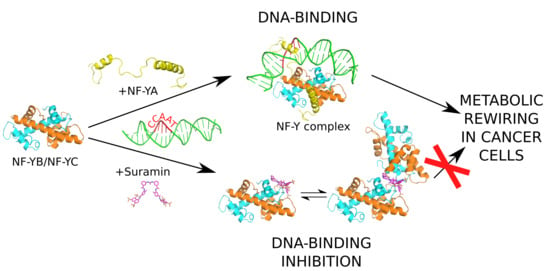
Structural Basis of Inhibition of the Pioneer Transcription Factor NF-Y by Suramin
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Search for NF-Y Inhibitors
2.2. Protein Expression and Purification
2.3. Electrophoretic Mobility Shift Assays (EMSA)
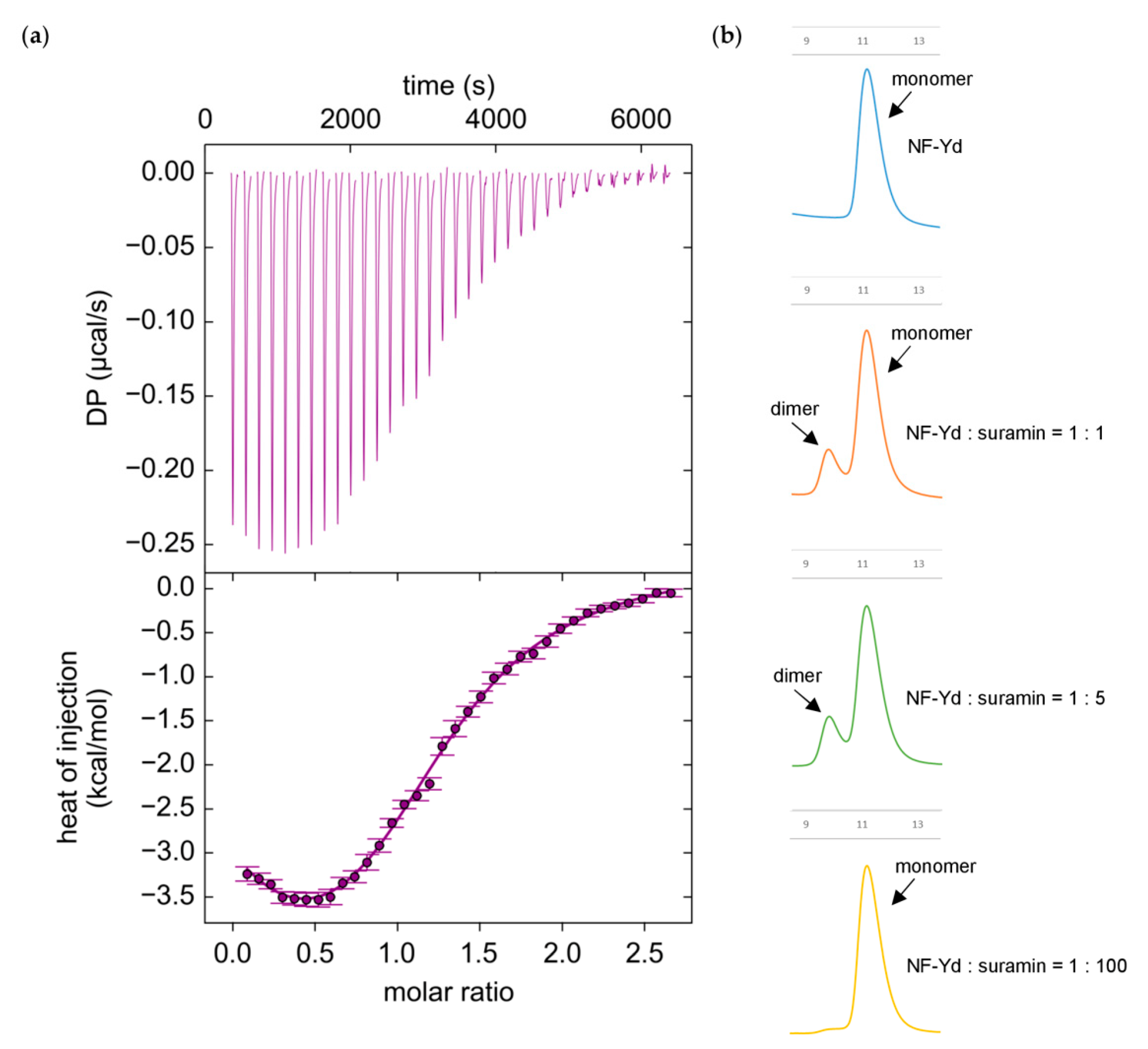
2.4. Isothermal Titration Calorimetry (ITC)
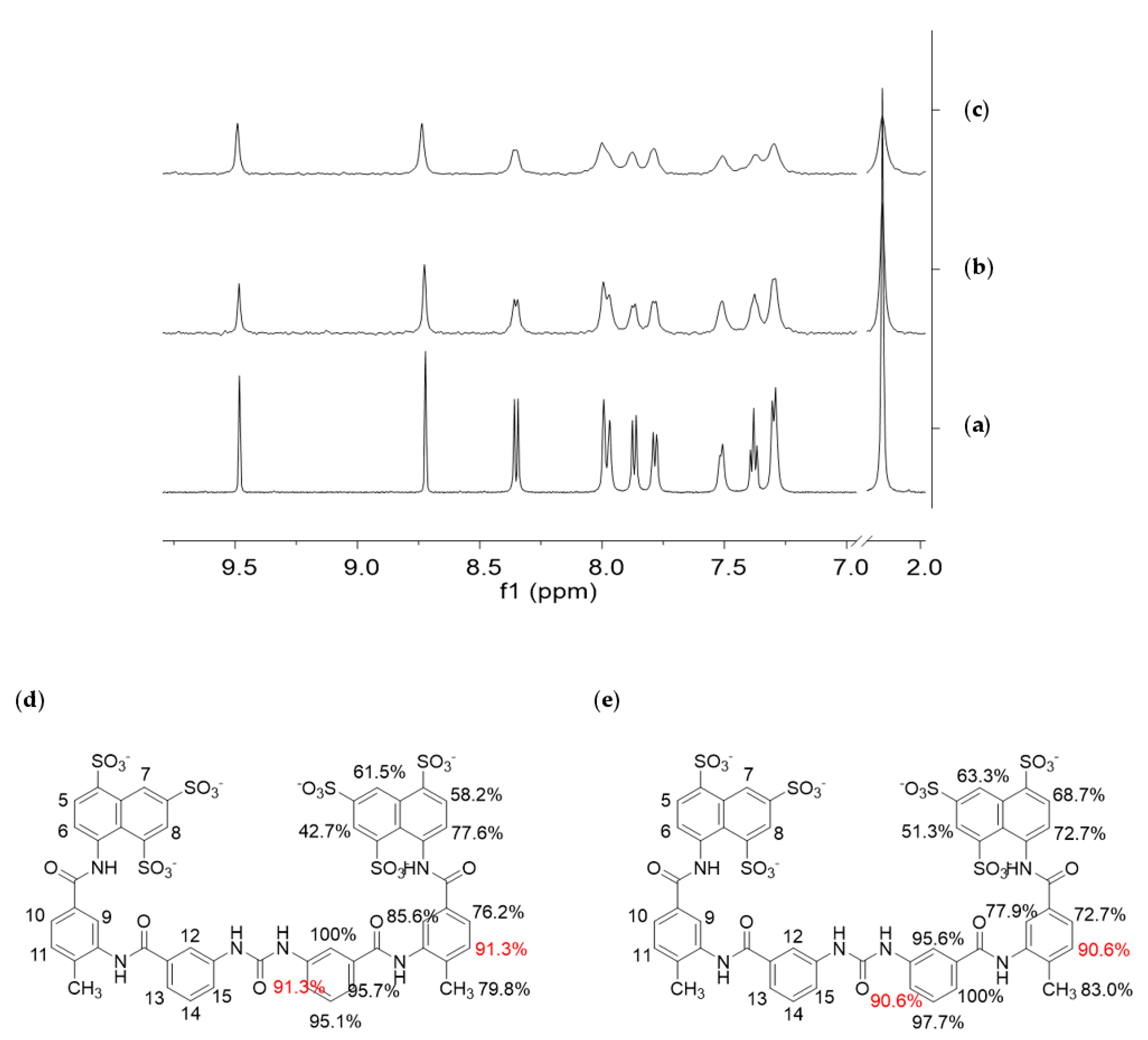
2.5. Saturation-Transfer Difference (STD) NMR
2.6. Crystallization, Data Collection, Structure Determination and Refinement
2.7. Molecular Dynamics (MD) Simulations
3. Results
3.1. Identifying Suramin as a Compound Binding to NF-Y
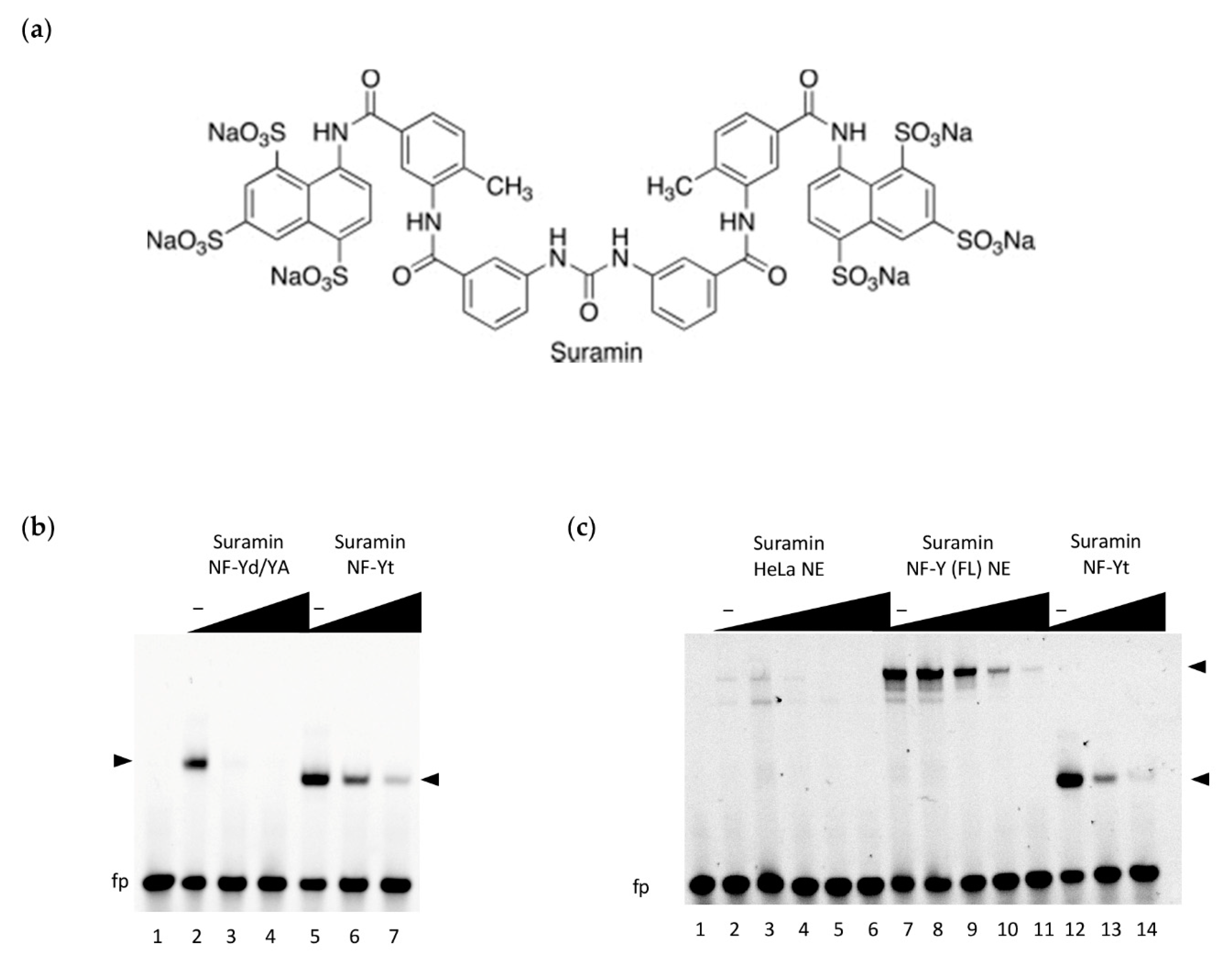
3.2. Inhibition of NF-Y DNA-Binding by Suramin
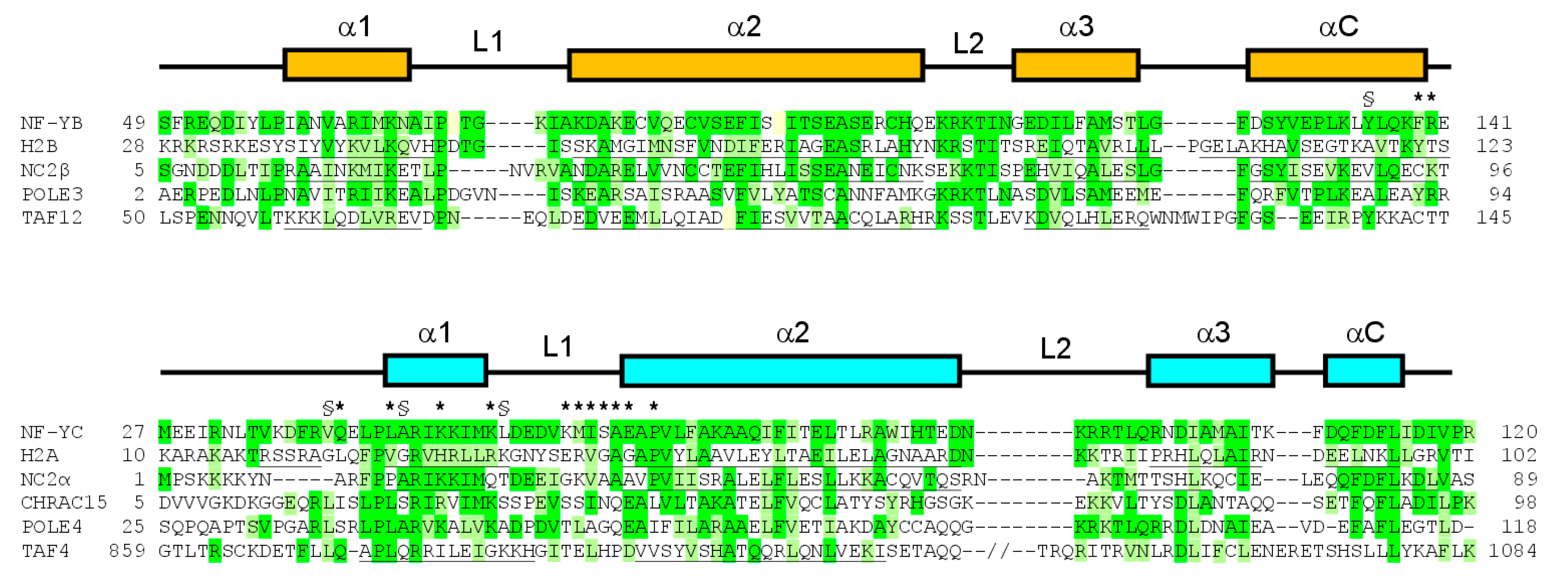
3.3. Interaction between NF-Yd and Suramin
3.4. STD NMR Binding Experiments
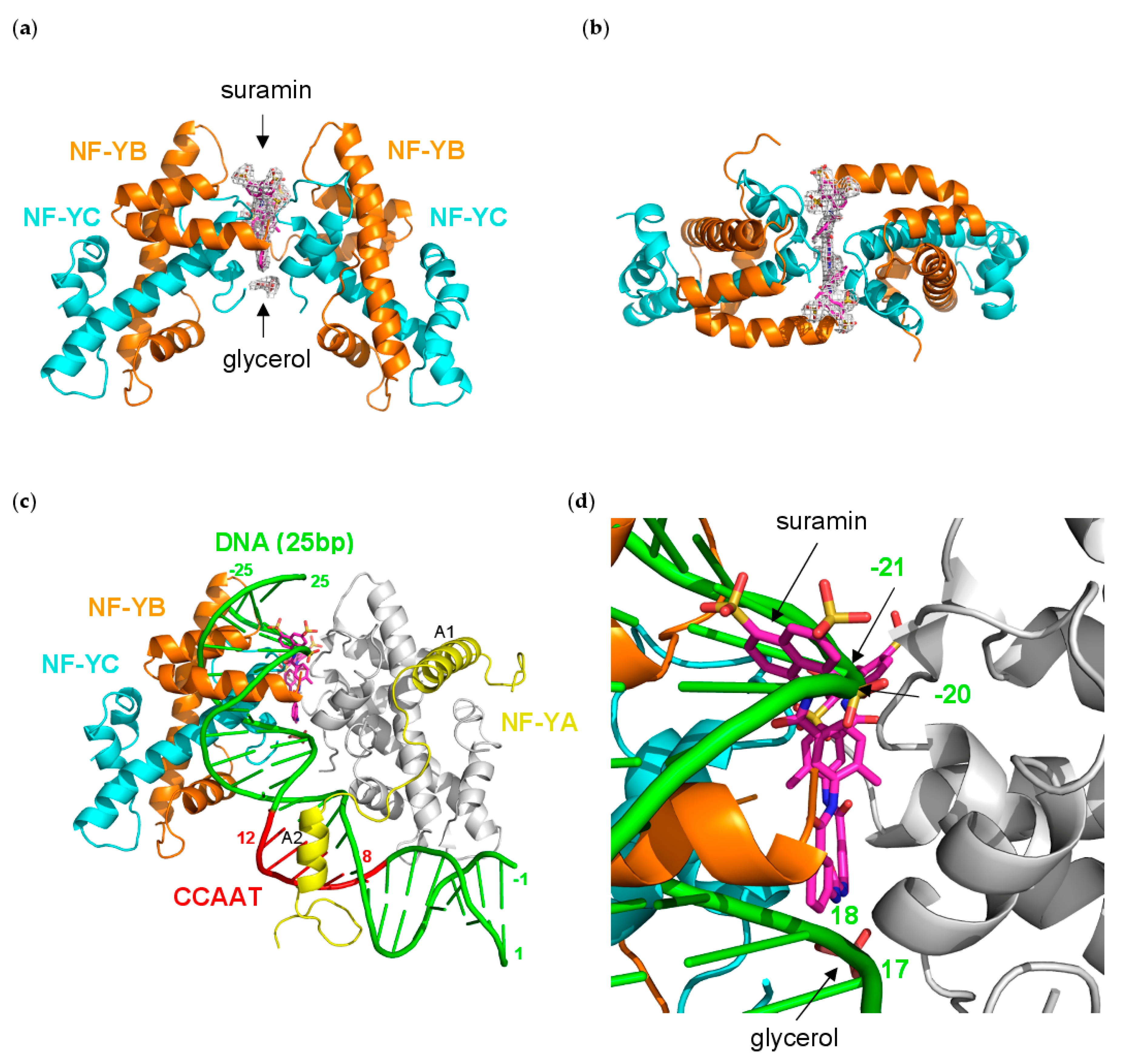
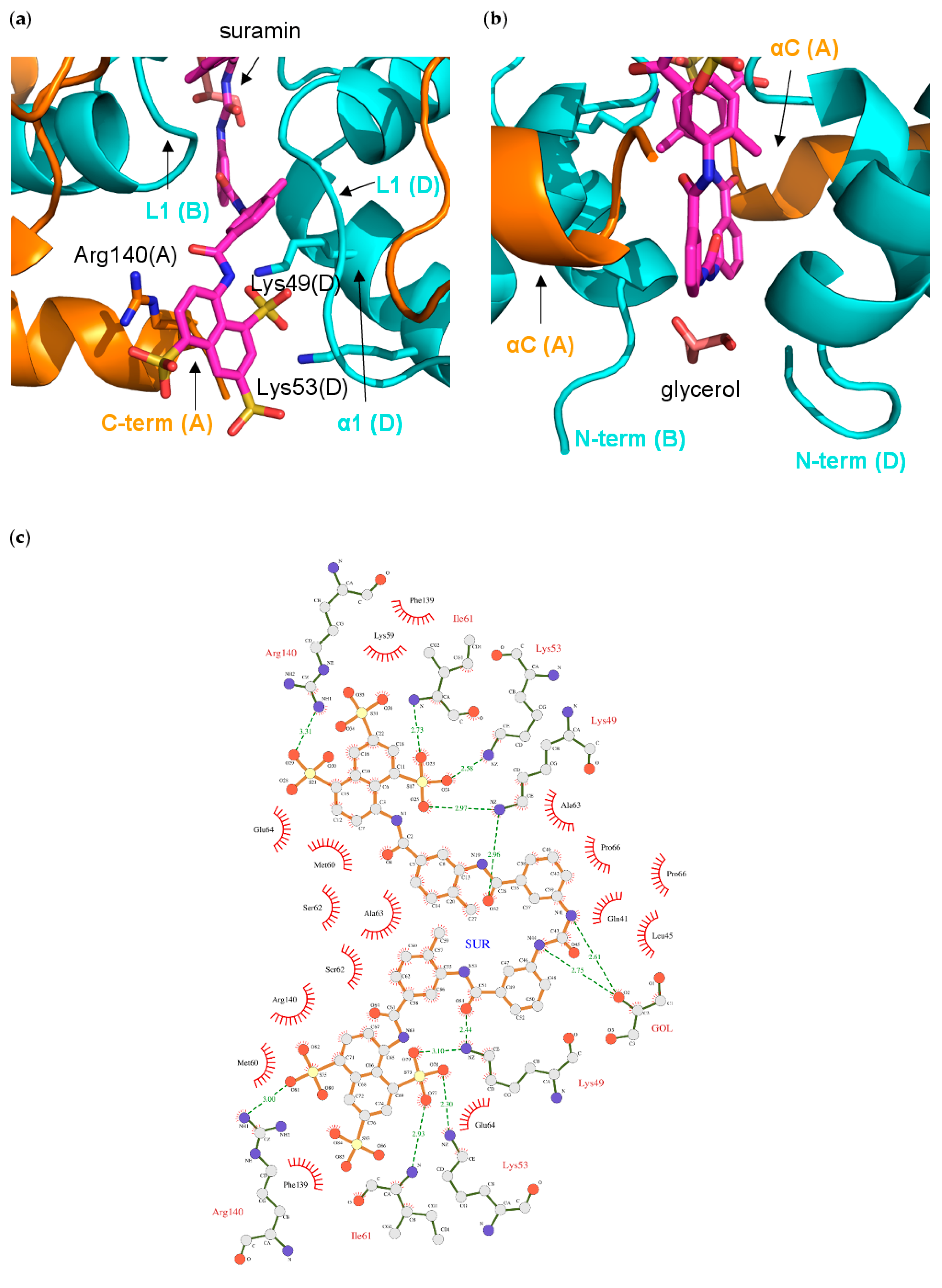
3.5. NF-Yd–Suramin Complex
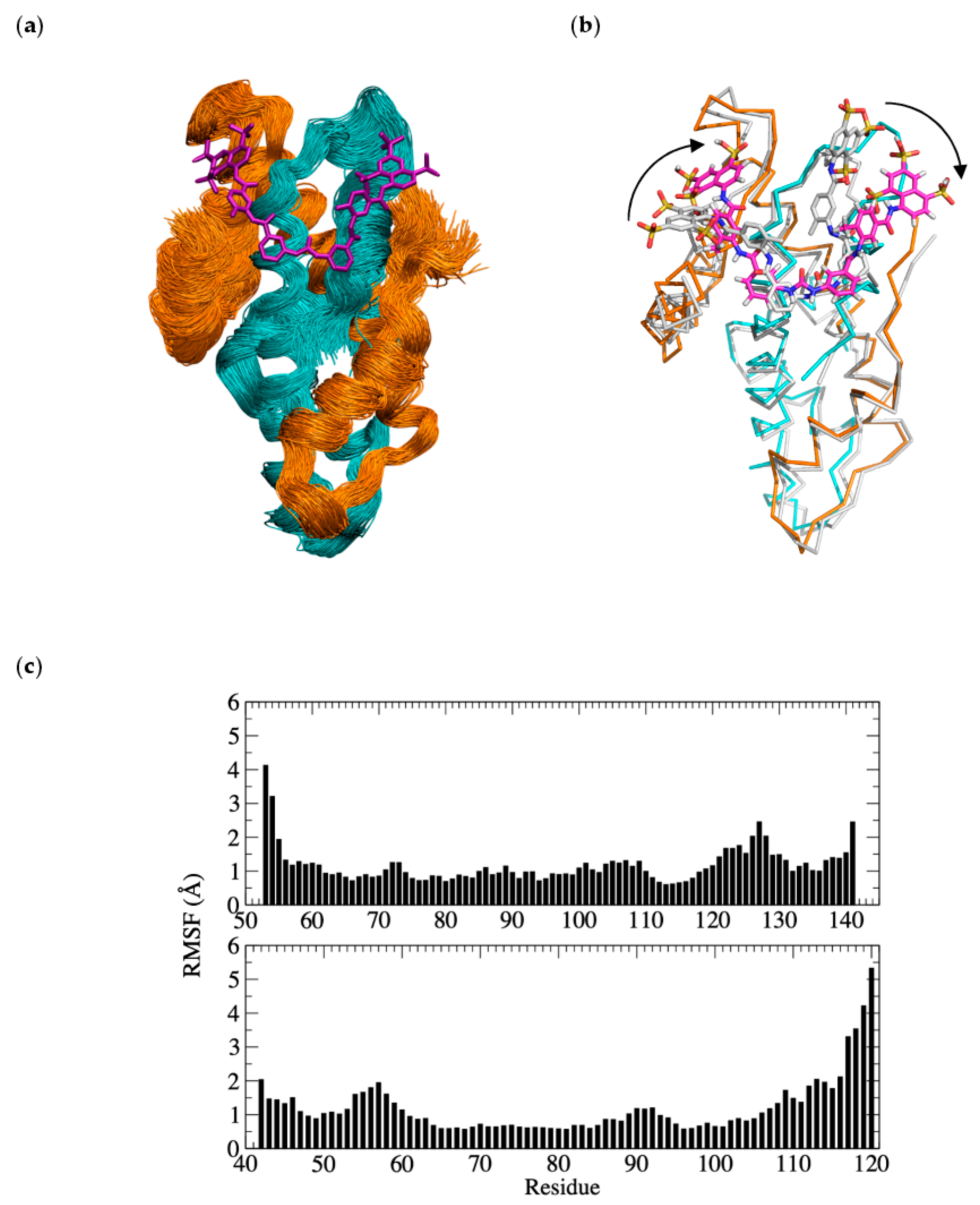
3.6. Molecular Dynamics Simulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mantovani, R. The molecular biology of the CCAAT-binding factor NF-Y. Gene 1999, 239, 15–27. [Google Scholar] [CrossRef]
- Dolfini, D.; Zambelli, F.; Pavesi, G.; Mantovani, R. A perspective of promoter architecture from the CCAAT box. Cell Cycle 2009, 8, 4127–4137. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Bolotin, E.; Jiang, T.; Sladek, F.M.; Martinez, E. Prevalence of the initiator over the TATA box in human and yeast genes and identification of DNA motifs enriched in human TATA-less core promoters. Gene 2007, 389, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Dolfini, D.; Mantovani, R. Targeting the Y/CCAAT box in cancer: YB-1 (YBX1) or NF-Y? Cell Death Differ. 2013, 20, 676–685. [Google Scholar] [CrossRef]
- Bergh, K.T.; Litzka, O.; Brakhage, A.A. Identification of a major cis-acting DNA element controlling the bidirectionally transcribed penicillin biosynthesis genes acvA (pcbAB) and ipnA (pcbC) of Aspergillus nidulans. J. Bacteriol. 1996, 178, 3908–3916. [Google Scholar] [CrossRef][Green Version]
