Unveiling Human Non-Random Genome Editing Mechanisms Activated in Response to Chronic Environmental Changes: I. Where Might These Mechanisms Come from and What Might They Have Led To?
Abstract
1. Waiting for Beneficial Random Mutations within the Vast Eukaryotic Cell Genome: Is It Like “Waiting for Godot”?
1.1. Random Mutations: A Weak Point in the Modern Synthesis of Evolution
1.2. The Complex Protein–Protein Steric Interactions of Eukaryotic Cells Originate from the Translation of Unrelated Genes: The Needs of Gene Editing Feedback Mechanisms
1.3. Environmental Changes Drive Organisms to Evolve Rapidly: The Evolvability Trait Was Likely Selected Early on in the Evolution of Life
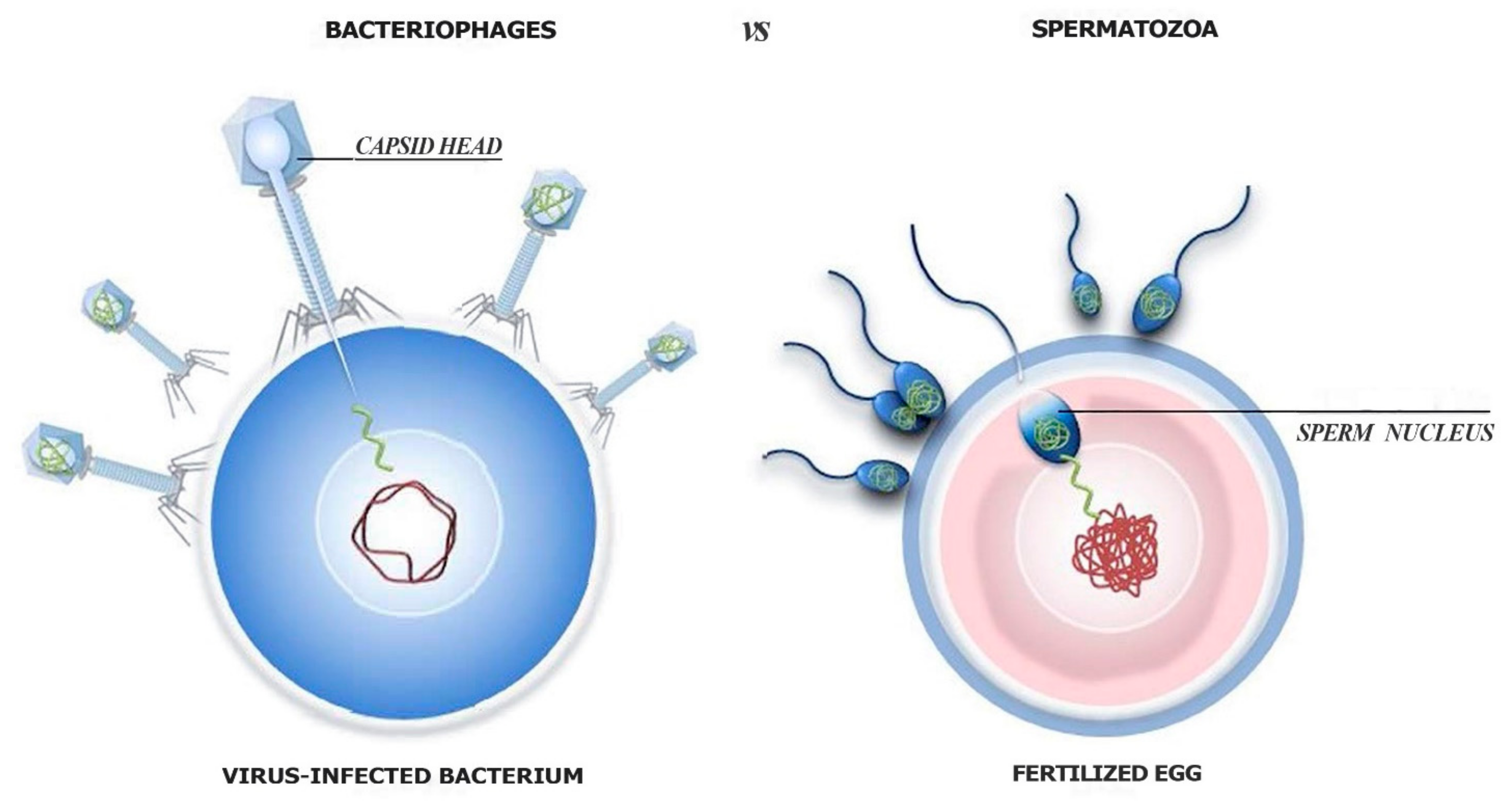
1.4. Why Should Eukaryotic Organisms Survive Viruses?
1.5. The Open Questions of the “Ecological” and Punctuated Equilibrium Views of Evolution
1.6. Immunoglobulin Somatic Hypermutation as a Mechanism Model for Eukaryotic Gene/Cell Evolution
2. Are Viral Nucleic Acid Insertions in the Prokaryotic Cells Always Harmful or Can They Also Be Useful to Prokaryotic Cells? The Example of CRISPR-Cas Systems
2.1. Selection of Virus–Cell Mating Pairs: A Primordial Form of “Sexuality” and Symbiosis
2.2. The Meanings of Virus Fragments in Lamarckian CRISPR-Cas Systems
3. Viral Nucleic Acid Insertions from the Segregated CRISPR-Cas Sites of Prokaryotes Then Spread into Coding Regions of the Eukaryotic Cell Genome. Are They Harmful or Useful to Eukaryotic Cells? The Hypothesis of Retrotransposon-Guided Human APOBEC Antiviral Activity
