Glutamatergic Receptor Trafficking and Delivery: Role of the Exocyst Complex
Abstract
1. Introduction
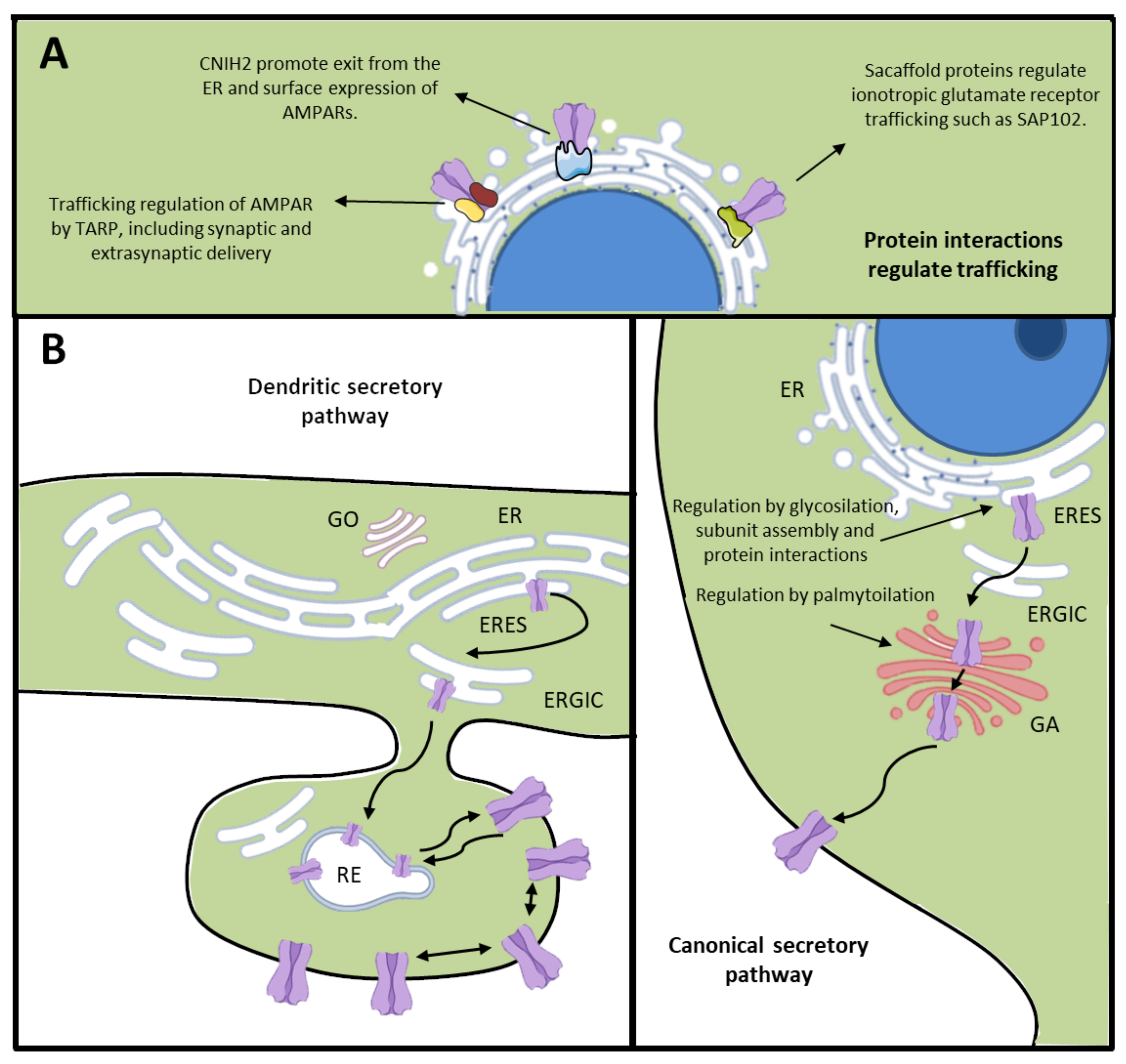
2. Membrane Trafficking of Ionotropic Glutamate Receptors: From ER to Plasma Membrane
2.1. Membrane Trafficking in Neurons
2.2. Membrane Trafficking of Ionotropic Glutamate Receptors
2.3. Membrane Trafficking in Synaptic Plasticity
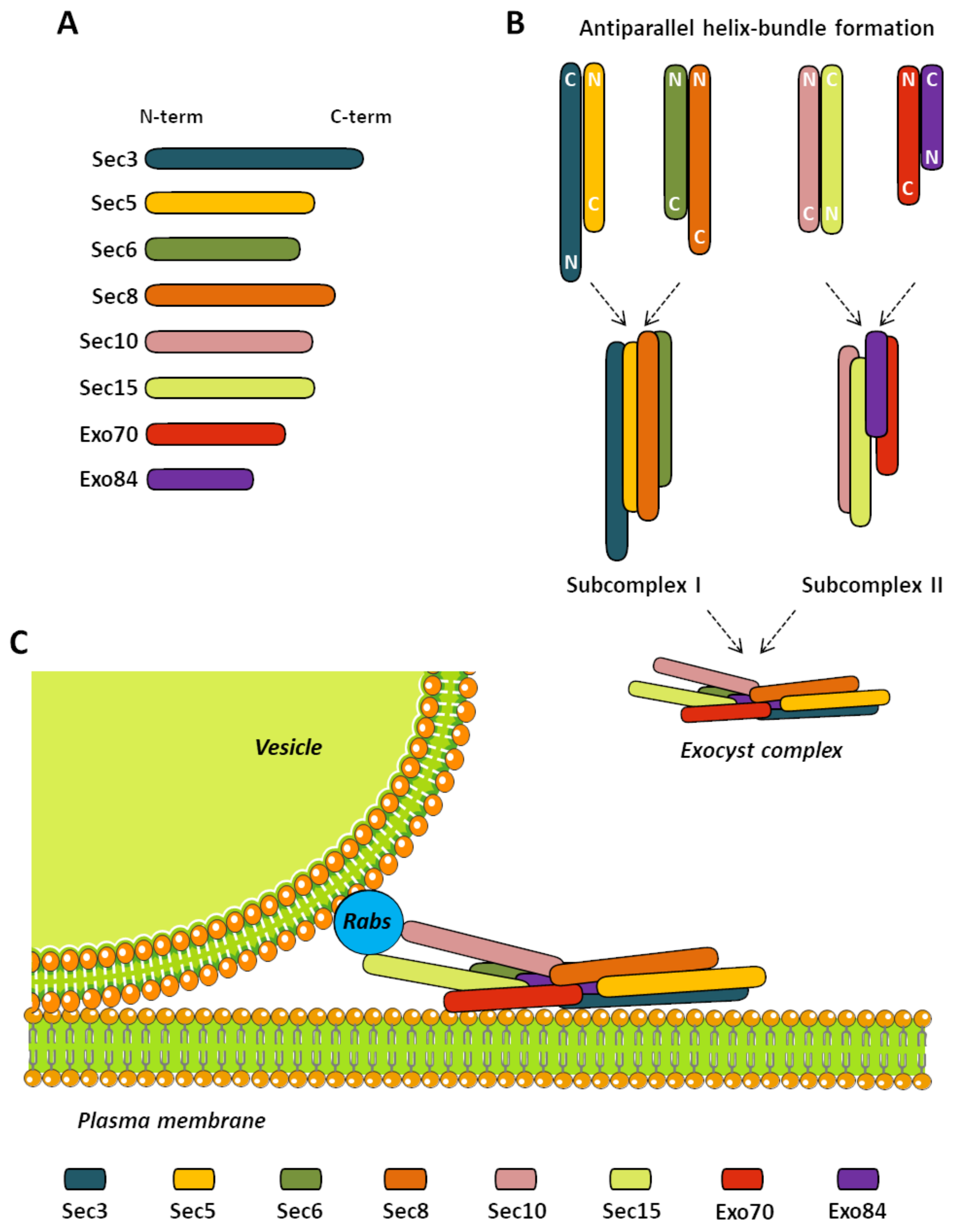
3. The Exocyst Complex
Exocyst in Neurons
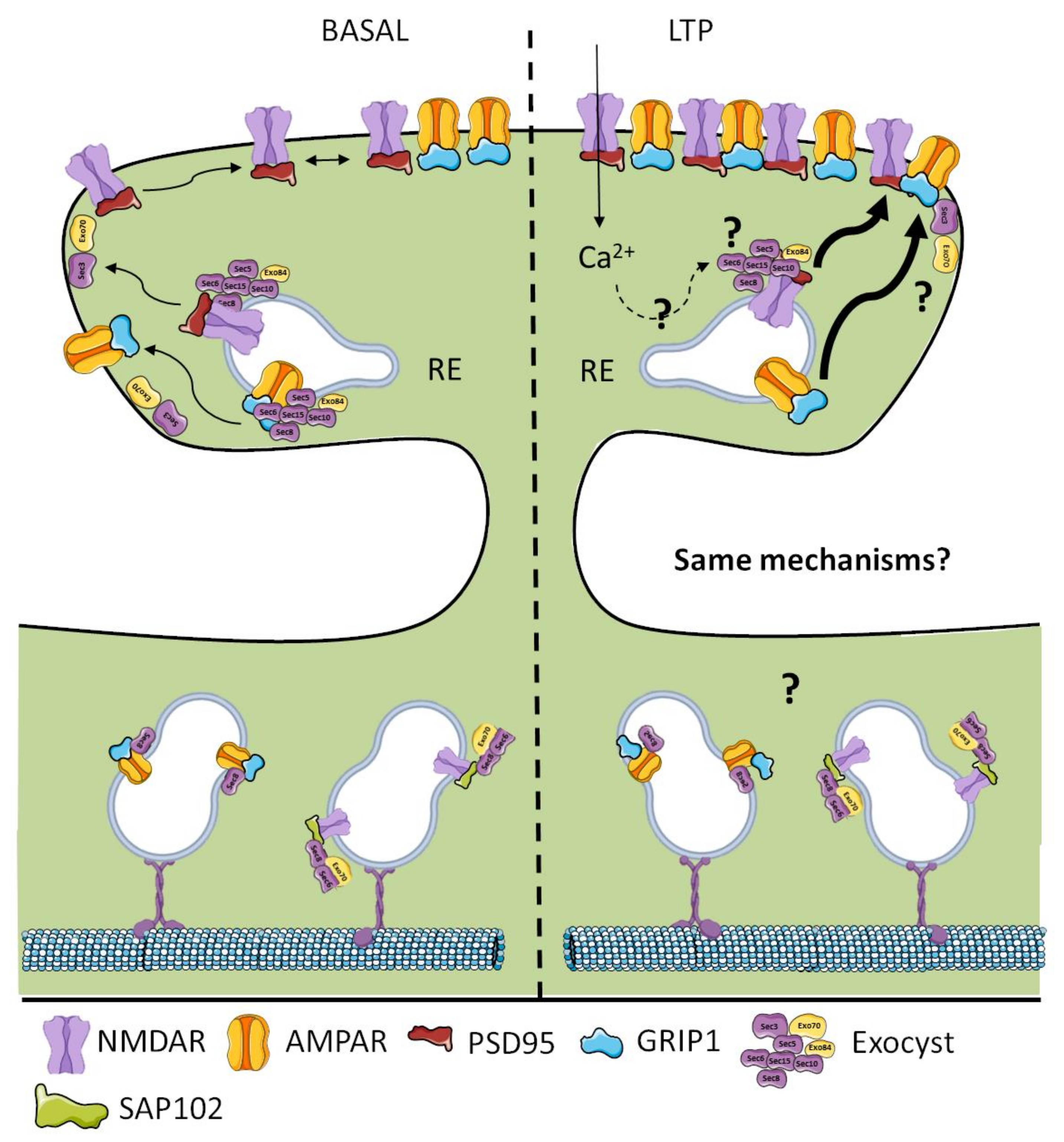
4. Exocyst in Ionotropic Glutamatergic Receptor Trafficking and Delivery
5. Glutamatergic Receptor Trafficking: When and Where to Choose the Exocyst
6. Conclusion and Projections
Author Contributions
Funding
Conflicts of Interest
References
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Hering, H.; Sheng, M. Dendritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.T.; Ueda, T. Glutamate Release. Neurochem. Res. 2015, 40, 2443–2460. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, L.A.; Page, A.J.; Young, R.L. Metabotropic glutamate receptors as novel therapeutic targets on visceral sensory pathways. Front. Neurosci. 2011, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Jakowec, M.W.; Jackson-Lewis, V.; Chen, X.; Langston, J.W.; Przedborski, S. The postnatal development of AMPA receptor subunits in the basal ganglia of the rat. Dev. Neurosci. 1998, 20, 19–33. [Google Scholar] [CrossRef]
- Sillevis Smitt, P.; Kinoshita, A.; De Leeuw, B.; Moll, W.; Coesmans, M.; Jaarsma, D.; Henzen-Logmans, S.; Vecht, C.; De Zeeuw, C.; Sekiyama, N.; et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N. Engl. J. Med. 2000, 342, 21–27. [Google Scholar] [CrossRef]
- Testa, C.M.; Friberg, I.K.; Weiss, S.W.; Standaert, D.G. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J. Comp. Neurol. 1998, 390, 5–19. [Google Scholar] [CrossRef]
- Fukaya, M.; Kato, A.; Lovett, C.; Tonegawa, S.; Watanabe, M. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc. Natl. Acad. Sci. USA 2003, 100, 4855–4860. [Google Scholar] [CrossRef]
- Dunah, A.W.; Luo, J.; Wang, Y.H.; Yasuda, R.P.; Wolfe, B.B. Subunit composition of N-methyl-D-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol. Pharmacol. 1998, 53, 429–437. [Google Scholar] [CrossRef]
- Nishi, M.; Hinds, H.; Lu, H.P.; Kawata, M.; Hayashi, Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, RC185. [Google Scholar] [CrossRef]
- Habermacher, C.; Angulo, M.C.; Benamer, N. Glutamate versus GABA in neuron-oligodendroglia communication. Glia 2019, 67, 2092–2106. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.R. The role of glutamate in central nervous system health and disease—A review. Vet. J. 2007, 173, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.; Jasti, J.; Beich-Frandsen, M.; Gouaux, E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell 2006, 127, 85–97. [Google Scholar] [CrossRef]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192. [Google Scholar] [CrossRef]
- Papouin, T.; Ladepeche, L.; Ruel, J.; Sacchi, S.; Labasque, M.; Hanini, M.; Groc, L.; Pollegioni, L.; Mothet, J.P.; Oliet, S.H. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 2012, 150, 633–646. [Google Scholar] [CrossRef]
- Kristiansen, L.V.; Huerta, I.; Beneyto, M.; Meador-Woodruff, J.H. NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 2007, 7, 48–55. [Google Scholar] [CrossRef]
- Blanke, M.L.; VanDongen, A.M.J. Activation Mechanisms of the NMDA Receptor. In Biology of the NMDA Receptor; Van Dongen, A.M., Ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Maritzen, T.; Haucke, V. Coupling of exocytosis and endocytosis at the presynaptic active zone. Neurosci. Res. 2018, 127, 45–52. [Google Scholar] [CrossRef]
- Valenzuela, J.I.; Perez, F. Diversifying the secretory routes in neurons. Front. Neurosci. 2015, 9, 358. [Google Scholar] [CrossRef]
- Hanus, C.; Ehlers, M.D. Specialization of biosynthetic membrane trafficking for neuronal form and function. Curr. Opin. Neurobiol. 2016, 39, 8–16. [Google Scholar] [CrossRef]
- Luscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harbor Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R. Transport-vesicle targeting: Tethers before SNAREs. Nat. Cell Biol. 1999, 1, E17–E22. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.R.; Munro, S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 2002, 115, 2627–2637. [Google Scholar] [PubMed]
- Guo, W.; Sacher, M.; Barrowman, J.; Ferro-Novick, S.; Novick, P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000, 10, 251–255. [Google Scholar] [CrossRef]
- TerBush, D.R.; Maurice, T.; Roth, D.; Novick, P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996, 15, 6483–6494. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Grant, A.; Novick, P. Exo84p is an exocyst protein essential for secretion. J. Biol. Chem. 1999, 274, 23558–23564. [Google Scholar] [CrossRef]
- Hsu, S.C.; Ting, A.E.; Hazuka, C.D.; Davanger, S.; Kenny, J.W.; Kee, Y.; Scheller, R.H. The mammalian brain rsec6/8 complex. Neuron 1996, 17, 1209–1219. [Google Scholar] [CrossRef]
- Martin-Urdiroz, M.; Deeks, M.J.; Horton, C.G.; Dawe, H.R.; Jourdain, I. The Exocyst Complex in Health and Disease. Front. Cell Dev. Biol. 2016, 4, 24. [Google Scholar] [CrossRef]