- Ceribelli, M.; Dolfini, D.; Merico, D.; Gatta, R.; Viganò, A.M.; Pavesi, G.; Mantovani, R. The histone-like NF-Y is a bifunctional transcription factor. Mol. Cell. Biol. 2008, 28, 2047–2058. [Google Scholar] [CrossRef]
- Deng, H.; Sun, Y.; Zhang, Y.; Luo, X.; Hou, W.; Yan, L.; Chen, Y.; Tian, E.; Han, J.; Zhang, H. Transcription factor NFY globally represses the expression of the C. elegans Hox gene abdominal-B homolog egl-5. Dev. Biol. 2007, 308, 583–592. [Google Scholar] [CrossRef]
- Littlejohn, T.G.; Hynes, M.J. Analysis of the site of action of the amdR product for regulation of the amdS gene of Aspergillus nidulans. Mol. Gen. Genet. 1992, 235, 81–88. [Google Scholar] [CrossRef]
- Litzka, O.; Bergh, K.T.; Brakhage, A.A. The Aspergillus nidulans penicillin biosynthesis gene aat (penDE) is controlled by a CCAAT-containing DNA element. Eur. J. Biochem. 1996, 238, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Steidl, S.; Hynes, M.J.; Brakhage, A.A. The Aspergillus nidulans multimeric CCAAT binding complex AnCF is negatively autoregulated via its hapB subunit gene. J. Mol. Biol. 2001, 306, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Bolotin-Fukuhara, M. Thirty years of the HAP2/3/4/5 complex. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 543–559. [Google Scholar] [CrossRef]
- Benatti, P.; Chiaramonte, M.L.; Lorenzo, M.; Hartley, J.A.; Hochhauser, D.; Gnesutta, N.; Mantovani, R.; Imbriano, C.; Dolfini, D. NF-Y activates genes of metabolic pathways altered in cancer cells. Oncotarget 2016, 7, 1633–1650. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, H.; Elemento, O.; Tavazoie, S. Revealing global regulatory perturbations across human cancers. Mol. Cell 2009, 36, 900–911. [Google Scholar] [CrossRef]
- Cicchillitti, L.; Corrado, G.; Carosi, M.; Dabrowska, M.E.; Loria, R.; Falcioni, R.; Cutillo, G.; Piaggio, G.; Vizza, E. Prognostic role of NF-YA splicing isoforms and Lamin A status in low grade endometrial cancer. Oncotarget 2017, 8, 7935–7945. [Google Scholar] [CrossRef]
- Dolfini, D.; Andrioletti, V.; Mantovani, R. Overexpression and alternative splicing of NF-YA in breast cancer. Sci. Rep. 2019, 9, 12955. [Google Scholar] [CrossRef]