3.1. The Retrotransposon-Guided APOBEC Enzyme Hypothesis
3.2. How Have Endogenous Viral Sequences Spread into the Coding Regions of the Eukaryotic Cell Genome?
3.3. Antiviral Activity of Retrotransposon-Guided APOBECs
3.4. The Eukaryotic Gene Development Hypothesis
3.5. Cell Integration of Viral Elements: The Richness of Foreigner-Migrant “Cultures” Following Unproductive Struggles
4. Exaptation of Human Retrotransposon-APOBEC Systems: From Antiviral Activity to Chronic Stress-Induced Site-Specific Genome Editing
4.1. Transposon- and APOBEC-Mediated Genome Editing Mechanisms: A Possible Link with Environmental Stress Conditions
4.2. Stress-Induced Transposon Mobilization: The Intronic Origin Hypothesis
4.3. Molecular Concepts of the Cellular Environment and Cell Environmental Stress Conditions: An “Ecological” View of Cells and Tissues
4.4. Cellular Homeostatic Mechanisms Regulate Optimal Cellular Protein Concentrations in Normal Environmental Fluctuations
 ) that regulates its transcription is free to induce its gene transcription. The subsequent translation into proteins increases the protein amount and, by binding with a second protein (P1), the pathway functional activity in which the protein (P) is involved (see Figure 2B). To fine-tune protein expression, cells have developed negative feedback mechanisms through repressors (
) that regulates its transcription is free to induce its gene transcription. The subsequent translation into proteins increases the protein amount and, by binding with a second protein (P1), the pathway functional activity in which the protein (P) is involved (see Figure 2B). To fine-tune protein expression, cells have developed negative feedback mechanisms through repressors ( ) able to inhibit the activity of transcription factors (
) able to inhibit the activity of transcription factors ( ): the higher the expression of the protein (P) bound to its partner protein (P1) along their signal pathway, the higher the gene repression (
): the higher the expression of the protein (P) bound to its partner protein (P1) along their signal pathway, the higher the gene repression ( ), which inhibits gene transcription. Finally, lowering protein synthesis, protein degradation produces both low levels of protein (P) and repressors (Figure 2C), similar to the initial condition (Figure 2A). The cycle restarts and the amount of protein (P) fluctuates around the optimal needs for cell functionality according to the environmental conditions. Usually, cell epigenetic homeostatic responses counteract normal environmental fluctuations through negative feedback loops that tend to reduce external environmental molecular changes around a normal fluctuating range. Normal environmental fluctuations induce either an increase or a decrease in gene transcription and protein expression and stability. However, a major problem arises when a new environmental context induces cell homeostatic responses which, though reaching their maximum activity, are not sufficient to meet the new cell needs. This occurs when the demand surpasses the potential ability to synthesise and stabilize a specific protein, i.e., the cell does not find the solution/balance among the epigenetic mechanisms. If the new environmental condition is temporary, it may produce a temporary cell stress; however, when the environmental changes are persistent and/or increasing, chronically stressed cells have to activate different mechanisms in order to survive.
), which inhibits gene transcription. Finally, lowering protein synthesis, protein degradation produces both low levels of protein (P) and repressors (Figure 2C), similar to the initial condition (Figure 2A). The cycle restarts and the amount of protein (P) fluctuates around the optimal needs for cell functionality according to the environmental conditions. Usually, cell epigenetic homeostatic responses counteract normal environmental fluctuations through negative feedback loops that tend to reduce external environmental molecular changes around a normal fluctuating range. Normal environmental fluctuations induce either an increase or a decrease in gene transcription and protein expression and stability. However, a major problem arises when a new environmental context induces cell homeostatic responses which, though reaching their maximum activity, are not sufficient to meet the new cell needs. This occurs when the demand surpasses the potential ability to synthesise and stabilize a specific protein, i.e., the cell does not find the solution/balance among the epigenetic mechanisms. If the new environmental condition is temporary, it may produce a temporary cell stress; however, when the environmental changes are persistent and/or increasing, chronically stressed cells have to activate different mechanisms in order to survive.4.5. Putative Cellular Mechanisms of Eukaryotic Non-Random Genome Editing in Response to Chronic Stress Conditions: Chronic Stress-Induced Gene Duplication and Site-Specific Mutagenesis
4.6. Learning from the Immune System: The Example of AID-Mediated Immunoglobulin Recombination
5. Environmentally Induced Adaptation of Eukaryotic Inadequate/Hyper-Functional Genes by Retrotransposon-Guided Mutagenic Enzymes: What Might It Have Led To?
5.1. Retrotransposon-Guided Mutagenic Enzymes: An Ideal “Lamarckian” Tool of Evolvability and Plasticity. The Example of Polyethylene Metabolising Moth Larvae
5.2. Non-random Genome Editing Mechanisms: Tools to Transform Pollutants into Essential Components of Life and Possible Links of Neotenic Traits to “Neotenic” Environments
5.3. The New Hypothesis of Giraffe Evolution and the Fascinating Hypothesis of “Selfish” Endogenous Retrovirus Reactivation by Starvation-Induced Gene Hyper-Transcription
5.4. Abundance of Ancient Pollutants/New Nutrients Could Lead to the Rise of “Clades” and Altruistic Biological Behaviours by Mean of Non-Random Genome and Epigenome Editing Mechanisms