- Hanus, C.; Ehlers, M.D. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic 2008, 9, 1437–1445. [Google Scholar] [CrossRef]
- Aridor, M.; Guzik, A.K.; Bielli, A.; Fish, K.N. Endoplasmic reticulum export site formation and function in dendrites. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 3770–3776. [Google Scholar] [CrossRef]
- Viotti, C. ER to Golgi-Dependent Protein Secretion: The Conventional Pathway. Methods Mol. Biol. 2016, 1459, 3–29. [Google Scholar] [CrossRef]
- Kappeler, F.; Klopfenstein, D.R.; Foguet, M.; Paccaud, J.P.; Hauri, H.P. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J. Biol. Chem. 1997, 272, 31801–31808. [Google Scholar] [CrossRef]
- Krijnse-Locker, J.; Parton, R.G.; Fuller, S.D.; Griffiths, G.; Dotti, C.G. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol. Biol. Cell 1995, 6, 1315–1332. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Mayer, T.; McCarthy, J.B. Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr. Biol. CB 2001, 11, 351–355. [Google Scholar] [CrossRef]
- Mu, Y.; Otsuka, T.; Horton, A.C.; Scott, D.B.; Ehlers, M.D. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 2003, 40, 581–594. [Google Scholar] [CrossRef]
- Bowen, A.B.; Bourke, A.M.; Hiester, B.G.; Hanus, C.; Kennedy, M.J. Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. eLife 2017, 6. [Google Scholar] [CrossRef]
- Mignogna, M.L.; D’Adamo, P. Critical importance of RAB proteins for synaptic function. Small GTPases 2018, 9, 145–157. [Google Scholar] [CrossRef]
- Veleri, S.; Punnakkal, P.; Dunbar, G.L.; Maiti, P. Molecular Insights into the Roles of Rab Proteins in Intracellular Dynamics and Neurodegenerative Diseases. Neuromol. Med. 2018, 20, 18–36. [Google Scholar] [CrossRef]
- Ungermann, C.; Kummel, D. Structure of membrane tethers and their role in fusion. Traffic 2019, 20, 479–490. [Google Scholar] [CrossRef]
- Madrigal, M.P.; Portales, A.; San Juan, M.P.; Jurado, S. Postsynaptic SNARE Proteins: Role in Synaptic Transmission and Plasticity. Neurosci. 2019, 420, 12–21. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Davison, I.G.; Robinson, C.G.; Ehlers, M.D. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell 2010, 141, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.H.; Terashima, A.; Petralia, R.S.; Wenthold, R.J.; Isaac, J.T.; Roche, K.W.; Roche, P.A. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat. Neurosci. 2010, 13, 338–343. [Google Scholar] [CrossRef]
- Hussain, S.; Davanger, S. Postsynaptic VAMP/Synaptobrevin Facilitates Differential Vesicle Trafficking of GluA1 and GluA2 AMPA Receptor Subunits. PLoS ONE 2015, 10, e0140868. [Google Scholar] [CrossRef]
- Gu, Y.; Chiu, S.L.; Liu, B.; Wu, P.H.; Delannoy, M.; Lin, D.T.; Wirtz, D.; Huganir, R.L. Differential vesicular sorting of AMPA and GABAA receptors. Proc. Natl. Acad. Sci. USA 2016, 113, E922–E931. [Google Scholar] [CrossRef] [PubMed]
- Wenthold, R.J.; Prybylowski, K.; Standley, S.; Sans, N.; Petralia, R.S. Trafficking of NMDA receptors. Ann. Rev. Pharmacol. Toxicol. 2003, 43, 335–358. [Google Scholar] [CrossRef]
- Ju, W.; Morishita, W.; Tsui, J.; Gaietta, G.; Deerinck, T.J.; Adams, S.R.; Garner, C.C.; Tsien, R.Y.; Ellisman, M.H.; Malenka, R.C. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat. Neurosci. 2004, 7, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Khatri, L.; Ziff, E.B. Trafficking of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptor subunit GluA2 from the endoplasmic reticulum is stimulated by a complex containing Ca2+/calmodulin-activated kinase II (CaMKII) and PICK1 protein and by release of Ca2+ from internal stores. J. Biol. Chem. 2014, 289, 19218–19230. [Google Scholar] [CrossRef]
- Jeyifous, O.; Waites, C.L.; Specht, C.G.; Fujisawa, S.; Schubert, M.; Lin, E.I.; Marshall, J.; Aoki, C.; de Silva, T.; Montgomery, J.M.; et al. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat. Neurosci. 2009, 12, 1011–1019. [Google Scholar] [CrossRef]
- Shi, S.; Hayashi, Y.; Esteban, J.A.; Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 2001, 105, 331–343. [Google Scholar] [CrossRef]
- Buonarati, O.R.; Hammes, E.A.; Watson, J.F.; Greger, I.H.; Hell, J.W. Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Jackson, A.C.; Bjorgan, K.; During, M.J.; Sprengel, R.; Seeburg, P.H.; Nicoll, R.A. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 2009, 62, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, E.; von Engelhardt, J. AMPA receptor complex constituents: Control of receptor assembly, membrane trafficking and subcellular localization. Mol. Cell. Neurosci. 2018, 91, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Furukawa, H.; Traynelis, S.F. Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol. Pharmacol. 2010, 78, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Meddows, E.; Le Bourdelles, B.; Grimwood, S.; Wafford, K.; Sandhu, S.; Whiting, P.; McIlhinney, R.A. Identification of molecular determinants that are important in the assembly of N-methyl-D-aspartate receptors. J. Biol. Chem. 2001, 276, 18795–18803. [Google Scholar] [CrossRef]