- Bie, L.Y.; Li, D.; Mu, Y.; Wang, S.; Chen, B.B.; Lyu, H.F.; Han, L.L.; Nie, C.Y.; Yang, C.C.; Wang, L.; et al. Analysis of cyclin E co-expression genes reveals nuclear transcription factor Y subunit alpha is an oncogene in gastric cancer. Chronic Dis. Transl. Med. 2018, 5, 44–52. [Google Scholar] [CrossRef]
- Bezzecchi, E.; Ronzio, M.; Dolfini, D.; Mantovani, R. NF-YA Overexpression in Lung Cancer: LUSC. Genes 2019, 10, 937. [Google Scholar] [CrossRef]
- Bezzecchi, E.; Ronzio, M.; Semeghini, V.; Andrioletti, V.; Mantovani, R.; Dolfini, D. NF-YA Overexpression in Lung Cancer: LUAD. Genes 2020, 11, 198. [Google Scholar] [CrossRef]
- Tsigelny, I.F.; Mukthavaram, R.; Kouznetsova, V.L.; Chao, Y.; Babic, I.; Nurmemmedov, E.; Pastorino, S.; Jiang, P.; Calligaris, D.; Agar, N.; et al. Multiple spatially related pharmacophores define small molecule inhibitors of OLIG2 in glioblastoma. Oncotarget 2017, 8, 22370–22384. [Google Scholar] [CrossRef]
- Bykov, V.J.; Wiman, K.G. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett. 2014, 588, 2622–2627. [Google Scholar] [CrossRef]
- Gayvert, K.M.; Dardenne, E.; Cheung, C.; Boland, M.R.; Lorberbaum, T.; Wanjala, J.; Chen, Y.; Rubin, M.A.; Tatonetti, N.P.; Rickman, D.S.; et al. A Computational Drug Repositioning Approach for Targeting Oncogenic Transcription Factors. Cell Rep. 2016, 15, 2348–2356. [Google Scholar] [CrossRef]
- Hochhauser, D.; Kotecha, M.; O’hare, C.; Morris, P.J.; Hartley, J.M.; Taherbhai, Z.; Harris, D.; Forni, C.; Mantovani, R.; Lee, M.; et al. Modulation of topoisomerase IIalpha expression by a DNA sequence-specific polyamide. Mol. Cancer Ther. 2007, 6, 346–354. [Google Scholar] [CrossRef]
- Kotecha, M.; Kluza, J.; Wells, G.; O’Hare, C.C.; Forni, C.; Mantovani, R.; Howard, P.W.; Morris, P.; Thurston, D.E.; Hartley, J.A.; et al. Inhibition of DNA binding of the NF-Y transcription factor by the pyrrolobenzodiazepine-polyamide conjugate GWL-78. Mol. Cancer Ther. 2008, 7, 1319–1328. [Google Scholar] [CrossRef]