5.5. Chronic Diseases as by-Products of Environmentally-Induced Non-Random Genome Editing Mechanisms in Environmentally Inadequate Genomes
6. Exploiting Cancer as a Model to Study Non-Random Genome Editing Mechanisms and Eukaryotic Cell Evolution
6.1. A Cell Species That Exploits Non-Random Genome Editing Mechanisms to Survive in Chronic Stress Conditions: Cancer
6.2. Non-Random Retro-Transposition of Repetitive Elements and Non-Random Genome Editing of Regulatory Sequences and Transcription Factors Account for Non-Random Novel Regulatory Gene Networks and Epigenome Editing/Remodelling and Enheritance: The Example of Melanism in the British Peppered Moth
6.3. Non-Random Chromosomal Rearrangements and the Involvement of Non-Random Epigenome Editing Mechanisms in Cancer Cells Following Exposure to Chemical and Physical Stressors
6.4. Solid Cancer/Leukaemia/Lymphoma as Models of “Cell Speciation” and the Example of Mouse Speciation in Seveso
7. Sexual Symbiosis: An Altruistic Cooperation of Distinct Sexual Functions in Order to Transfer Environmentally Induced Acquired Novelties to Progeny
7.1. Non-Mendelian Transgenerational Inheritance Induced by Chronic Environmental Changes: The Central Role of Male Germline Cells
7.2. The Fascinating Role of “Female”in the Embryo-Foetal Development and the Revisiting of Haeckel’s Evolutionary Theory: The Involvement of Epigenetic Memories
8. Conclusions and Future Perspectives
8.1. Non-Random Genome Editing Mechanisms: A Link between Environmental Changes and Eukaryotic Biological Novelties
8.2. A Symbiotic Reconciliation of the Darwinian and Lamarckian Perspectives
8.3. Non-Random Genome Editing Mechanisms Reconcile Gene-Centred and “Ecological” Theories of Evolution: Thinking of a New “Hyper-Modern” Synthesis of Evolutionary Theory, Eco-Evo-Memo-Poly
8.4. Multilevel Biological Organisations and Multilevel Selections: A “Philosophical” Structuring of Life
8.5. Biological Systems as Fractal Systems with a Nested “Matryoshka Doll” Structure
8.6. On the Origin of Biomolecules and Virus-Like Structures
8.7. The Evolution of Viruses as Migrating “Organelles” of Cells: Implications for Human Brain Evolution
8.8. Conclusive Chemical and Physical Considerations on Life: The Big Bang of Life as a Gravitation-Like Force of Aggregation, a Gravitational Singularity with a Black Hole-Like Behaviour
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Activation-induced deaminase | AID |
| Adenosine | A |
| Adenosine deaminase enzymes acting on RNA | ADARs |
| Androgen receptor | AR |
| Androgen response elements | AREs |
| Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like | APOBEC |
| Clustered, regularly interspaced, short, palindromic repeats | CRISPR |
| Cytosine | C |
| CRISPR-associated | Cas |
| CRISPR-associated complex for antiviral defence | Cascade |
| CRISPR RNAs | crRNAs |
| Dihydrotestosterone | DHT |
| Ecological evolutionary developmental biology | Eco-Evo-Devo |
| Endogenous retroviruses | ERVs |
| Gene copy number variations | CNV |
| Guanosine | G |
| Haemoglobin | Hb |
| Heavy-chain class-switch recombination | CSR |
| Immunoglobulin | Ig |
| Inosine | I |
| Interferons | IFNs |
| Long interspersed nuclear elements | LINEs |
| Long-terminal repeats | LTR |
| Open reading frame 1 and 2 proteins | ORF1p and ORF2p |
| Protein | P |
| Short interspersed nuclear elements | SINEs |
| Single nucleotide polymorphisms | SNP |
| Single-stranded DNA | ssDNA |
| Somatic hypermutation | SHM |
| Stress induced mutagenesis | SIM |
| Switch | S |
| Thymidine | T |
References
- Holmes, E.C. What Does Virus Evolution Tell Us about Virus Origins? J. Virol. 2011, 85, 5247–5251. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Gago-Zachert, S.; Serra, P.; Sanjuán, R.; Elena, S.F. Viroids: Survivors from the RNA world? Annu. Rev. Microbiol. 2014, 68, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Durzyńska, J.; Goździcka-Józefiak, A. Viruses and cells intertwined since the dawn of evolution. Virol. J. 2015, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Galhardo, R.S.; Hastings, P.J.; Rosenberg, S.M. Mutation as a Stress Response and the Regulation of Evolvability. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 399–435. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.M.; Hastings, P.; Rosenberg, S.M. Stress-Induced Mutagenesis: Implications in Cancer and Drug Resistance. Annu. Rev. Cancer Biol. 2017, 1, 119–140. [Google Scholar] [CrossRef]
- Koonin, E.V.; Wolf, Y.I. Is evolution Darwinian or/and Lamarckian? Biol. Direct 2009, 4, 42. [Google Scholar] [CrossRef]
- Katsnelson, M.I.; Wolf, Y.I.; Koonin, E.V. On the feasibility of saltational evolution. Proc. Natl. Acad. Sci. USA 2019, 116, 21068–21075. [Google Scholar] [CrossRef]
- Wright, B.E. A Biochemical Mechanism for Nonrandom Mutations and Evolution. J. Bacteriol. 2000, 182, 2993–3001. [Google Scholar] [CrossRef]
- Steele, E.J.; Gorczynski, R.M.; Lindley, R.A.; Liu, Y.; Temple, R.; Tokoro, G.; Wickramasinghe, D.T.; Wickramasinghe, N.C. Lamarck and Panspermia—On the Efficient Spread of Living Systems Throughout the Cosmos. Prog. Biophys. Mol. Biol. 2019, 149, 10–32. [Google Scholar] [CrossRef]
- Franklin, A.; Steele, E.J.; Lindley, R.A. A proposed reverse transcription mechanism for (CAG)n and similar expandable repeats that cause neurological and other diseases. Heliyon 2020, 6, e03258. [Google Scholar] [CrossRef]
- Shapiro, J.A. Living Organisms Author Their Read-Write Genomes in Evolution. Biology 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G. The Yin and Yang of anti-Darwinian epigenetics and Darwinian genetics. Riv. Biol. 2008, 100, 361–402. [Google Scholar]
- Agrawal, L.; Lu, X.; Qingwen, J.; VanHorn-Ali, Z.; Nicolescu, I.V.; McDermott, D.H.; Murphy, P.M.; Alkhatib, G. Role for CCR5Δ32 Protein in Resistance to R5, R5 × 4, and X4 Human Immunodeficiency Virus Type 1 in Primary CD4+. J. Virol. 2004, 78, 2277–2287. [Google Scholar] [CrossRef][Green Version]
