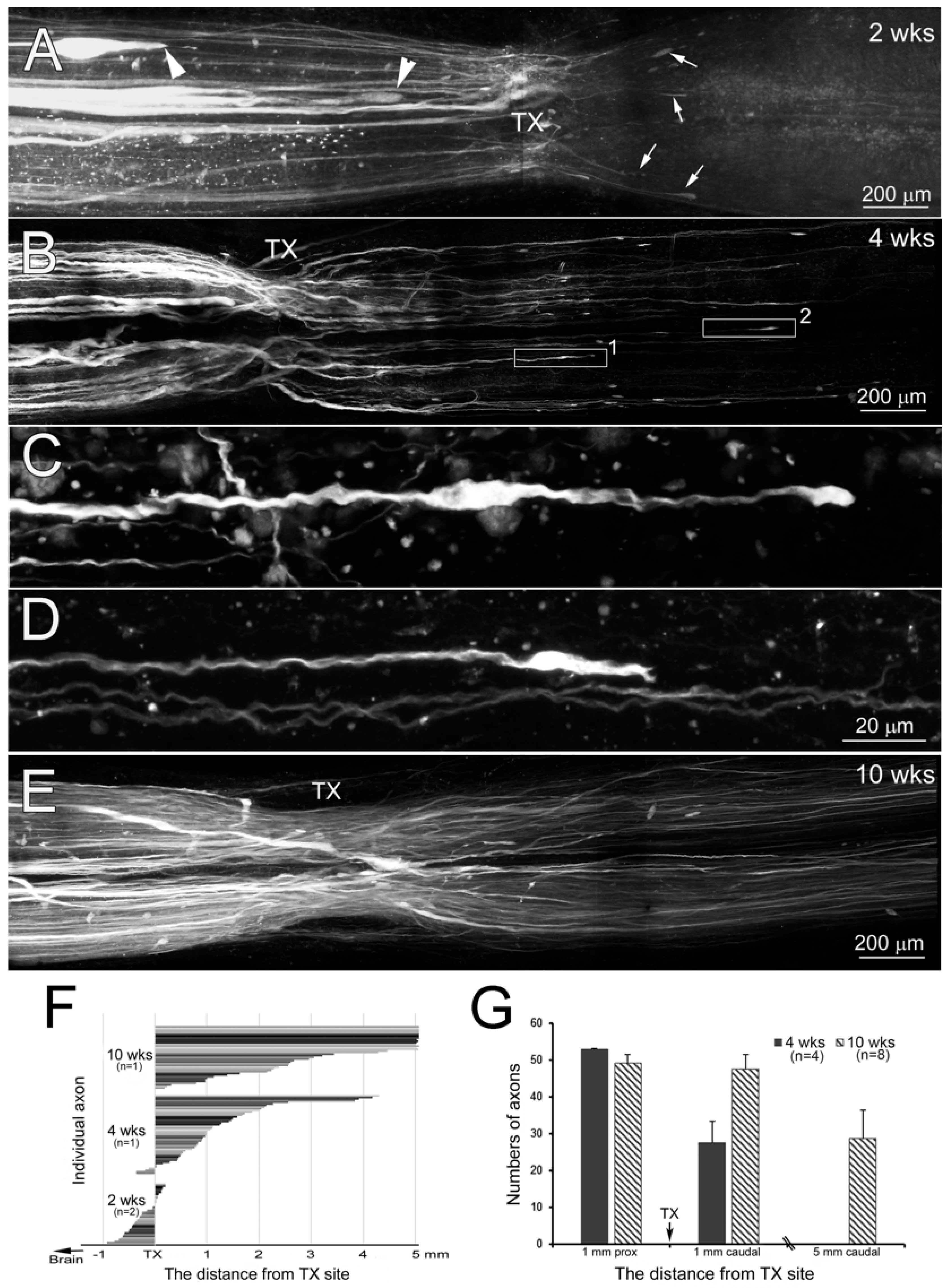
3.1. Axons Bridge the Lesion by Two Weeks Post-TX
3.2. BABB Clearing Improves Structural Resolution of Retrogradely Labeled Neurons
3.3. Application of BABB Clearing after Imaging with FLICA to Assess Regeneration and Apoptotic Signaling in the Same Preparation
3.4. BABB Clearing Increases the Apparent Number of Neurons with Regenerated Axons
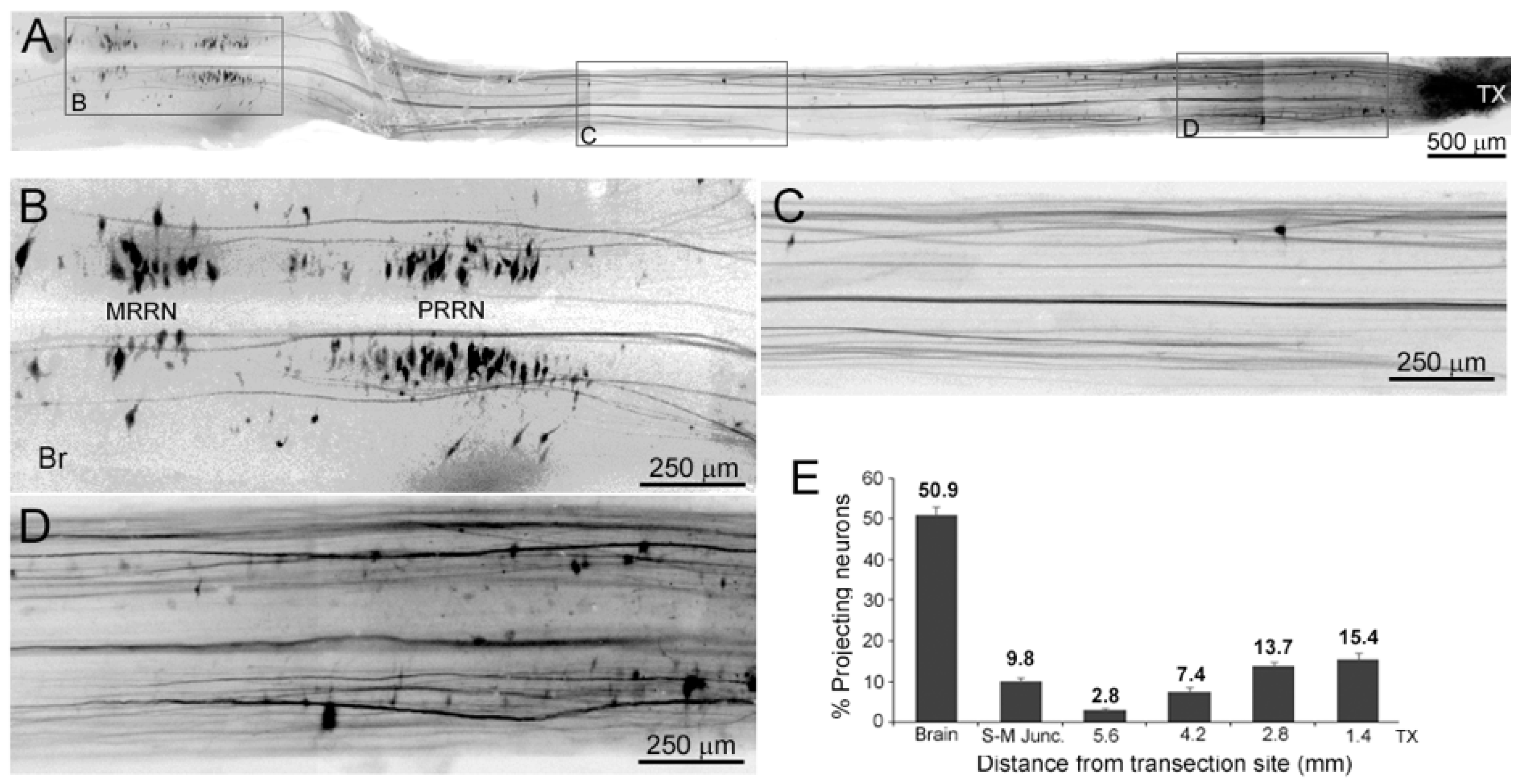
3.5. Early Regenerating Axons Arise from Small RS Neurons and Small Propriospinal Neurons Near the TX
3.6. Axon Retraction Is Greater in Large Caliber RS Axons than in Narrow Ones
3.7. Time Course of Axon Regeneration Demonstrated in SC Wholemounts by Anterograde Labeling
4. Discussion
4.1. BABB Clearing
4.2. Early Regenerating RS Axons
4.3. Anterograde Tracing of Descending Axons in CNS Wholemounts
4.4. Initial Axon Retraction
4.5. Time Course of Regenerating RS Axons
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shik, M.L.; Orlovsky, G.N. Neurophysiology of locomotor automatism. Physiol. Rev. 1976, 56, 465–501. [Google Scholar] [CrossRef]
- Jacobs, A.J.; Swain, G.P.; Snedeker, J.A.; Pijak, D.S.; Gladstone, L.J.; Selzer, M.E. Recovery of Neurofilament Expression Selectively in Regenerating Reticulospinal Neurons. J. Neurosci. 1997, 17, 5206–5220. [Google Scholar] [CrossRef] [PubMed]
- Rovainen, C.M. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). II. Dorsal cells and giant interneurons. J. Neurophysiol. 1967, 30, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.P.; Snedeker, J.A.; Ayers, J.; Selzer, M.E. Cytoarchitecture of spinal-projecting neurons in the brain of the larval sea lamprey. J. Comp. Neurol. 1993, 336, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Rovainen, C.M. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mauthner cells. J. Neurophysiol. 1967, 30, 1000–1023. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.; McClellan, A.D. Long Distance Axonal Regeneration of Identified Lamprey Reticulospinal Neurons. Exp. Neurol. 1994, 127, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Rovainen, C.M. Regeneration of Müller and Mauthner axons after spinal transection in larval lampreys. J. Comp. Neurol. 1976, 168, 545–554. [Google Scholar] [CrossRef]
- Yin, H.; Selzer, M. Axonal regeneration in lamprey spinal cord. J. Neurosci. 1983, 3, 1135–1144. [Google Scholar] [CrossRef]