- Schuler, T.; Mesic, I.; Madry, C.; Bartholomaus, I.; Laube, B. Formation of NR1/NR2 and NR1/NR3 heterodimers constitutes the initial step in N-methyl-D-aspartate receptor assembly. J. Biol. Chem. 2008, 283, 37–46. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Ehlers, M.D. Organelles and trafficking machinery for postsynaptic plasticity. Ann. Rev. Neurosci. 2006, 29, 325–362. [Google Scholar] [CrossRef]
- Scott, D.B.; Blanpied, T.A.; Swanson, G.T.; Zhang, C.; Ehlers, M.D. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 3063–3072. [Google Scholar] [CrossRef]
- Kandel, M.B.; Yamamoto, S.; Midorikawa, R.; Morise, J.; Wakazono, Y.; Oka, S.; Takamiya, K. N-glycosylation of the AMPA-type glutamate receptor regulates cell surface expression and tetramer formation affecting channel function. J. Neurochem. 2018, 147, 730–747. [Google Scholar] [CrossRef]
- Lichnerova, K.; Kaniakova, M.; Park, S.P.; Skrenkova, K.; Wang, Y.X.; Petralia, R.S.; Suh, Y.H.; Horak, M. Two N-glycosylation Sites in the GluN1 Subunit Are Essential for Releasing N-methyl-d-aspartate (NMDA) Receptors from the Endoplasmic Reticulum. J. Biol. Chem. 2015, 290, 18379–18390. [Google Scholar] [CrossRef]
- Skrenkova, K.; Lee, S.; Lichnerova, K.; Kaniakova, M.; Hansikova, H.; Zapotocky, M.; Suh, Y.H.; Horak, M. N-Glycosylation Regulates the Trafficking and Surface Mobility of GluN3A-Containing NMDA Receptors. Front. Mol. Neurosci. 2018, 11, 188. [Google Scholar] [CrossRef]
- Sohn, H.; Park, M. Palmitoylation-mediated synaptic regulation of AMPA receptor trafficking and function. Arch. Pharm. Res. 2019, 42, 426–435. [Google Scholar] [CrossRef]
- Mattison, H.A.; Hayashi, T.; Barria, A. Palmitoylation at two cysteine clusters on the C-terminus of GluN2A and GluN2B differentially control synaptic targeting of NMDA receptors. PLoS ONE 2012, 7, e49089. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.A.; Cho, T.; Lee, D.Z.; Lee, J.J.; Lee, B.; Kim, S.W.; Shin, H.S.; Kang, M.G. LARGE, an intellectual disability-associated protein, regulates AMPA-type glutamate receptor trafficking and memory. Proc. Natl. Acad. Sci. USA 2018, 115, 7111–7116. [Google Scholar] [CrossRef]
- Standley, S.; Petralia, R.S.; Gravell, M.; Hamilton, R.; Wang, Y.X.; Schubert, M.; Wenthold, R.J. Trafficking of the NMDAR2B receptor subunit distal cytoplasmic tail from endoplasmic reticulum to the synapse. PLoS ONE 2012, 7, e39585. [Google Scholar] [CrossRef] [PubMed]
- Standley, S.; Roche, K.W.; McCallum, J.; Sans, N.; Wenthold, R.J. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron 2000, 28, 887–898. [Google Scholar] [CrossRef]
- Xia, H.; Hornby, Z.D.; Malenka, R.C. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharmacology 2001, 41, 714–723. [Google Scholar] [CrossRef]
- Sans, N.; Prybylowski, K.; Petralia, R.S.; Chang, K.; Wang, Y.X.; Racca, C.; Vicini, S.; Wenthold, R.J. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. 2003, 5, 520–530. [Google Scholar] [CrossRef]
- Sans, N.; Racca, C.; Petralia, R.S.; Wang, Y.X.; McCallum, J.; Wenthold, R.J. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 7506–7516. [Google Scholar] [CrossRef]
- El-Husseini Ael, D.; Schnell, E.; Dakoji, S.; Sweeney, N.; Zhou, Q.; Prange, O.; Gauthier-Campbell, C.; Aguilera-Moreno, A.; Nicoll, R.A.; Bredt, D.S. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 2002, 108, 849–863. [Google Scholar] [CrossRef]
- Chen, L.; Chetkovich, D.M.; Petralia, R.S.; Sweeney, N.T.; Kawasaki, Y.; Wenthold, R.J.; Bredt, D.S.; Nicoll, R.A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000, 408, 936–943. [Google Scholar] [CrossRef]
- Dong, H.; O’Brien, R.J.; Fung, E.T.; Lanahan, A.A.; Worley, P.F.; Huganir, R.L. GRIP: A synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 1997, 386, 279–284. [Google Scholar] [CrossRef]
- DeSouza, S.; Fu, J.; States, B.A.; Ziff, E.B. Differential palmitoylation directs the AMPA receptor-binding protein ABP to spines or to intracellular clusters. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 3493–3503. [Google Scholar] [CrossRef]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef] [PubMed]
- Payne, H.L. The role of transmembrane AMPA receptor regulatory proteins (TARPs) in neurotransmission and receptor trafficking (Review). Mol. Membr. Biol. 2008, 25, 353–362. [Google Scholar] [CrossRef]
- Tomita, S.; Chen, L.; Kawasaki, Y.; Petralia, R.S.; Wenthold, R.J.; Nicoll, R.A.; Bredt, D.S. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 2003, 161, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.S.; Siuda, E.R.; Nisenbaum, E.S.; Bredt, D.S. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron 2008, 59, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Rouach, N.; Byrd, K.; Petralia, R.S.; Elias, G.M.; Adesnik, H.; Tomita, S.; Karimzadegan, S.; Kealey, C.; Bredt, D.S.; Nicoll, R.A. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat. Neurosci. 2005, 8, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, M.; Tsujita, M.; Yamazaki, M.; Kushiya, E.; Abe, M.; Akashi, K.; Natsume, R.; Kano, M.; Kamiya, H.; Watanabe, M.; et al. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur. J. Neurosci. 2006, 24, 2177–2190. [Google Scholar] [CrossRef]
- Menuz, K.; O’Brien, J.L.; Karmizadegan, S.; Bredt, D.S.; Nicoll, R.A. TARP redundancy is critical for maintaining AMPA receptor function. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 8740–8746. [Google Scholar] [CrossRef]