- Pope, K.O.; D’Hondt, S.L.; Marshall, C.R. Meteorite impact and the mass extinction of species at the Cretaceous/Tertiary boundary. Proc. Natl. Acad. Sci. USA 1998, 95, 11028–11029. [Google Scholar] [CrossRef]
- Kerr, R.A. Mega-Eruptions Drove the Mother of Mass Extinctions. Science 2013, 342, 1424. [Google Scholar] [CrossRef]
- Shapiro, J.A. No genome is an island: Toward a 21st century agenda for evolution. Ann. N. Y. Acad. Sci. 2019, 1447, 21–52. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F.; Bosch, T.C.G.; Ledón-Rettig, C. Eco-Evo-Devo: Developmental symbiosis and developmental plasticity as evolutionary agents. Nat. Rev. Genet. 2015, 16, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Laland, K.N.; Uller, T.; Feldman, M.; Sterelny, K.; Müller, G.B.; Moczek, A.; Jablonka, E.; Odling-Smee, J.; Wray, G.A.; Hoekstra, H.E.; et al. Does evolutionary theory need a rethink? Nat. Cell Biol. 2014, 514, 161–164. [Google Scholar] [CrossRef]
- Gould, S.J.; Eldredge, N. Punctuated equilibrium comes of age. Nat. Cell Biol. 1993, 366, 223–227. [Google Scholar] [CrossRef]
- Garagna, S.; Zuccotti, M.; Redi, C.A.; Capanna, E. Trapping speciation. Nat. Cell Biol. 1997, 390, 241–242. [Google Scholar] [CrossRef]
- Hof, A.V.; Campagne, P.; Rigden, D.J.; Yung, C.J.; Lingley, J.; Quail, M.A.; Hall, N.; Darby, A.C.; Saccheri, I.J. The industrial melanism mutation in British peppered moths is a transposable element. Nat. Cell Biol. 2016, 534, 102–105. [Google Scholar] [CrossRef]
- Skinner, M.K. Environmental Epigenetics and a Unified Theory of the Molecular Aspects of Evolution: A Neo-Lamarckian Concept that Facilitates Neo-Darwinian Evolution. Genome Biol. Evol. 2015, 7, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, L.; Fanti, L.; Specchia, V.; Bozzetti, M.P.; Berloco, M.; Palumbo, G.; Pimpinelli, S. Transposons, environmental changes, and heritable induced phenotypic variability. Chromosoma 2014, 123, 345–354. [Google Scholar] [CrossRef] [PubMed]
- McHeyzer-Williams, M.; Okitsu, S.; Wang, N.; McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 2011, 12, 24–34. [Google Scholar] [CrossRef]
- Waddington, C.H. Canalization of Development and Genetic Assimilation of Acquired Characters. Nat. Cell Biol. 1959, 183, 1654–1655. [Google Scholar] [CrossRef]
- Callier, V. Core Concept: Gene transfers from bacteria and viruses may be shaping complex organisms. Proc. Natl. Acad. Sci. USA 2019, 116, 13714–13716. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.V.; Nuñez, J.K.; Doudna, J.A. Biology and Applications of CRISPR Systems: Harnessing Nature’s Toolbox for Genome Engineering. Cell 2016, 164, 29–44. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef]
- Ratner, H.K.; Sampson, T.R.; Weiss, D.S. I can see CRISPR now, even when phage are gone: A view on alternative CRISPR-Cas functions from the prokaryotic envelope. Curr. Opin. Infect. Dis. 2015, 28, 267–274. [Google Scholar] [CrossRef][Green Version]
- He, F.; Vestergaard, G.; Peng, W.; She, Q.; Peng, X. CRISPR-Cas type I-A Cascade complex couples viral infection surveillance to host transcriptional regulation in the dependence of Csa3b. Nucleic Acids Res. 2016, 45, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Jangam, D.; Feschotte, C.; Betrán, E. Transposable Element Domestication As an Adaptation to Evolutionary Conflicts. Trends Genet. 2017, 33, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Makarova, K.S.; Wolf, Y.I.; Krupovic, M. Evolutionary entanglement of mobile genetic elements and host defence systems: Guns for hire. Nat. Rev. Genet. 2019, 21, 119–131. [Google Scholar] [CrossRef]
- Seed, K.D.; Lazinski, D.W.; Calderwood, S.B.; Camilli, A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nat. Cell Biol. 2013, 494, 489–491. [Google Scholar] [CrossRef]
- Haerter, J.O.; Sneppen, K. Spatial Structure and Lamarckian Adaptation Explain Extreme Genetic Diversity at CRISPR Locus. mBio 2012, 3, e00126-12. [Google Scholar] [CrossRef] [PubMed]
- Salter, J.D.; Bennett, R.P.; Smith, H.C. The APOBEC Protein Family: United by Structure, Divergent in Function. Trends Biochem. Sci. 2016, 41, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.; Ross, S.R. APOBEC3 Proteins in Viral Immunity. J. Immunol. 2015, 195, 4565–4570. [Google Scholar] [CrossRef]
- Zhang, W.; Du, J.; Yu, K.; Wang, T.; Yong, X.; Yu, X.-F. Association of Potent Human Antiviral Cytidine Deaminases with 7SL RNA and Viral RNP in HIV-1 Virions. J. Virol. 2010, 84, 12903–12913. [Google Scholar] [CrossRef]
- Senavirathne, G.; Bertram, J.G.; Jaszczur, M.; Chaurasiya, K.R.; Pham, P.; Mak, C.H.; Goodman, M.F.; Rueda, D. Activation-induced deoxycytidine deaminase (AID) co-transcriptional scanning at single-molecule resolution. Nat. Commun. 2015, 6, 10209. [Google Scholar] [CrossRef]
- Hulme, A.E.; Bogerd, H.P.; Cullen, B.R.; Moran, J.V. Selective inhibition of Alu retrotransposition by APOBEC3G. Gene 2007, 390, 199–205. [Google Scholar] [CrossRef]
- Roy-Engel, A.M. LINEs, SINEs and other retroelements: Do birds of a feather flock together? Front. Biosci. 2012, 17, 1345–1361. [Google Scholar] [CrossRef]
- Chiu, Y.-L.; Witkowska, H.E.; Hall, S.C.; Santiago, M.L.; Soros, V.B.; Esnault, C.; Heidmann, T.; Greene, W.C. High-molecular-mass APOBEC3G complexes restrict Alu retrotransposition. Proc. Natl. Acad. Sci. USA 2006, 103, 15588–15593. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, M.D.; Harris, R.S. APOBEC3B and APOBEC3F Inhibit L1 Retrotransposition by a DNA Deamination-independent Mechanism. J. Biol. Chem. 2006, 281, 16837–16841. [Google Scholar] [CrossRef]
- Bogerd, H.P.; Wiegand, H.L.; Hulme, A.E.; Garcia-Perez, J.L.; O’Shea, K.S.; Moran, J.V.; Cullen, B.R. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc. Natl. Acad. Sci. USA 2006, 103, 8780–8785. [Google Scholar] [CrossRef] [PubMed]