- Roederer, E.; Goldberg, N.; Cohen, M. Modification of retrograde degeneration in transected spinal axons of the lamprey by applied DC current. J. Neurosci. 1983, 3, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.R.; Troxel, M.T.; Kohler, V.J.; Grossmann, E.M.; McClellan, A.D. Time course of locomotor recovery and functional regeneration in spinal-transected lamprey: Kinematics and electromyography. Exp. Brain Res. 1993, 97, 83–95. [Google Scholar] [CrossRef]
- McClellan, A.D. Time course of locomotor recovery and functional regeneration in spinal cord-transected lamprey: In vitro preparations. J. Neurophysiol. 1994, 72, 847–860. [Google Scholar] [CrossRef]
- Oliphint, P.A.; Alieva, N.; Foldes, A.E.; Tytell, E.D.; Lau, B.Y.B.; Pariseau, J.S.; Cohen, A.H.; Morgan, J.R. Regenerated synapses in lamprey spinal cord are sparse and small even after functional recovery from injury. J. Comp. Neurol. 2010, 518, 2854–2872. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.R.; McClellan, A.D. Extent and time course of restoration of descending brainstem projections in spinal cord-transected lamprey. J. Comp. Neurol. 1994, 344, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Lurie, D.I.; Pijak, D.S.; Selzer, M.E. Structure of reticulospinal axon growth cones and their cellular environment during regeneration in the lamprey spinal cord. J. Comp. Neurol. 1994, 344, 559–580. [Google Scholar] [CrossRef]
- Hall, G.; Cohen, M. Extensive dendritic sprouting induced by close axotomy of central neurons in the lamprey. Science 1983, 222, 518–521. [Google Scholar] [CrossRef]
- Davis, G.; McClellan, A.D. Time course of anatomical regeneration of descending brainstem neurons and behavioral recovery in spinal-transected lamprey. Brain Res. 1993, 602, 131–137. [Google Scholar] [CrossRef]
- Tang, D.; Selzer, M.E. Projections of lamprey spinal neurons determined by the retrograde axonal transport of horseradish peroxidase. J. Comp. Neurol. 1979, 188, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jin, L.-Q.; Sul, J.-Y.; Haydon, P.G.; Selzer, M.E. Live Imaging of Regenerating Lamprey Spinal Axons. Neurorehabilit. Neural Repair 2005, 19, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, G.; Selzer, M.E. Activated caspase detection in living tissue combined with subsequent retrograde labeling, immunohistochemistry or in situ hybridization in whole-mounted lamprey brains. J. Neurosci. Methods 2013, 220, 92–98. [Google Scholar] [CrossRef][Green Version]
- Nikitina, N.; Bronner, M.E.; Sauka-Spengler, T. Immunostaining of Whole-Mount and Sectioned Lamprey Embryos. Cold Spring Harb. Protoc. 2009, 2009, 5126. [Google Scholar] [CrossRef]
- Hill, A.S.; Nishino, A.; Nakajo, K.; Zhang, G.; Fineman, J.R.; Selzer, M.E.; Okamura, Y.; Cooper, E.C. Ion Channel Clustering at the Axon Initial Segment and Node of Ranvier Evolved Sequentially in Early Chordates. PLoS Genet. 2008, 4, e1000317. [Google Scholar] [CrossRef]