- Mackay, H.; Brown, T.; Sexton, J.S.; Kotecha, M.; Nguyen, B.; Wilson, W.D.; Kluza, J.; Savic, B.; O’Hare, C.; Hochhauser, D.; et al. Targeting the inverted CCAAT Box-2 of the topoisomerase IIalpha gene: DNA sequence selective recognition by a polyamide-intercalator as a staggered dimer. Bioorgan. Med. Chem. 2008, 16, 2093–2102. [Google Scholar] [CrossRef]
- Franks, A.; Tronrud, C.; Kiakos, K.; Kluza, J.; Munde, M.; Brown, T.; Mackay, H.; Wilson, W.D.; Hochhauser, D.; Hartley, J.A.; et al. Targeting the ICB2 site of the topoisomerase IIalpha promoter with a formamido-pyrrole-imidazole-pyrrole H-pin polyamide. Bioorgan. Med. Chem. 2010, 18, 5553–5561. [Google Scholar] [CrossRef]
- Brucoli, F.; Hawkins, R.M.; James, C.H.; Jackson, P.J.; Wells, G.; Jenkins, T.C.; Ellis, T.; Kotecha, M.; Hochhauser, D.; Hartley, J.A.; et al. An extended pyrrolobenzodiazepine-polyamide conjugate with selectivity for a DNA sequence containing the ICB2 transcription factor binding site. J. Med. Chem. 2013, 56, 6339–6351. [Google Scholar] [CrossRef]
- Pett, L.; Kiakos, K.; Satam, V.; Patil, P.; Laughlin-Toth, S.; Gregory, M.; Bowerman, M.; Olson, K.; Savagian, M.; Lee, M.; et al. Modulation of topoisomerase IIα expression and chemosensitivity through targeted inhibition of NF-Y: DNA binding by a diamino p-anisyl-benzimidazole (Hx) polyamide. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 617–629. [Google Scholar] [CrossRef]
- Nardini, M.; Gnesutta, N.; Donati, G.; Gatta, R.; Forni, C.; Fossati, A.; Vonrhein, C.; Moras, D.; Romier, C.; Bolognesi, M.; et al. Sequence-specific transcription factor NF-Y displays histone-like DNA binding and H2B-like ubiquitination. Cell 2013, 152, 132–143. [Google Scholar] [CrossRef]
- Huber, E.M.; Scharf, D.H.; Hortschansky, P.; Groll, M.; Brakhage, A.A. DNA minor groove sensing and widening by the CCAAT-binding complex. Structure 2012, 20, 1757–1768. [Google Scholar] [CrossRef]
- Chaves-Sanjuan, A.; Gnesutta, N.; Gobbini, A.; Martignago, D.; Bernardini, A.; Fornara, F.; Mantovani, R.; Nardini, M. Structural determinants for NF-Y subunit organization and NF-Y/DNA association in plants. Plant J. 2020, accepted. [Google Scholar] [CrossRef]