- Letts, V.A.; Mahaffey, C.L.; Beyer, B.; Frankel, W.N. A targeted mutation in Cacng4 exacerbates spike-wave seizures in stargazer (Cacng2) mice. Proc. Natl. Acad. Sci. USA 2005, 102, 2123–2128. [Google Scholar] [CrossRef]
- Bissen, D.; Foss, F.; Acker-Palmer, A. AMPA receptors and their minions: Auxiliary proteins in AMPA receptor trafficking. Cell. Mol. Life Sci. CMLS 2019, 76, 2133–2169. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, J.; Harmel, N.; Zolles, G.; Bildl, W.; Kulik, A.; Heimrich, B.; Chisaka, O.; Jonas, P.; Schulte, U.; Fakler, B.; et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 2009, 323, 1313–1319. [Google Scholar] [CrossRef]
- Harmel, N.; Cokic, B.; Zolles, G.; Berkefeld, H.; Mauric, V.; Fakler, B.; Stein, V.; Klocker, N. AMPA receptors commandeer an ancient cargo exporter for use as an auxiliary subunit for signaling. PLoS ONE 2012, 7, e30681. [Google Scholar] [CrossRef]
- Herring, B.E.; Shi, Y.; Suh, Y.H.; Zheng, C.Y.; Blankenship, S.M.; Roche, K.W.; Nicoll, R.A. Cornichon proteins determine the subunit composition of synaptic AMPA receptors. Neuron 2013, 77, 1083–1096. [Google Scholar] [CrossRef]
- Mignogna, M.L.; Giannandrea, M.; Gurgone, A.; Fanelli, F.; Raimondi, F.; Mapelli, L.; Bassani, S.; Fang, H.; Van Anken, E.; Alessio, M.; et al. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat. Commun. 2015, 6, 6504. [Google Scholar] [CrossRef] [PubMed]
- Gerges, N.Z.; Backos, D.S.; Esteban, J.A. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J. Biol. Chem. 2004, 279, 43870–43878. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.C.; Correia, S.S.; Petrok, C.N.; Esteban, J.A. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 13311–13315. [Google Scholar] [CrossRef]
- Hausser, A.; Schlett, K. Coordination of AMPA receptor trafficking by Rab GTPases. Small GTPases 2019, 10, 419–432. [Google Scholar] [CrossRef]
- Wang, J.; Lv, X.; Wu, Y.; Xu, T.; Jiao, M.; Yang, R.; Li, X.; Chen, M.; Yan, Y.; Chen, C.; et al. Postsynaptic RIM1 modulates synaptic function by facilitating membrane delivery of recycling NMDARs in hippocampal neurons. Nat. Commun. 2018, 9, 2267. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Mattison, H.A.; Cerpa, W. Role of NMDA Receptor-Mediated Glutamatergic Signaling in Chronic and Acute Neuropathologies. Neural Plaast. 2016, 2016, 2701526. [Google Scholar] [CrossRef]
- Sheng, M.; Kim, M.J. Postsynaptic signaling and plasticity mechanisms. Science 2002, 298, 776–780. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, F.L.; Sun, X.; Wen, L.; Mei, L.; Tang, B.S.; Xiong, W.C. VPS35-deficiency results in an impaired AMPA receptor trafficking and decreased dendritic spine maturation. Mol. Brain 2015, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Savas, J.N.; Ribeiro, L.F.; Wierda, K.D.; Wright, R.; DeNardo-Wilke, L.A.; Rice, H.C.; Chamma, I.; Wang, Y.Z.; Zemla, R.; Lavallee-Adam, M.; et al. The Sorting Receptor SorCS1 Regulates Trafficking of Neurexin and AMPA Receptors. Neuron 2015, 87, 764–780. [Google Scholar] [CrossRef]
- Herring, B.E.; Nicoll, R.A. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Ann. Rev. Physiol. 2016, 78, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.S.; Royston, S.E.; Xu, J.; Cavaretta, J.P.; Vest, M.O.; Lee, K.Y.; Lee, S.; Jeong, H.G.; Lombroso, P.J.; Chung, H.J. Regulation of STEP61 and tyrosine-phosphorylation of NMDA and AMPA receptors during homeostatic synaptic plasticity. Mol. Brain 2015, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Jurado, S.; Benoist, M.; Lario, A.; Knafo, S.; Petrok, C.N.; Esteban, J.A. PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 2010, 29, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Bacaj, T.; Morishita, W.; Goswami, D.; Arendt, K.L.; Xu, W.; Chen, L.; Malenka, R.C.; Sudhof, T.C. Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature 2017, 544, 316–321. [Google Scholar] [CrossRef]
- Jurado, S.; Goswami, D.; Zhang, Y.; Molina, A.J.; Sudhof, T.C.; Malenka, R.C. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron 2013, 77, 542–558. [Google Scholar] [CrossRef]
- Bin, N.R.; Ma, K.; Harada, H.; Tien, C.W.; Bergin, F.; Sugita, K.; Luyben, T.T.; Narimatsu, M.; Jia, Z.; Wrana, J.L.; et al. Crucial Role of Postsynaptic Syntaxin 4 in Mediating Basal Neurotransmission and Synaptic Plasticity in Hippocampal CA1 Neurons. Cell Rep. 2018, 23, 2955–2966. [Google Scholar] [CrossRef]
- Zheng, N.; Jeyifous, O.; Munro, C.; Montgomery, J.M.; Green, W.N. Synaptic activity regulates AMPA receptor trafficking through different recycling pathways. eLife 2015, 4. [Google Scholar] [CrossRef]
- Mao, L.; Takamiya, K.; Thomas, G.; Lin, D.T.; Huganir, R.L. GRIP1 and 2 regulate activity-dependent AMPA receptor recycling via exocyst complex interactions. Proc. Natl. Acad. Sci. USA 2010, 107, 19038–19043. [Google Scholar] [CrossRef]
- Moretto, E.; Passafaro, M. Recent Findings on AMPA Receptor Recycling. Front. Cell. Neurosci. 2018, 12, 286. [Google Scholar] [CrossRef]