- Muckenfuss, H.; Hamdorf, M.; Held, U.; Perković, M.; Löwer, J.; Cichutek, K.; Flory, E.; Schumann, G.G.; Münk, C. APOBEC3 Proteins Inhibit Human LINE-1 Retrotransposition. J. Biol. Chem. 2006, 281, 22161–22172. [Google Scholar] [CrossRef] [PubMed]
- Kinomoto, M.; Kanno, T.; Shimura, M.; Ishizaka, Y.; Kojima, A.; Kurata, T.; Sata, T.; Tokunaga, K. All APOBEC3 family proteins differentially inhibit LINE-1 retrotransposition. Nucleic Acids Res. 2007, 35, 2955–2964. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.; Montano, M.; Garcia-Perez, J.L.; Moran, J.V.; Greene, W.C. Endogenous APOBEC3B Restricts LINE-1 Retrotransposition in Transformed Cells and Human Embryonic Stem Cells. J. Biol. Chem. 2011, 286, 36427–36437. [Google Scholar] [CrossRef]
- Horn, A.V.; Klawitter, S.; Held, U.; Berger, A.; Vasudevan, A.A.J.; Bock, A.; Hofmann, H.; Hanschmann, K.-M.O.; Trösemeier, J.-H.; Flory, E.; et al. Human LINE-1 restriction by APOBEC3C is deaminase independent and mediated by an ORF1p interaction that affects LINE reverse transcriptase activity. Nucleic Acids Res. 2013, 42, 396–416. [Google Scholar] [CrossRef]
- Feng, Y.; Goubran, M.H.; Follack, T.B.; Chelico, L. Deamination-independent restriction of LINE-1 retrotransposition by APOBEC3H. Sci. Rep. 2017, 7, 10881. [Google Scholar] [CrossRef]
- Mills, R.E.; Bennett, E.A.; Iskow, R.C.; Devine, S.E. Which transposable elements are active in the human genome? Trends Genet. 2007, 23, 183–191. [Google Scholar] [CrossRef]
- Koito, A.; Ikeda, T. Intrinsic immunity against retrotransposons by APOBEC cytidine deaminases. Front. Microbiol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 2016, 351, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Reddy, R.; Henning, D.; Epstein, P.; Busch, H. Nucleotide sequence of 7 S RNA. Homology to Alu DNA and La 4.5 S RNA. J. Biol. Chem. 1982, 257, 5136–5142. [Google Scholar] [PubMed]
- Bach, D.; Peddi, S.; Mangeat, B.; Lakkaraju, A.; Strub, K.; Trono, D. Characterization of APOBEC3G binding to 7SL RNA. Retrovirology 2008, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wildschutte, J.H.; Williams, Z.H.; Montesion, M.; Subramanian, R.P.; Kidd, J.M.; Coffin, J.M. Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc. Natl. Acad. Sci. USA 2016, 113, E2326–E2334. [Google Scholar] [CrossRef]
- Koonin, E.V.; Krupovic, M. Evolution of adaptive immunity from transposable elements combined with innate immune systems. Nat. Rev. Genet. 2014, 16, 184–192. [Google Scholar] [CrossRef]
- Medstrand, P.; Van De Lagemaat, L.N.; Mager, D.L. Retroelement Distributions in the Human Genome: Variations Associated With Age and Proximity to Genes. Genome Res. 2002, 12, 1483–1495. [Google Scholar] [CrossRef]
- Diener, T.O. Circular RNAs: Relics of precellular evolution? Proc. Natl. Acad. Sci. USA 1989, 86, 9370–9374. [Google Scholar] [CrossRef]
- Polak, P.; Domany, E. Alu elements contain many binding sites for transcription factors and may play a role in regulation of developmental processes. BMC Genom. 2006, 7, 1–15. [Google Scholar] [CrossRef]
- Feschotte, C. The contribution of transposable elements to the evolution of regulatory networks. Nat. Rev. Genet. 2008, 9, 397–405. [Google Scholar] [CrossRef]
- Miller, W.J.; McDonald, J.F.; Pinsker, W. Molecular domestication of mobile elements. Genet. 1997, 100, 261–270. [Google Scholar] [CrossRef]
- Volff, J.-N. Turning junk into gold: Domestication of transposable elements and the creation of new genes in eukaryotes. BioEssays 2006, 28, 913–922. [Google Scholar] [CrossRef]
- Iyer, L.M.; Zhang, D.; Rogozin, I.B.; Aravind, L. Evolution of the deaminase fold and multiple origins of eukaryotic editing and mutagenic nucleic acid deaminases from bacterial toxin systems. Nucleic Acids Res. 2011, 39, 9473–9497. [Google Scholar] [CrossRef] [PubMed]
- Imamichi, H.; Dewar, R.L.; Adelsberger, J.W.; Rehm, C.A.; O’Doherty, U.; Paxinos, E.E.; Fauci, A.S.; Lane, H.C. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2016, 113, 8783–8788. [Google Scholar] [CrossRef]
- Orlandi, C.; Canovari, B.; Bozzano, F.; Marras, F.; Pasquini, Z.; Barchiesi, F.; De Maria, A.; Magnani, M.; Casabianca, A. A comparative analysis of unintegrated HIV-1 DNA measurement as a potential biomarker of the cellular reservoir in the blood of patients controlling and non-controlling viral replication. J. Transl. Med. 2020, 18, 1–16. [Google Scholar] [CrossRef]
- Lee, Y.; Rio, D.C. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.G.; Klug, A. Ribozymes: Structure and mechanism in RNA catalysis. Trends Biochem. Sci. 1996, 21, 220–224. [Google Scholar] [CrossRef]
- Ajon, M.; Fröls, S.; Van Wolferen, M.; Stoecker, K.; Teichmann, D.; Driessen, A.J.M.; Grogan, D.W.; Albers, S.-V.; Schleper, C. UV-inducible DNA exchange in hyperthermophilic archaea mediated by type IV pili. Mol. Microbiol. 2011, 82, 807–817. [Google Scholar] [CrossRef]
- Pinto, Y.; Gabay, O.; Arbiza, L.; Sams, A.J.; Keinan, A.; Levanon, E.Y. Clustered mutations in hominid genome evolution are consistent with APOBEC3G enzymatic activity. Genome Res. 2016, 26, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Gordenin, D.A. Clusters of Multiple Mutations: Incidence and Molecular Mechanisms. Annu. Rev. Genet. 2015, 49, 243–267. [Google Scholar] [CrossRef]
- Platt, R.N.; Vandewege, M.W.; Ray, D.A. Mammalian transposable elements and their impacts on genome evolution. Chromosom. Res. 2018, 26, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Mourier, T.; Nielsen, L.P.; Hansen, A.J.; Willerslev, E. Transposable elements in cancer as a by-product of stress-induced evolvability. Front. Genet. 2014, 5, 156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Westra, E.R.; Buckling, A.; Fineran, P.C. CRISPR–Cas systems: Beyond adaptive immunity. Nat. Rev. Genet. 2014, 12, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Dimitriu, T.; Ashby, B.; Westra, E.R. Transposition: A CRISPR Way to Get Around. Curr. Biol. 2019, 29, R886–R889. [Google Scholar] [CrossRef]