- Jeganathan, S.; Wendt, M.; Kiehstaller, S.; Brancacci, D.; Kuepper, A.; Pospiech, N.; Carotenuto, A.; Novellino, E.; Hennig, S.; Grossmann, T.N. Constrained peptides with fine-tuned flexibility inhibit NF-Y transcription factor assembly. Angew. Chem. Int. Ed. Engl. 2019, 58, 17351–17358. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Bernardini, A.; Lorenzo, M.; Nardini, M.; Mantovani, R.; Gnesutta, N. The phosphorylatable Ser320 of NF-YA is involved in DNA binding of the NF-Y trimer. FASEB J. 2019, 33, 4790–4801. [Google Scholar] [CrossRef]
- Diebold, M.L.; Fribourg, S.; Koch, M.; Metzger, T.; Romier, C. Deciphering correct strategies for multiprotein complex assembly by co-expression: Application to complexes as large as the histone octamer. J. Struct. Biol. 2011, 175, 178–188. [Google Scholar] [CrossRef]
- Romier, C.; Cocchiarella, F.; Mantovani, R.; Moras, D. The NF-YB/NF-YC structure gives insight into DNA binding and transcription regulation by CCAAT factor NF-Y. J. Biol. Chem. 2003, 278, 1336–1345. [Google Scholar] [CrossRef]
- Ceribelli, M.; Benatti, P.; Imbriano, C.; Mantovani, R. NF-YC complexity is generated by dual promoters and alternative splicing. J. Biol. Chem. 2009, 284, 34189–34200. [Google Scholar] [CrossRef]
- Saponaro, A. Isothermal Titration Calorimetry: A Biophysical Method to Characterize the Interaction between Label-free Biomolecules in Solution. Bio-Protocol 2018, 8, e2957. [Google Scholar] [CrossRef]
- Brautigam, C.A.; Zhao, H.; Vargas, C.; Keller, S.; Schuck, P. Integration and global analysis of isothermal titration calorimetry data for studying macromolecular interactions. Nat. Protoc. 2016, 11, 882–894. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 72–82. [Google Scholar] [CrossRef]
- Storoni, L.C.; McCoy, A.J.; Read, R.J. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 432–438. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Vagin, A.A.; Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997, 53, 240–255. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A.; Simmerling, C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 2006, 65, 712–725. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Fawzi, M.