- Chiu, S.L.; Diering, G.H.; Ye, B.; Takamiya, K.; Chen, C.M.; Jiang, Y.; Niranjan, T.; Schwartz, C.E.; Wang, T.; Huganir, R.L. GRASP1 Regulates Synaptic Plasticity and Learning through Endosomal Recycling of AMPA Receptors. Neuron 2017, 93, 1405–1419.e8. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Ramachandran, B.; Ahmed, S.; Benito, E.; Shinoda, Y.; Nitzan, N.; Heukamp, A.; Rannio, S.; Martens, H.; Barth, J.; et al. Synaptotagmin-3 drives AMPA receptor endocytosis, depression of synapse strength, and forgetting. Science 2019, 363. [Google Scholar] [CrossRef] [PubMed]
- Roche, K.W.; Standley, S.; McCallum, J.; Dune Ly, C.; Ehlers, M.D.; Wenthold, R.J. Molecular determinants of NMDA receptor internalization. Nat. Neurosci. 2001, 4, 794–802. [Google Scholar] [CrossRef]
- Petralia, R.S.; Wang, Y.X.; Wenthold, R.J. Internalization at glutamatergic synapses during development. Eur. J. Neurosci. 2003, 18, 3207–3217. [Google Scholar] [CrossRef] [PubMed]
- Matern, H.T.; Yeaman, C.; Nelson, W.J.; Scheller, R.H. The Sec6/8 complex in mammalian cells: Characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells. Proc. Natl. Acad. Sci. USA 2001, 98, 9648–9653. [Google Scholar] [CrossRef]
- Heider, M.R.; Gu, M.; Duffy, C.M.; Mirza, A.M.; Marcotte, L.L.; Walls, A.C.; Farrall, N.; Hakhverdyan, Z.; Field, M.C.; Rout, M.P.; et al. Subunit connectivity, assembly determinants and architecture of the yeast exocyst complex. Nat. Struct. Mol. Biol. 2016, 23, 59–66. [Google Scholar] [CrossRef]
- Katoh, Y.; Nozaki, S.; Hartanto, D.; Miyano, R.; Nakayama, K. Architectures of multisubunit complexes revealed by a visible immunoprecipitation assay using fluorescent fusion proteins. J. Cell Sci. 2015, 128, 2351–2362. [Google Scholar] [CrossRef]
- Yanay, C.; Morpurgo, N.; Linial, M. Evolution of insect proteomes: Insights into synapse organization and synaptic vesicle life cycle. Genome Biol. 2008, 9, R27. [Google Scholar] [CrossRef] [PubMed]
- Lepore, D.M.; Martinez-Nunez, L.; Munson, M. Exposing the Elusive Exocyst Structure. Trends Biochem. Sci. 2018, 43, 714–725. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Nishida-Fukuda, H.; Li, Y.; McDonald, W.H.; Gradinaru, C.C.; Macara, I.G. Exocyst dynamics during vesicle tethering and fusion. Nat. Commun. 2018, 9, 5140. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; TerBush, D.; Abraham, M.; Guo, W. The exocyst complex in polarized exocytosis. Int. Rev. Cytol. 2004, 233, 243–265. [Google Scholar] [CrossRef]
- Hsu, S.C.; Hazuka, C.D.; Roth, R.; Foletti, D.L.; Heuser, J.; Scheller, R.H. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron 1998, 20, 1111–1122. [Google Scholar] [CrossRef]
- Munson, M.; Novick, P. The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 2006, 13, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Picco, A.; Irastorza-Azcarate, I.; Specht, T.; Boke, D.; Pazos, I.; Rivier-Cordey, A.S.; Devos, D.P.; Kaksonen, M.; Gallego, O. The In Vivo Architecture of the Exocyst Provides Structural Basis for Exocytosis. Cell 2017, 168, 400–412.e18. [Google Scholar] [CrossRef]
- Mei, K.; Li, Y.; Wang, S.; Shao, G.; Wang, J.; Ding, Y.; Luo, G.; Yue, P.; Liu, J.J.; Wang, X.; et al. Cryo-EM structure of the exocyst complex. Nat. Struct. Mol. Biol. 2018, 25, 139–146. [Google Scholar] [CrossRef]
- Mei, K.; Guo, W. The exocyst complex. Curr. Biol. CB 2018, 28, R922–R925. [Google Scholar] [CrossRef]
- Kulich, I.; Pecenkova, T.; Sekeres, J.; Smetana, O.; Fendrych, M.; Foissner, I.; Hoftberger, M.; Zarsky, V. Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 2013, 14, 1155–1165. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Macara, I.G. The Par3 polarity protein is an exocyst receptor essential for mammary cell survival. Nat. Commun. 2017, 8, 14867. [Google Scholar] [CrossRef] [PubMed]
- Moskalenko, S.; Tong, C.; Rosse, C.; Mirey, G.; Formstecher, E.; Daviet, L.; Camonis, J.; White, M.A. Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 2003, 278, 51743–51748. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, J.; Guo, W. The role of Sec3p in secretory vesicle targeting and exocyst complex assembly. Mol. Biol. Cell 2014, 25, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- TerBush, D.R.; Novick, P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 1995, 130, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.; Hughes, T.; Pypaert, M.; Novick, P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 2004, 167, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Mehta, S.Q.; Pichaud, F.; Bellen, H.J.; Quiocho, F.A. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol. Biol. 2005, 12, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Ellis, S.; Sriratana, A.; Mitchell, C.A.; Rowe, T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 2004, 279, 43027–43034. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Nikolova, L.S.; Schjelderup, A.; Metzstein, M.M. Exocyst-mediated membrane trafficking is required for branch outgrowth in Drosophila tracheal terminal cells. Dev. Biol. 2014, 390, 41–50. [Google Scholar] [CrossRef]
- Donovan, K.W.; Bretscher, A. Myosin-V is activated by binding secretory cargo and released in coordination with Rab/exocyst function. Dev. Cell 2012, 23, 769–781. [Google Scholar] [CrossRef]
- Rivera-Molina, F.; Toomre, D. Live-cell imaging of exocyst links its spatiotemporal dynamics to various stages of vesicle fusion. J. Cell Biol. 2013, 201, 673–680. [Google Scholar] [CrossRef]