- Klompe, S.E.; Vo, P.L.H.; Halpin-Healy, T.S.; Sternberg, S.H. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nat. Cell Biol. 2019, 571, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Strecker, J.; Ladha, A.; Gardner, Z.; Schmid-Burgk, J.L.; Makarova, K.S.; Koonin, E.V.; Zhang, F. RNA-guided DNA insertion with CRISPR-associated transposases. Science 2019, 365, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yang, L.; Tanasa, B.; Hutt, K.; Ju, B.-G.; Ohgi, K.A.; Zhang, J.; Rose, D.W.; Fu, X.-D.; Glass, C.K.; et al. Nuclear Receptor-Induced Chromosomal Proximity and DNA Breaks Underlie Specific Translocations in Cancer. Cell 2009, 139, 1069–1083. [Google Scholar] [CrossRef]
- Jeffs, A.R.; Benjes, S.M.; Smith, T.L.; Sowerby, S.J.; Morris, C.M. The BCR gene recombines preferentially with Alu elements in complex BCR- ABL translocations of chronic myeloid leukaemia. Hum. Mol. Genet. 1998, 7, 767–776. [Google Scholar] [CrossRef]
- Lozynskyy, R.; Lozynska, M. The study of the mechanisms of the different phenotypical manifestations in patients with reciprocal translocations. Photonics Eur. 2006, 6191, 61911. [Google Scholar] [CrossRef]
- Song, J.; Li, X.; Sun, L.; Xu, S.; Liu, N.; Yao, Y.; Liu, Z.; Wang, W.; Rong, H.; Wang, B. A family with Robertsonian translocation: A potential mechanism of speciation in humans. Mol. Cytogenet. 2016, 9, 48. [Google Scholar] [CrossRef]
- García-Nieto, P.E.; Morrison, A.J.; Fraser, H.B. The somatic mutation landscape of the human body. Genome Biol. 2019, 20, 298. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. The significance of responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Romanish, M.T.; Mager, D.L. Distributions of Transposable Elements Reveal Hazardous Zones in Mammalian Introns. PLoS Comput. Biol. 2011, 7, e1002046. [Google Scholar] [CrossRef] [PubMed]
- Hesselberth, J.R. Lives that introns lead after splicing. Wiley Interdiscip. Rev. RNA 2013, 4, 677–691. [Google Scholar] [CrossRef]
- Talhouarne, G.J.S.; Gall, J.G. Lariat intronic RNAs in the cytoplasm of vertebrate cells. Proc. Natl. Acad. Sci. USA 2018, 115, E7970–E7977. [Google Scholar] [CrossRef]
- Laurent, G.S.; Shtokalo, D.N.; Tackett, M.R.; Yang, Z.; Eremina, T.; Wahlestedt, C.; Urcuqui-Inchima, S.; Seilheimer, B.; McCaffrey, T.; Kapranov, P. Intronic RNAs constitute the major fraction of the non-coding RNA in mammalian cells. BMC Genom. 2012, 13, 504. [Google Scholar] [CrossRef]
- Karst, S.M.; Rütz, M.-L.; Menees, T.M. The Yeast Retrotransposons Ty1 and Ty3 Require the RNA Lariat Debranching Enzyme, Dbr1p, for Efficient Accumulation of Reverse Transcripts. Biochem. Biophys. Res. Commun. 2000, 268, 112–117. [Google Scholar] [CrossRef]
- Cheng, Z.; Menees, T.M. RNA Branching and Debranching in the Yeast Retrovirus-like Element Ty. Science 2004, 303, 240–243. [Google Scholar] [CrossRef]
- Bianchi, M.; Crinelli, R.; Giacomini, E.; Carloni, E.; Radici, L.; Scarpa, E.; Tasini, F.; Magnani, M. A negative feedback mechanism links UBC gene expression to ubiquitin levels by affecting RNA splicing rather than transcription. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Perry, G.H.; Dominy, N.J.; Claw, K.G.; Lee, A.S.; Fiegler, H.; Redon, R.; Werner, J.; Villanea, F.A.; Mountain, J.L.; Misra, R.; et al. Diet and the evolution of human amylase gene copy number variation. Nat. Genet. 2007, 39, 1256–1260. [Google Scholar] [CrossRef]
- Gottlieb, B.; Beitel, L.K.; Alvarado, C.; Trifiro, M.A. Selection and mutation in the “new” genetics: An emerging hypothesis. Qual. Life Res. 2010, 127, 491–501. [Google Scholar] [CrossRef]
- Abyzov, A.; Mariani, J.; Palejev, D.; Zhang, Y.; Haney, M.S.; Tomasini, L.; Ferrandino, A.F.; Belmaker, L.A.R.; Szekely, A.; Wilson, M.; et al. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nat. Cell Biol. 2012, 492, 438–442. [Google Scholar] [CrossRef] [PubMed]
- O’Huallachain, M.; Karczewski, K.J.; Weissman, S.M.; Urban, A.E.; Snyder, M.P. Extensive genetic variation in somatic human tissues. Proc. Natl. Acad. Sci. USA 2012, 109, 18018–18023. [Google Scholar] [CrossRef]
- Gabrielli, S.; Ortolani, C.; Del Zotto, G.; Luchetti, F.; Canonico, B.; Buccella, F.; Artico, M.; Papa, S.; Zamai, L. The Memories of NK Cells: Innate-Adaptive Immune Intrinsic Crosstalk. J. Immunol. Res. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Zheng, S.; Vuong, B.Q.; Vaidyanathan, B.; Lin, J.-Y.; Huang, F.-T.; Chaudhuri, J. Non-coding RNA Generated following Lariat Debranching Mediates Targeting of AID to DNA. Cell 2015, 161, 762–773. [Google Scholar] [CrossRef]
- Steele, E.J.; Franklin, A.; Blanden, R.V. Genesis of the strand-biased signature in somatic hypermutation of rearranged immunoglobulin variable genes. Immunol. Cell Biol. 2004, 82, 209–218. [Google Scholar] [CrossRef]
- Zheng, Y.; Lorenzo, C.; Beal, P.A. DNA editing in DNA/RNA hybrids by adenosine deaminases that act on RNA. Nucleic Acids Res. 2017, 45, 3369–3377. [Google Scholar] [CrossRef] [PubMed]
- Enard, D.; Cai, L.; Gwennap, C.; Petrov, D.A. Viruses are a dominant driver of protein adaptation in mammals. eLife 2016, 5, e12469. [Google Scholar] [CrossRef] [PubMed]
- Demogines, A.; Abraham, J.; Choe, H.; Farzan, M.; Sawyer, S.L. Dual Host-Virus Arms Races Shape an Essential Housekeeping Protein. PLoS Biol. 2013, 11, e1001571. [Google Scholar] [CrossRef]
- Bombelli, P.; Howe, C.J.; Bertocchini, F. Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Curr. Biol. 2017, 27, R292–R293. [Google Scholar] [CrossRef] [PubMed]
- Ingram, W.R. Studies on Amphibian Neoteny. I. The Metamorphosis of the Colorado Axolotl by Injection of Inorganic Iodine. Physiol. Zoöl. 1929, 2, 149–156. [Google Scholar] [CrossRef]
- Ho, K.Y.; Veldhuis, J.D.; Johnson, M.L.; Furlanetto, R.; Evans, W.S.; Alberti, K.G.; Thorner, M.O. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J. Clin. Investig. 1988, 81, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hoo, R.L.-C.; Konishi, M.; Itoh, N.; Lee, P.-C.; Ye, H.-Y.; Lam, K.S.; Xu, A. Growth Hormone Induces Hepatic Production of Fibroblast Growth Factor 21 through a Mechanism Dependent on Lipolysis in Adipocytes. J. Biol. Chem. 2011, 286, 34559–34566. [Google Scholar] [CrossRef] [PubMed]