- Shifman, M.I.; Selzer, M.E. In situ hybridization in wholemounted lamprey spinal cord: Localization of netrin mRNA expression. J. Neurosci. Methods 2000, 104, 19–25. [Google Scholar] [CrossRef]
- Swain, G.P.; Jacobs, A.J.; Frei, E.; Selzer, M.E. A Method for in Situ Hybridization in Wholemounted Lamprey Brain: Neurofilament Expression in Larvae and Adults. Exp. Neurol. 1994, 126, 256–269. [Google Scholar] [CrossRef]
- Dodt, H.-U.; Leischner, U.; Schierloh, A.; Jährling, N.; Mauch, C.P.; Deininger, K.; Deussing, J.M.; Eder, M.; Zieglgänsberger, W.; Becker, K. Ultramicroscopy: Three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 2007, 4, 331–336. [Google Scholar] [CrossRef]
- Becker, K.; Jährling, N.; Kramer, E.R.; Schnorrer, F.; Dodt, H.-U. Ultramicroscopy: 3D reconstruction of large microscopical specimens. J. Biophotonics 2008, 1, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Jahrling, N.; Saghafi, S.; Weiler, R.; Dodt, H.-U. Chemical clearing and dehydration of GFP expressing mouse brains. PLoS ONE 2012, 7, e33916. [Google Scholar] [CrossRef]
- Dent, J.A.; Polson, A.G.; Klymkowsky, M.W. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development 1989, 105, 61–74. [Google Scholar]
- Zukor, K.A.; Kent, D.T.; Odelberg, S.J. Fluorescent whole-mount method for visualizing three-dimensional relationships in intact and regenerating adult newt spinal cords. Dev. Dyn. 2010, 239, 3048–3057. [Google Scholar] [CrossRef] [PubMed]
- Ivins, S.; Roberts, C.; Vernay, B.; Scambler, P.J. Analysis of Coronary Vessels in Cleared Embryonic Hearts. J. Vis. Exp. 2016, 2016, e54800. [Google Scholar] [CrossRef]
- Zucker, R.M. Whole insect and mammalian embryo imaging with confocal microscopy: Morphology and apoptosis. Cytom. Part. A 2006, 69, 1143–1152. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, L.-Q.; Hu, J.; Rodemer, W.; Selzer, M.E. Antisense Morpholino Oligonucleotides Reduce Neurofilament Synthesis and Inhibit Axon Regeneration in Lamprey Reticulospinal Neurons. PLoS ONE 2015, 10, e0137670. [Google Scholar] [CrossRef]
- Selzer, M.E. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J. Physiol. 1978, 277, 395–408. [Google Scholar] [CrossRef]
- Wood, M.; Cohen, M. Synaptic regeneration in identified neurons of the lamprey spinal cords. Science 1979, 206, 344–347. [Google Scholar] [CrossRef]
- Cohen, A.H.; Mackler, S.A.; Selzer, M.E. Behavioral recovery following spinal transection: Functional regeneration in the lamprey CNS. Trends Neurosci. 1988, 11, 227–231. [Google Scholar] [CrossRef]