- Shin, D.M.; Zhao, X.S.; Zeng, W.; Mozhayeva, M.; Muallem, S. The mammalian Sec6/8 complex interacts with Ca(2+) signaling complexes and regulates their activity. J. Cell Biol. 2000, 150, 1101–1112. [Google Scholar] [CrossRef]
- Yeaman, C.; Grindstaff, K.K.; Wright, J.R.; Nelson, W.J. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J. Cell Biol. 2001, 155, 593–604. [Google Scholar] [CrossRef]
- Lipschutz, J.H.; Guo, W.; O’Brien, L.E.; Nguyen, Y.H.; Novick, P.; Mostov, K.E. Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol. Biol. Cell 2000, 11, 4259–4275. [Google Scholar] [CrossRef] [PubMed]
- Grindstaff, K.K.; Yeaman, C.; Anandasabapathy, N.; Hsu, S.C.; Rodriguez-Boulan, E.; Scheller, R.H.; Nelson, W.J. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 1998, 93, 731–740. [Google Scholar] [CrossRef]
- Anitei, M.; Ifrim, M.; Ewart, M.A.; Cowan, A.E.; Carson, J.H.; Bansal, R.; Pfeiffer, S.E. A role for Sec8 in oligodendrocyte morphological differentiation. J. Cell Sci. 2006, 119, 807–818. [Google Scholar] [CrossRef]
- Inoue, M.; Chiang, S.H.; Chang, L.; Chen, X.W.; Saltiel, A.R. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol. Biol. Cell 2006, 17, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Ravier, M.A.; Xie, H.; Ewart, M.A.; Gould, G.W.; Baldwin, S.A.; Rutter, G.A. Mammalian exocyst complex is required for the docking step of insulin vesicle exocytosis. J. Biol. Chem. 2005, 280, 25565–25570. [Google Scholar] [CrossRef]
- Vega, I.E.; Hsu, S.C. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 3839–3848. [Google Scholar] [CrossRef]
- Wang, S.; Hsu, S.C. Immunological characterization of exocyst complex subunits in cell differentiation. Hybrid. Hybrid. 2003, 22, 159–164. [Google Scholar] [CrossRef]
- Yeaman, C.; Grindstaff, K.K.; Nelson, W.J. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J. Cell Sci. 2004, 117, 559–570. [Google Scholar] [CrossRef]
- Zajac, A.; Sun, X.; Zhang, J.; Guo, W. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol. Biol. Cell 2005, 16, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Adamson, C.L.; Valdez, G.; Guo, W.; Hsu, S.C. The mammalian exocyst, a complex required for exocytosis, inhibits tubulin polymerization. J. Biol. Chem. 2004, 279, 35958–35966. [Google Scholar] [CrossRef]
- Zakharenko, S.; Popov, S. Dynamics of axonal microtubules regulate the topology of new membrane insertion into the growing neurites. J. Cell Biol. 1998, 143, 1077–1086. [Google Scholar] [CrossRef]
- Tan, X.; Thapa, N.; Sun, Y.; Anderson, R.A. A kinase-independent role for EGF receptor in autophagy initiation. Cell 2015, 160, 145–160. [Google Scholar] [CrossRef]
- Cvrckova, F.; Zarsky, V. Old AIMs of the exocyst: Evidence for an ancestral association of exocyst subunits with autophagy-associated Atg8 proteins. Plant Signal. Behav. 2013, 8, e27099. [Google Scholar] [CrossRef]
- Singh, S.; Kumari, R.; Chinchwadkar, S.; Aher, A.; Matheshwaran, S.; Manjithaya, R. Exocyst Subcomplex Functions in Autophagosome Biogenesis by Regulating Atg9 Trafficking. J. Mol. Biol. 2019, 431, 2821–2834. [Google Scholar] [CrossRef]
- Tzfadia, O.; Galili, G. The Arabidopsis exocyst subcomplex subunits involved in a golgi-independent transport into the vacuole possess consensus autophagy-associated atg8 interacting motifs. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Hazuka, C.D.; Foletti, D.L.; Hsu, S.C.; Kee, Y.; Hopf, F.W.; Scheller, R.H. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 1324–1334. [Google Scholar] [CrossRef]
- Murthy, M.; Garza, D.; Scheller, R.H.; Schwarz, T.L. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 2003, 37, 433–447. [Google Scholar] [CrossRef]
- Murthy, M.; Ranjan, R.; Denef, N.; Higashi, M.E.; Schupbach, T.; Schwarz, T.L. Sec6 mutations and the Drosophila exocyst complex. J. Cell Sci. 2005, 118, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, G.A.; Hildebrand, J.D.; Soriano, P. The secretory protein Sec8 is required for paraxial mesoderm formation in the mouse. Dev. Biol. 1997, 192, 364–374. [Google Scholar] [CrossRef]
- Mehta, S.Q.; Hiesinger, P.R.; Beronja, S.; Zhai, R.G.; Schulze, K.L.; Verstreken, P.; Cao, Y.; Zhou, Y.; Tepass, U.; Crair, M.C.; et al. Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron 2005, 46, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lee, J.; Rowland, K.; Wen, Y.; Hua, H.; Carlson, N.; Lavania, S.; Parrish, J.Z.; Kim, M.D. Regulation of dendrite growth and maintenance by exocytosis. J. Cell Sci. 2015, 128, 4279–4292. [Google Scholar] [CrossRef] [PubMed]
- Lalli, G. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J. Cell Sci. 2009, 122, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Lira, M.; Arancibia, D.; Orrego, P.R.; Montenegro-Venegas, C.; Cruz, Y.; Garcia, J.; Leal-Ortiz, S.; Godoy, J.A.; Gundelfinger, E.D.; Inestrosa, N.C.; et al. The Exocyst Component Exo70 Modulates Dendrite Arbor Formation, Synapse Density, and Spine Maturation in Primary Hippocampal Neurons. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef]
- Dupraz, S.; Grassi, D.; Bernis, M.E.; Sosa, L.; Bisbal, M.; Gastaldi, L.; Jausoro, I.; Caceres, A.; Pfenninger, K.H.; Quiroga, S. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 13292–13301. [Google Scholar] [CrossRef]