- Agaba, M.; Ishengoma, E.; Miller, W.C.; McGrath, B.C.; Hudson, C.N.; Reina, O.C.B.; Ratan, A.; Burhans, R.; Chikhi, R.; Medvedev, P.; et al. Giraffe genome sequence reveals clues to its unique morphology and physiology. Nat. Commun. 2016, 7, 11519. [Google Scholar] [CrossRef]
- Palmisano, I.; Della Chiara, G.; D’Ambrosio, R.L.; Huichalaf, C.; Brambilla, P.; Corbetta, S.; Riba, M.; Piccirillo, R.; Valente, S.; Casari, G.; et al. Amino acid starvation induces reactivation of silenced transgenes and latent HIV-1 provirus via down-regulation of histone deacetylase 4 (HDAC4). Proc. Natl. Acad. Sci. USA 2012, 109, E2284–E2293. [Google Scholar] [CrossRef]
- De Vito, A.; Lazzaro, M.; Palmisano, I.; Cittaro, D.; Riba, M.; Lazarevic, D.; Bannai, M.; Gabellini, D.; Schiaffino, M.V. Amino acid deprivation triggers a novel GCN2-independent response leading to the transcriptional reactivation of non-native DNA sequences. PLoS ONE 2018, 13, e0200783. [Google Scholar] [CrossRef]
- Foster, K.R.; Parkinson, K.; Thompson, C.R.L. What can microbial genetics teach sociobiology? Trends Genet. 2007, 23, 74–80. [Google Scholar] [CrossRef]
- Herb, B.R.; Wolschin, F.; Hansen, K.D.; Aryee, M.J.; Langmead, B.; Irizarry, R.A.; Amdam, G.V.; Feinberg, A.P. Reversible switching between epigenetic states in honeybee behavioral subcastes. Nat. Neurosci. 2012, 15, 1371–1373. [Google Scholar] [CrossRef]
- D’Urso, A.; Brickner, J.H. Mechanisms of epigenetic memory. Trends Genet. 2014, 30, 230–236. [Google Scholar] [CrossRef]
- Power, M.C.; Adar, S.D.; Yanosky, J.D.; Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. NeuroToxicology 2016, 56, 235–253. [Google Scholar] [CrossRef]
- Underwood, E. The polluted brain. Science 2017, 355, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Siddoway, B.; Kaeser, G.E.; Segota, I.; Rivera, R.; Romanow, W.J.; Liu, C.S.; Park, C.; Kennedy, G.; Long, T.; et al. Somatic APP gene recombination in Alzheimer’s disease and normal neurons. Nat. Cell Biol. 2018, 563, 639–645. [Google Scholar] [CrossRef]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Vitale, M. NK cells and cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar] [CrossRef]
- Rajagopal, C.; Harikumar, K.B. The Origin and Functions of Exosomes in Cancer. Front. Oncol. 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.J.; Abi-Rached, L.; Gendzekhadze, K.; Korbel, D.; Gleimer, M.; Rowley, D.; Bruno, D.; Carrington, C.V.F.; Chandanayingyong, D.; Chang, Y.H.; et al. Unusual selection on the KIR3DL1/S1 natural killer cell receptor in Africans. Nat. Genet. 2007, 39, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Navin, N.; Kendall, J.; Troge, J.; Andrews, P.; Rodgers, L.; McIndoo, J.; Cook, K.; Stepansky, A.; Levy, D.; Esposito, D.; et al. Tumor Evolution Inferred by Single Cell Sequencing. Nature 2011, 472, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Tsou, S.-H.; Chen, T.-M.; Hsiao, H.-T.; Chen, Y.-H. A Critical Dose of Doxorubicin Is Required to Alter the Gene Expression Profiles in MCF-7 Cells Acquiring Multidrug Resistance. PLoS ONE 2015, 10, e0116747. [Google Scholar] [CrossRef]
- Balcer-Kubiczek, E.K.; Yin, J.; Lin, K.; Harrison, G.H.; Abraham, J.M.; Meltzer, S.J.; Meltzer, J.M.A.J. p53 Mutational Status and Survival of Human Breast Cancer MCF-7 Cell Variants after Exposure to X Rays or Fission Neutrons. Radiat. Res. 1995, 142, 256. [Google Scholar] [CrossRef]
- Chan, K.; Roberts, S.A.; Klimczak, L.J.; Sterling, J.F.; Saini, N.; Malc, E.P.; Kim, J.; Kwiatkowski, D.J.; Fargo, D.C.; Mieczkowski, P.A.; et al. An APOBEC3A hypermutation signature is distinguishable from the signature of background mutagenesis by APOBEC3B in human cancers. Nat. Genet. 2015, 47, 1067–1072. [Google Scholar] [CrossRef]
- Kanu, N.; Cerone, M.A.; Goh, G.; Zalmas, L.-P.; Bartkova, J.; Dietzen, M.; McGranahan, N.; Rogers, R.; Law, E.K.; Gromova, I.; et al. DNA replication stress mediates APOBEC3 family mutagenesis in breast cancer. Genome Biol. 2016, 17, 1–15. [Google Scholar] [CrossRef]
- Skinner, M.K.; Guerrero-Bosagna, C.; Haque, M.M. Environmentally induced epigenetic transgenerational inheritance of sperm epimutations promote genetic mutations. Epigenetics 2015, 10, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. A proposed selective mechanism based on metal chelation in industrial melanic moths. Biol. J. Linn. Soc. 2013, 109, 298–301. [Google Scholar] [CrossRef]
- Cook, L.M. The Rise and Fall of theCarbonariaForm of the Peppered Moth. Q. Rev. Biol. 2003, 78, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Fu, Y.; Kao, S.-C.A.; Yang, H.; Chen, X.S. Family-Wide Comparative Analysis of Cytidine and Methylcytidine Deamination by Eleven Human APOBEC Proteins. J. Mol. Biol. 2017, 429, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Abraham, B.J.; Berezovskaya, A.; Farah, N.; Liu, Y.; Leon, T.; Fielding, A.; Tan, S.H.; Sanda, T.; Weintraub, A.S.; et al. APOBEC signature mutation generates an oncogenic enhancer that drives LMO1 expression in T-ALL. Leukemia 2017, 31, 2057–2064. [Google Scholar] [CrossRef]
- Periyasamy, M.; Patel, H.; Lai, C.-F.; Nguyen, V.T.; Nevedomskaya, E.; Harrod, A.; Russell, R.; Reményi, J.; Ochocka, A.M.; Thomas, R.S.; et al. APOBEC3B-Mediated Cytidine Deamination Is Required for Estrogen Receptor Action in Breast Cancer. Cell Rep. 2015, 13, 108–121. [Google Scholar] [CrossRef]
- Caval, V.; Suspène, R.; Shapira, M.; Vartanian, J.-P.; Wain-Hobson, S. A prevalent cancer susceptibility APOBEC3A hybrid allele bearing APOBEC3B 3′UTR enhances chromosomal DNA damage. Nat. Commun. 2014, 5, 5129. [Google Scholar] [CrossRef]
- Li, Q.; Wrange, Ö.; Eriksson, P. The role of chromatin in transcriptional regulation. Int. J. Biochem. Cell Biol. 1997, 29, 731–742. [Google Scholar] [CrossRef]