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single Crystals: A New Molecular Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, T.-C.; Valiyaveettil, S.; Go, M.L.; Manjunatha Kini, R.; Velazquez-Campoy, A.; Sivaraman, J. Structural Characterization of Myotoxic Ecarpholin S from Echis carinatus Venom. Biophys. J. 2008, 95, 3366–3380. [Google Scholar] [CrossRef]
- Salvador, G.H.; Dreyer, T.R.; Cavalcante, W.L.; Matioli, F.F.; Dos Santos, J.I.; Velazquez-Campoy, A.; Gallacci, M.; Fontes, M.R. Structural and functional evidence for membrane docking and disruption sites on phospholipase A2-like proteins revealed by complexation with the inhibitor suramin. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 2066–2078. [Google Scholar] [CrossRef]
- Mayer, M.; Meyer, B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. Int. Ed. 1999, 38, 1784–1788. [Google Scholar] [CrossRef]
- Peri, F.; Airoldi, C.; Colombo, S.; Mari, S.; Jiménez-Barbero, J.; Martegani, E.; Nicotra, F. Sugar-Derived Ras Inhibitors: Group Epitope Mapping by NMR Spectroscopy and Biological Evaluation. Eur. J. Org. Chem. 2006, 16, 3707–3720. [Google Scholar] [CrossRef]
- Airoldi, C.; Merlo, S.; Sironi, E. NMR Molecular Recognition Studies for the Elucidation of Protein and Nucleic Acid Structure and Function. In Applications of NMR Spectroscopy; Rahman, A.U., Iqbal Choudhary, M., Eds.; Bentham Science Publishers Ltd.: Sharjah, UAE, 2015; Volume 2, pp. 147–219. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Maity, S.N. NF-Y (CBF) regulation in specific cell types and mouse models. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 598–603. [Google Scholar] [CrossRef]
- Dolfini, D.; Minuzzo, M.; Sertic, S.; Mantovani, R. NF-YA overexpression protects from glutamine deprivation. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118571. [Google Scholar] [CrossRef] [PubMed]
- Voogd, T.E.; Vansterkenburg, E.L.; Wilting, J.; Janssen, L.H. Recent research on the biological activity of suramin. Pharmacol. Rev. 1993, 45, 177–203. [Google Scholar] [PubMed]