- Mackler, S.; Selzer, M.; Hess, J.; Clements, J.; Narayan, O. Regeneration of functional synapses between individual recognizable neurons in the lamprey spinal cord. Science 1985, 229, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Mackler, S.A.; Selzer, M.E. Specificity of synaptic regeneration in the spinal cord of the larval sea lamprey. J. Physiol. 1987, 388, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, K.L.; Allen, S.R.; Harkenrider, T.L.; Fogerson, S.M.; Guadarrama, E.; Morgan, J.R. Regenerative capacity in the lamprey spinal cord is not altered after a repeated transection. PLoS ONE 2019, 14, e0204193. [Google Scholar] [CrossRef]
- Lau, B.Y.B.; Fogerson, S.M.; Walsh, R.B.; Morgan, J.R. Cyclic AMP promotes axon regeneration, lesion repair and neuronal survival in lampreys after spinal cord injury. Exp. Neurol. 2013, 250, 31–42. [Google Scholar] [CrossRef]
- Barreiro-Iglesias, A.; Zhang, G.; Selzer, M.E.; Shifman, M.I. Complete Spinal Cord Injury and Brain Dissection Protocol for Subsequent Wholemount In Situ Hybridization in Larval Sea Lamprey. J. Vis. Exp. 2014, 2014, 51494. [Google Scholar] [CrossRef]
- Barreiro-Iglesias, A.; Shifman, M.I. Use of Fluorochrome-Labeled Inhibitors of Caspases to Detect Neuronal Apoptosis in the Whole-Mounted Lamprey Brain after Spinal Cord Injury. Enzym. Res. 2012, 2012, 1–7. [Google Scholar] [CrossRef][Green Version]
- Hu, J.; Zhang, G.; Rodemer, W.; Jin, L.-Q.; Shifman, M.; Selzer, M.E. The role of RhoA in retrograde neuronal death and axon regeneration after spinal cord injury. Neurobiol. Dis. 2017, 98, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Rovainen, C.M. Synaptic interactions of identified nerve cells in the spinal cord of the sea lamprey. J. Comp. Neurol. 1974, 154, 189–206. [Google Scholar] [CrossRef]
- Rovainen, C.M. Neurobiology of lampreys. Physiol. Rev. 1979, 59, 1007–1077. [Google Scholar] [CrossRef]
- Buchanan, J.T. Electrophysiological properties of identified classes of lamprey spinal neurons. J. Neurophysiol. 1993, 70, 2313–2325. [Google Scholar] [CrossRef]
- Buchanan, J.T. Contributions of identifiable neurons and neuron classes to lamprey vertebrate neurobiology. Prog. Neurobiol. 2001, 63, 441–466. [Google Scholar] [CrossRef]
- Hall, G.; Poulos, A.; Cohen, M. Sprouts emerging from the dendrites of axotomized lamprey central neurons have axonlike ultrastructure. J. Neurosci. 1989, 9, 588–599. [Google Scholar] [CrossRef]