- Pommereit, D.; Wouters, F.S. An NGF-induced Exo70-TC10 complex locally antagonises Cdc42-mediated activation of N-WASP to modulate neurite outgrowth. J. Cell Sci. 2007, 120, 2694–2705. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, J.; Yang, C.; Capraro, B.R.; Baumgart, T.; Bradley, R.P.; Ramakrishnan, N.; Xu, X.; Radhakrishnan, R.; Svitkina, T.; et al. Exo70 generates membrane curvature for morphogenesis and cell migration. Dev. Cell 2013, 26, 266–278. [Google Scholar] [CrossRef]
- Zuo, X.; Zhang, J.; Zhang, Y.; Hsu, S.C.; Zhou, D.; Guo, W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat. Cell Biol. 2006, 8, 1383–1388. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Sun, Y.; He, B.; Yang, C.; Svitkina, T.; Goldman, Y.E.; Guo, W. Exo70 stimulates the Arp2/3 complex for lamellipodia formation and directional cell migration. Curr. Biol. CB 2012, 22, 1510–1515. [Google Scholar] [CrossRef]
- Taylor, C.A.; Yan, J.; Howell, A.S.; Dong, X.; Shen, K. RAB-10 Regulates Dendritic Branching by Balancing Dendritic Transport. PLoS Genet. 2015, 11, e1005695. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Yadav, S.; DeVault, L.; Nung Jan, Y.; Sherwood, D.R. RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization. PLoS Genet. 2015, 11, e1005484. [Google Scholar] [CrossRef]
- Fujita, A.; Koinuma, S.; Yasuda, S.; Nagai, H.; Kamiguchi, H.; Wada, N.; Nakamura, T. GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70. PLoS ONE 2013, 8, e79689. [Google Scholar] [CrossRef]
- Riefler, G.M.; Balasingam, G.; Lucas, K.G.; Wang, S.; Hsu, S.C.; Firestein, B.L. Exocyst complex subunit sec8 binds to postsynaptic density protein-95 (PSD-95): A novel interaction regulated by cypin (cytosolic PSD-95 interactor). Biochem. J. 2003, 373, 49–55. [Google Scholar] [CrossRef]
- Cubelos, B.; Gimenez, C.; Zafra, F. The glycine transporter GLYT1 interacts with Sec3, a component of the exocyst complex. Neuropharmacology 2005, 49, 935–944. [Google Scholar] [CrossRef]
- Teodoro, R.O.; Pekkurnaz, G.; Nasser, A.; Higashi-Kovtun, M.E.; Balakireva, M.; McLachlan, I.G.; Camonis, J.; Schwarz, T.L. Ral mediates activity-dependent growth of postsynaptic membranes via recruitment of the exocyst. EMBO J. 2013, 32, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Andrews, H.K.; Zhang, Y.Q.; Trotta, N.; Broadie, K. Drosophila sec10 is required for hormone secretion but not general exocytosis or neurotransmission. Traffic 2002, 3, 906–921. [Google Scholar] [CrossRef]
- Schwenger, D.B.; Kuner, T. Acute genetic perturbation of exocyst function in the rat calyx of Held impedes structural maturation, but spares synaptic transmission. Eur. J. Neurosci. 2010, 32, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Parthier, D.; Kuner, T.; Korber, C. The presynaptic scaffolding protein Piccolo organizes the readily releasable pool at the calyx of Held. J. Physiol. 2018, 596, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Babai, N.; Kaeser, P.; Sudhof, T.C.; Schneggenburger, R. RIM1 and RIM2 redundantly determine Ca2+ channel density and readily releasable pool size at a large hindbrain synapse. J. Neurophysiol. 2015, 113, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, M.; Renden, R.; Horstmann, H.; Gitler, D.; Kuner, T. Overexpression of synapsin Ia in the rat calyx of Held accelerates short-term plasticity and decreases synaptic vesicle volume and active zone area. Front. Cell. Neurosci. 2013, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, C. Molecularly and structurally distinct synapses mediate reliable encoding and processing of auditory information. Hear. Res. 2015, 330, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Gerges, N.Z.; Backos, D.S.; Rupasinghe, C.N.; Spaller, M.R.; Esteban, J.A. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 2006, 25, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Mellone, M.; Gardoni, F. Modulation of NMDA receptor at the synapse: Promising therapeutic interventions in disorders of the nervous system. Eur. J. Pharmacol. 2013, 719, 75–83. [Google Scholar] [CrossRef]
- Washbourne, P.; Liu, X.B.; Jones, E.G.; McAllister, A.K. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 8253–8264. [Google Scholar] [CrossRef]
- Hirokawa, N.; Takemura, R. Molecular motors in neuronal development, intracellular transport and diseases. Curr. Opin. Neurobiol. 2004, 14, 564–573. [Google Scholar] [CrossRef]
- Setou, M.; Seog, D.H.; Tanaka, Y.; Kanai, Y.; Takei, Y.; Kawagishi, M.; Hirokawa, N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 2002, 417, 83–87. [Google Scholar] [CrossRef]
- Heisler, F.F.; Lee, H.K.; Gromova, K.V.; Pechmann, Y.; Schurek, B.; Ruschkies, L.; Schroeder, M.; Schweizer, M.; Kneussel, M. GRIP1 interlinks N-cadherin and AMPA receptors at vesicles to promote combined cargo transport into dendrites. Proc. Natl. Acad. Sci. USA 2014, 111, 5030–5035. [Google Scholar] [CrossRef]
- Hoogenraad, C.C.; Milstein, A.D.; Ethell, I.M.; Henkemeyer, M.; Sheng, M. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nature Neurosci. 2005, 8, 906–915. [Google Scholar] [CrossRef]