- Mitsuya, H.; Popovic, M.; Yarchoan, R.; Matsushita, S.; Gallo, R.C.; Broder, S. Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III. Science 1984, 226, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Villalona-Calero, M.A.; Wientjes, M.G.; Otterson, G.A.; Kanter, S.; Young, D.; Murgo, A.J.; Fischer, B.; DeHoff, C.; Chen, D.; Yeh, T.K.; et al. Phase I study of low-dose suramin as a chemosensitizer in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2003, 9, 3303–3311. [Google Scholar] [PubMed]
- Wiedemar, N.; Hauser, D.A.; Mäser, P. 100 Years of Suramin. Antimicrob. Agents Chemother. 2020, 64, e01168-19. [Google Scholar] [CrossRef]
- Larsen, A.K. Suramin: An anticancer drug with unique biological effects. Cancer Chemother. Pharmacol. 1993, 32, 96–98. [Google Scholar] [CrossRef]
- Nakajima, M.; De Chavigny, A.; Johnson, C.E.; Hamada, J.; Stein, C.A.; Nicolson, G.L. Suramin. A potent inhibitor of melanoma heparanase and invasion. J. Biol. Chem. 1991, 266, 9661–9666. [Google Scholar]
- Firsching, A.; Nickel, P.; Mora, P.; Allolio, B. Antiproliferative and angiostatic activity of suramin analogues. Cancer Res. 1995, 55, 4957–4961. [Google Scholar]
- Parish, C.R.; Freeman, C.; Brown, K.J.; Francis, D.J.; Cowden, W.B. Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res. 1999, 59, 3433–3441. [Google Scholar]
- Marchetti, D.; Reiland, J.; Erwin, B.; Roy, M. Inhibition of heparanase activity and heparanase-induced angiogenesis by suramin analogues. Int. J. Cancer 2003, 104, 167–174. [Google Scholar] [CrossRef]
- Su, L.; Bryan, N.; Battista, S.; Freitas, J.; Garabedian, A.; D’Alessio, F.; Romano, M.; Falanga, F.; Fusco, A.; Kos, L.; et al. Suramin potently inhibits binding of the mammalian high mobility group protein AT-hook 2 to DNA. BioRxiv 2019, 838656. [Google Scholar] [CrossRef]
- Schuetz, A.; Min, J.; Antoshenko, T.; Wang, C.L.; Allali-Hassani, A.; Dong, A.; Loppnau, P.; Vedadi, M.; Bochkarev, A.; Sternglanz, R.; et al. Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin. Structure 2007, 15, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Gnesutta, N.; Nardini, M.; Mantovani, R. The H2A/H2B-like histone-fold domain proteins at the crossroad between chromatin and different DNA metabolisms. Transcription 2013, 4, 114–119. [Google Scholar] [CrossRef] [PubMed]