- Jin, L.-Q.; Zhang, G.; Jamison, C.; Takano, H.; Haydon, P.G.; Selzer, M.E. Axon regeneration in the absence of growth cones; acceleration by cyclic AMP. J. Comp. Neurol. 2009, 515, 295–312. [Google Scholar] [CrossRef]
- McHale, M.K.; Hall, G.F.; Cohen, M.J. Early cytoskeletal changes following injury of giant spinal axons in the lamprey. J. Comp. Neurol. 1995, 353, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Mackler, S.; Yin, H.; Selzer, M. Determinants of directional specificity in the regeneration of lamprey spinal axons. J. Neurosci. 1986, 6, 1814–1821. [Google Scholar] [CrossRef]
- Yin, H.S.; Mackler, S.A.; Selzer, M.E. Directional specificity in the regeneration of lamprey spinal axons. Science 1984, 224, 894–896. [Google Scholar] [CrossRef]
- Lurie, D.I.; Selzer, M.E. Preferential regeneration of spinal axons through the scar in hemisected lamprey spinal cord. J. Comp. Neurol. 1991, 313, 669–679. [Google Scholar] [CrossRef]
- Currie, S.N.; Ayers, J. Regeneration of locomotor command systems in the sea lamprey. Brain Res. 1983, 279, 238–240. [Google Scholar] [CrossRef]
- McClellan, A.D. Brainstem command systems for locomotion in the lamprey: Localization of descending pathways in the spinal cord. Brain Res. 1988, 457, 338–349. [Google Scholar] [CrossRef]
- Richardson, D.S.; Lichtman, J.W. Clarifying Tissue Clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Azaripour, A.; Lagerweij, T.; Scharfbillig, C.; Jadczak, A.E.; Willerhausen, B.; Van Noorden, C.J.; Jadczak, L. A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog. Histochem. Cytochem. 2016, 51, 9–23. [Google Scholar] [CrossRef]
- Ariel, P. A beginner’s guide to tissue clearing. Int. J. Biochem. Cell Biol. 2017, 84, 35–39. [Google Scholar] [CrossRef]
- Murakami, Y.; Pasqualetti, M.; Takio, Y.; Hirano, S.; Rijli, F.M.; Kuratani, S. Segmental development of reticulospinal and branchiomotor neurons in lamprey: Insights into the evolution of the vertebrate hindbrain. Development 2004, 131, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.G. Two-headed mutants of the lamprey, a basal vertebrate. Zool. Lett. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Tada, M.N.; Kuratani, S. Evolutionary and developmental understanding of the spinal accessory nerve. Zool. Lett. 2015, 1, 4. [Google Scholar] [CrossRef]
- Hall, G.; Cohen, M. Dendritic amputation redistributes sprouting evoked by axotomy in lamprey central neurons. J. Neurosci. 1988, 8, 3598–3606. [Google Scholar] [CrossRef]