- Sans, N.; Wang, P.Y.; Du, Q.; Petralia, R.S.; Wang, Y.X.; Nakka, S.; Blumer, J.B.; Macara, I.G.; Wenthold, R.J. mPins modulates PSD-95 and SAP102 trafficking and influences NMDA receptor surface expression. Nat. Cell Biol. 2005, 7, 1179–1190. [Google Scholar] [CrossRef]
- Petralia, R.S.; Al-Hallaq, R.A.; Wenthold, R.J. Trafficking and Targeting of NMDA Receptors. In Biology of the NMDA Receptor; Van Dongen, A.M., Ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Prybylowski, K.; Fu, Z.; Losi, G.; Hawkins, L.M.; Luo, J.; Chang, K.; Wenthold, R.J.; Vicini, S. Relationship between availability of NMDA receptor subunits and their expression at the synapse. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 8902–8910. [Google Scholar] [CrossRef]
- Park, M. AMPA Receptor Trafficking for Postsynaptic Potentiation. Front. Cell. Neurosci. 2018, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Isaac, J.T.; Wang, Y.T. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 2004, 5, 952–962. [Google Scholar] [CrossRef]
- Lau, C.G.; Zukin, R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, A.J.; Choquet, D. Regulation of AMPA receptor lateral movements. Nature 2002, 417, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Tardin, C.; Cognet, L.; Bats, C.; Lounis, B.; Choquet, D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003, 22, 4656–4665. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Man, H.; Ju, W.; Trimble, W.S.; MacDonald, J.F.; Wang, Y.T. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 2001, 29, 243–254. [Google Scholar] [CrossRef]
- Pickard, L.; Noel, J.; Duckworth, J.K.; Fitzjohn, S.M.; Henley, J.M.; Collingridge, G.L.; Molnar, E. Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors at hippocampal neuronal plasma membranes. Neuropharmacology 2001, 41, 700–713. [Google Scholar] [CrossRef]
- Chia, P.Z.; Gleeson, P.A. Membrane tethering. F1000prime Rep. 2014, 6, 74. [Google Scholar] [CrossRef]
- Spang, A. Membrane Tethering Complexes in the Endosomal System. Front. Cell Dev. Biol. 2016, 4, 35. [Google Scholar] [CrossRef]
- Uhm, M.; Bazuine, M.; Zhao, P.; Chiang, S.H.; Xiong, T.; Karunanithi, S.; Chang, L.; Saltiel, A.R. Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Duan, Y.; Guo, Q.; Zhang, T.; Meng, Y.; Sun, D.; Luo, G.; Liu, Y. Cyclin-dependent kinase-mediated phosphorylation of the exocyst subunit Exo84 in late G1 phase suppresses exocytic secretion and cell growth in yeast. J. Biol. Chem. 2019, 294, 11323–11332. [Google Scholar] [CrossRef]
- Tay, Y.D.; Leda, M.; Spanos, C.; Rappsilber, J.; Goryachev, A.B.; Sawin, K.E. Fission Yeast NDR/LATS Kinase Orb6 Regulates Exocytosis via Phosphorylation of the Exocyst Complex. Cell Rep. 2019, 26, 1654–1667.e7. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhan, Y.Y.; Wu, B.; Yu, Q.; Xu, L.; Hong, X.; Zhong, L.; Mi, P.; Xiao, L.; Wang, X.; et al. ULK1 phosphorylates Exo70 to suppress breast cancer metastasis. Nat. Commun. 2020, 11, 117. [Google Scholar] [CrossRef]
- Ren, J.; Guo, W. ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Dev. Cell 2012, 22, 967–978. [Google Scholar] [CrossRef]
- Grosshans, D.R.; Clayton, D.A.; Coultrap, S.J.; Browning, M.D. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat. Neurosci. 2002, 5, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.Y.; Skeberdis, V.A.; Jover, T.; Grooms, S.Y.; Lin, Y.; Araneda, R.C.; Zheng, X.; Bennett, M.V.; Zukin, R.S. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 2001, 4, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Groc, L.; Heine, M.; Cognet, L.; Brickley, K.; Stephenson, F.A.; Lounis, B.; Choquet, D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 2004, 7, 695–696. [Google Scholar] [CrossRef]
- Taoufik, E.; Kouroupi, G.; Zygogianni, O.; Matsas, R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: An overview of induced pluripotent stem-cell-based disease models. Open Biol. 2018, 8. [Google Scholar] [CrossRef]
- Hasegawa, T.; Sugeno, N.; Kikuchi, A.; Baba, T.; Aoki, M. Membrane Trafficking Illuminates a Path to Parkinson’s Disease. Tohoku J. Exp. Med. 2017, 242, 63–76. [Google Scholar] [CrossRef][Green Version]
- Kiral, F.R.; Kohrs, F.E.; Jin, E.J.; Hiesinger, P.R. Rab GTPases and Membrane Trafficking in Neurodegeneration. Curr. Biol. CB 2018, 28, R471–R486. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lira, M.; Mira, R.G.; Carvajal, F.J.; Zamorano, P.; Inestrosa, N.C.; Cerpa, W. Glutamatergic Receptor Trafficking and Delivery: Role of the Exocyst Complex. Cells 2020, 9, 2402. https://doi.org/10.3390/cells9112402
Lira M, Mira RG, Carvajal FJ, Zamorano P, Inestrosa NC, Cerpa W. Glutamatergic Receptor Trafficking and Delivery: Role of the Exocyst Complex. Cells. 2020; 9(11):2402. https://doi.org/10.3390/cells9112402
Chicago/Turabian StyleLira, Matías, Rodrigo G. Mira, Francisco J. Carvajal, Pedro Zamorano, Nibaldo C. Inestrosa, and Waldo Cerpa. 2020. "Glutamatergic Receptor Trafficking and Delivery: Role of the Exocyst Complex" Cells 9, no. 11: 2402. https://doi.org/10.3390/cells9112402
APA StyleLira, M., Mira, R. G., Carvajal, F. J., Zamorano, P., Inestrosa, N. C., & Cerpa, W. (2020). Glutamatergic Receptor Trafficking and Delivery: Role of the Exocyst Complex. Cells, 9(11), 2402. https://doi.org/10.3390/cells9112402