| Data Collection | |
| Space group | P212121 |
| Cell dimensions | |
| a, b, c (Å) | 45.697, 61.213, 123.533 |
| α, β, γ (°) | 90.00, 90.00, 90.00 |
| Resolution (Å) | 45.7–2.7 (2.83–2.70) * |
| Unique reflections | 10061 (1299) |
| Rmerge (%) | 0.11 (0.75) |
| I/σ(I) | 16.2 (3.5) |
| Multiplicity | 12.1 (12.8) |
| Completeness (%) | 100 (100) |
| Refinement | |
| Rwork/Rfree (%) | 22.2/27.4 |
| No. residues/molecules | |
| NF-YB | 88 (A chain); 89 (C chain) |
| NF-YC | 79 (B chain); 80(D chain) |
| Suramin | 1 |
| Glycerol | 1 |
| Citrate | 1 |
| Water | 47 |
| B-factors (Å2) | 55.1 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.009 |
| Bond angles (°) | 1.41 |
| Ramachandran statistics | |
| allowed region (%) | 98.1 |
| favorably allowed region (%) | 1.9 |
| outliers | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardone, V.; Chaves-Sanjuan, A.; Lapi, M.; Airoldi, C.; Saponaro, A.; Pasqualato, S.; Dolfini, D.; Camilloni, C.; Bernardini, A.; Gnesutta, N.; et al. Structural Basis of Inhibition of the Pioneer Transcription Factor NF-Y by Suramin. Cells 2020, 9, 2370. https://doi.org/10.3390/cells9112370
Nardone V, Chaves-Sanjuan A, Lapi M, Airoldi C, Saponaro A, Pasqualato S, Dolfini D, Camilloni C, Bernardini A, Gnesutta N, et al. Structural Basis of Inhibition of the Pioneer Transcription Factor NF-Y by Suramin. Cells. 2020; 9(11):2370. https://doi.org/10.3390/cells9112370
Chicago/Turabian StyleNardone, Valentina, Antonio Chaves-Sanjuan, Michela Lapi, Cristina Airoldi, Andrea Saponaro, Sebastiano Pasqualato, Diletta Dolfini, Carlo Camilloni, Andrea Bernardini, Nerina Gnesutta, and et al. 2020. "Structural Basis of Inhibition of the Pioneer Transcription Factor NF-Y by Suramin" Cells 9, no. 11: 2370. https://doi.org/10.3390/cells9112370
APA StyleNardone, V., Chaves-Sanjuan, A., Lapi, M., Airoldi, C., Saponaro, A., Pasqualato, S., Dolfini, D., Camilloni, C., Bernardini, A., Gnesutta, N., Mantovani, R., & Nardini, M. (2020). Structural Basis of Inhibition of the Pioneer Transcription Factor NF-Y by Suramin. Cells, 9(11), 2370. https://doi.org/10.3390/cells9112370