- Hall, G.; Cohen, M. The pattern of dendritic sprouting and retraction induced by axotomy of lamprey central neurons. J. Neurosci. 1988, 8, 3584–3597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Rodemer, W.; Lee, T.; Hu, J.; Selzer, M. The Effect of Axon Resealing on Retrograde Neuronal Death after Spinal Cord Injury in Lamprey. Brain Sci. 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Laramore, C.; Maymind, E.; Shifman, M. Expression of neurotrophin and its tropomyosin-related kinase receptors (Trks) during axonal regeneration following spinal cord injury in larval lamprey. Neuroscience 2011, 183, 265–277. [Google Scholar] [CrossRef]
- Ertürk, A.; Becker, K.; Jährling, N.; Mauch, C.P.; Hojer, C.D.; Egen, J.G.; Hellal, F.; Bradke, F.; Sheng, M.; Dodt, H.-U. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 2012, 7, 1983–1995. [Google Scholar] [CrossRef]
- Susaki, E.A.; Tainaka, K.; Perrin, D.; Kishino, F.; Tawara, T.; Watanabe, T.M.; Yokoyama, C.; Onoe, H.; Eguchi, M.; Yamaguchi, S.; et al. Whole-Brain Imaging with Single-Cell Resolution Using Chemical Cocktails and Computational Analysis. Cell 2014, 157, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Hama, H.; Kurokawa, H.; Kawano, H.; Ando, R.; Shimogori, T.; Noda, H.; Fukami, K.; Sakaue-Sawano, A.; Miyawaki, A. Scale: A chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 2011, 14, 1481–1488. [Google Scholar] [CrossRef]
- Ke, M.-T.; Fujimoto, S.; Imai, T. SeeDB: A simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat. Neurosci. 2013, 16, 1154–1161. [Google Scholar] [CrossRef]
- Baek, K.; Jung, S.; Lee, J.; Min, E.; Jung, W.; Cho, H. Quantitative assessment of regional variation in tissue clearing efficiency using optical coherence tomography (OCT) and magnetic resonance imaging (MRI): A feasibility study. Sci. Rep. 2019, 9, 2923. [Google Scholar] [CrossRef]
- Parra, S.G.; Vesuna, S.S.; Murray, T.A.; Levene, M.J. Multiphoton Microscopy of Cleared Mouse Brain Expressing YFP. J. Vis. Exp. 2012, 2012, 3848. [Google Scholar] [CrossRef]
- McClellan, A. Locomotor recovery in spinal-transected lamprey: Role of functional regeneration of descending axons from brainstem locomotor command neurons. Neuroscience 1990, 37, 781–798. [Google Scholar] [CrossRef]
- Shifman, M.I.; Zhang, G.; Selzer, M. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. J. Comp. Neurol. 2008, 510, 269–282. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, J.; Li, S.; Huang, L.; Selzer, M.E. Selective expression of CSPG receptors PTPsigma and LAR in poorly regenerating reticulospinal neurons of lamprey. J. Comp. Neurol. 2014, 522, 2209–2229. [Google Scholar] [CrossRef]
- Vargas, M.E.; Barres, B.A. Why Is Wallerian Degeneration in the CNS So Slow? Annu. Rev. Neurosci. 2007, 30, 153–179. [Google Scholar] [CrossRef]
- Perry, V.H.; Brown, M.C.; Gordon, S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J. Exp. Med. 1987, 165, 1218–1223. [Google Scholar] [CrossRef]
- Hilton, B.J.; Blanquie, O.; Tedeschi, A.; Bradke, F. High-resolution 3D imaging and analysis of axon regeneration in unsectioned spinal cord with or without tissue clearing. Nat. Protoc. 2019, 14, 1235–1260. [Google Scholar] [CrossRef]
- Hall, G.F.; Lee, V.M.-Y. Neurofilament sidearm proteolysis is a prominent early effect of axotomy in lamprey giant central neurons. J. Comp. Neurol. 1995, 353, 38–49. [Google Scholar] [CrossRef]
- Cragg, B.G.; Thomas, P.K. Changes in conduction velocity and fibre size proximal to peripheral nerve lesions. J. Physiol. 1961, 157, 315–327. [Google Scholar] [CrossRef]
- Aitken, J.T.; Thomas, P.K. Retrograde changes in fibre size following nerve section. J. Anat. 1962, 96, 121–129. [Google Scholar]
- Armstrong, J.; Zhang, L.; McClellan, A.D. Axonal regeneration of descending and ascending spinal projection neurons in spinal cord-transected larval lamprey. Exp. Neurol. 2003, 180, 156–166. [Google Scholar] [CrossRef]

| Experiments | 3 days | 1 wk | 10 days | 2 wks | 4 wks | 6 wks | 10 wks | 13 wks |
|---|---|---|---|---|---|---|---|---|
| NF immunostaining | 2 | 2 | ||||||
| Retrograde labeling | 1 | 1 | 1 | |||||
| Re-TX | 6 | |||||||
| Regeneration | 1 (1 mm) | 1 (2 mm) | 1 (3 mm) | 6 (5 mm) | ||||
| Anterograde labeling | ||||||||
| Wallerian degeneration | 1 | 1 | 1 | |||||
| Retraction | 3 | 3 | 4 | 4 | ||||
| Regeneration | 2 | 5 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Rodemer, W.; Sinitsa, I.; Hu, J.; Selzer, M.E. Source of Early Regenerating Axons in Lamprey Spinal Cord Revealed by Wholemount Optical Clearing with BABB. Cells 2020, 9, 2427. https://doi.org/10.3390/cells9112427
Zhang G, Rodemer W, Sinitsa I, Hu J, Selzer ME. Source of Early Regenerating Axons in Lamprey Spinal Cord Revealed by Wholemount Optical Clearing with BABB. Cells. 2020; 9(11):2427. https://doi.org/10.3390/cells9112427
Chicago/Turabian StyleZhang, Guixin, William Rodemer, Isabelle Sinitsa, Jianli Hu, and Michael E. Selzer. 2020. "Source of Early Regenerating Axons in Lamprey Spinal Cord Revealed by Wholemount Optical Clearing with BABB" Cells 9, no. 11: 2427. https://doi.org/10.3390/cells9112427
APA StyleZhang, G., Rodemer, W., Sinitsa, I., Hu, J., & Selzer, M. E. (2020). Source of Early Regenerating Axons in Lamprey Spinal Cord Revealed by Wholemount Optical Clearing with BABB. Cells, 9(11), 2427. https://doi.org/10.3390/cells9112427