- Roukos, V.; Voss, T.C.; Schmidt, C.K.; Lee, S.; Wangsa, D.; Misteli, T. Spatial Dynamics of Chromosome Translocations in Living Cells. Science 2013, 341, 660–664. [Google Scholar] [CrossRef]
- Babich, V.; Aksenov, N.; Alexeenko, V.; Oei, S.; Buchlow, G.; Tomilin, N. Association of some potential hormone response elements in human genes with the Alu family repeats. Gene 1999, 239, 341–349. [Google Scholar] [CrossRef]
- Tsantoulis, P.K.; Kotsinas, A.; Sfikakis, P.P.; Evangelou, K.; Sideridou, M.; Levy, B.; Mo, L.; Kittas, C.; Wu, X.-R.; Papavassiliou, A.G.; et al. Oncogene-induced replication stress preferentially targets common fragile sites in preneoplastic lesions. A genome-wide study. Oncogene 2007, 27, 3256–3264. [Google Scholar] [CrossRef] [PubMed]
- Fungtammasan, A.; Walsh, E.; Chiaromonte, F.; Eckert, K.A.; Makova, K.D. A genome-wide analysis of common fragile sites: What features determine chromosomal instability in the human genome? Genome Res. 2012, 22, 993–1005. [Google Scholar] [CrossRef]
- Russell, L.B. X-ray-induced developmental abnormalities in the mouse and their use in the analysis of embryological patterns. II. Abnormalities of the vertebral column and thorax. J. Exp. Zoöl. 1956, 131, 329–395. [Google Scholar] [CrossRef]
- Olson, M.E.; Harris, R.S.; Harki, D.A. APOBEC Enzymes as Targets for Virus and Cancer Therapy. Cell Chem. Biol. 2018, 25, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Mercer, D.; Hu, X.; Liu, H.; Li, M.M. Common Leukemia- and Lymphoma-Associated Genetic Aberrations in Healthy Individuals. J. Mol. Diagn. 2011, 13, 213–219. [Google Scholar] [CrossRef]
- Barber, J.C.K. Directly transmitted unbalanced chromosome abnormalities and euchromatic variants. J. Med. Genet. 2005, 42, 609–629. [Google Scholar] [CrossRef][Green Version]
- Spadafora, C. Sperm-Mediated Transgenerational Inheritance. Front. Microbiol. 2017, 8, 2401. [Google Scholar] [CrossRef]
- Spadafora, C. The “evolutionary field” hypothesis. Non-Mendelian transgenerational inheritance mediates diversification and evolution. Prog. Biophys. Mol. Biol. 2018, 134, 27–37. [Google Scholar] [CrossRef]
- Evans, E.; Hogarth, C.; Mitchell, D.; Griswold, M.D. Riding the spermatogenic wave: Profiling gene expression within neonatal germ and sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol. Reprod. 2014, 90, 108. [Google Scholar] [CrossRef]
- Chan, J.C.; Morgan, C.P.; Leu, N.A.; Shetty, A.; Cisse, Y.M.; Nugent, B.M.; Morrison, K.E.; Jašarević, E.; Huang, W.; Kanyuch, N.; et al. Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q. 150 years of Darwin’s theory of intercellular flow of hereditary information. Nat. Rev. Mol. Cell Biol. 2018, 19, 749–750. [Google Scholar] [CrossRef]
- Vecoli, C.; Montano, L.; Andreassi, M.G. Environmental pollutants: Genetic damage and epigenetic changes in male germ cells. Environ. Sci. Pollut. Res. 2016, 23, 23339–23348. [Google Scholar] [CrossRef]
- Martin, M.P.; Gao, X.; Lee, J.-H.; Nelson, G.W.; Detels, R.; Goedert, J.J.; Buchbinder, S.; Hoots, K.; Vlahov, D.; Trowsdale, J.; et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 2002, 31, 429–434. [Google Scholar] [CrossRef]
- Martin, M.P.; Qi, Y.; Gao, X.; Yamada, E.; Martin, J.N.; Pereyra, F.; Colombo, S.; Brown, E.E.; Shupert, W.L.; Phair, J.; et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 2007, 39, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, B.L.; Ferrand, R.; Munyati, S.; Folkard, S.; Boyd, K.; Bandason, T.; Jallow, S.; Rowland-Jones, S.L.; Yindom, L.-M. HLA Correlates of Long-Term Survival in Vertically Infected HIV-1-Positive Adolescents in Harare, Zimbabwe. Aids Res. Hum. Retrovir. 2015, 31, 504–507. [Google Scholar] [CrossRef]
- Secco, V.D.; Riccioli, A.; Padula, F.; Ziparo, E.; Filippini, A. Mouse Sertoli Cells Display Phenotypical and Functional Traits of Antigen-Presenting Cells in Response to Interferon Gamma. Biol. Reprod. 2008, 78, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Seisenberger, S.; Peat, J.R.; Hore, T.A.; Santos, F.; Dean, W.; Reik, W. Reprogramming DNA methylation in the mammalian life cycle: Building and breaking epigenetic barriers. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110330. [Google Scholar] [CrossRef]
- O’Farrell, P.H.; Stumpff, J.; Su, T.T. Embryonic Cleavage Cycles: How Is a Mouse Like a Fly? Curr. Biol. 2004, 14, R35–R45. [Google Scholar] [CrossRef]
- Heyer, B.S.; Macauley, A.; Behrendtsen, O.; Werb, Z. Hypersensitivity to DNA damage leads to increased apoptosis during early mouse development. Genome Res. 2000, 14, 2072–2084. [Google Scholar] [PubMed]
- Jacobson, M.D.; Weil, M.; Raff, M.C. Programmed Cell Death in Animal Development. Cell 1997, 88, 347–354. [Google Scholar] [CrossRef]
- Chan, W.F.N.; Gurnot, C.; Montine, T.J.; Sonnen, J.A.; Guthrie, K.A.; Nelson, J.L. Male Microchimerism in the Human Female Brain. PLoS ONE 2012, 7, e45592. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.M.; Fairhurst, R.M. Malaria parasites and red cell variants: When a house is not a home. Curr. Opin. Hematol. 2014, 21, 193–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonçalves, B.P.; Sagara, I.; Coulibaly, M.; Wu, Y.; Assadou, M.H.; Guindo, A.; Ellis, R.D.; Diakite, M.; Gabriel, E.E.; Prevots, D.R.; et al. Hemoglobin variants shape the distribution of malaria parasites in human populations and their transmission potential. Sci. Rep. 2017, 7, 14267. [Google Scholar] [CrossRef]
- Palis, J. Primitive and definitive erythropoiesis in mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Joice, R.; Nilsson, S.K.; Montgomery, J.; Dankwa, S.; Egan, E.; Morahan, B.; Seydel, K.B.; Bertuccini, L.; Alano, P.; Williamson, K.C.; et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014, 6, 244re5. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, G.; Philip, N.; Reardon, J.; Lacsina, J.R.; Majoros, W.; Chapman, L.; Thornburg, C.D.; Telen, M.J.; Ohler, U.; Nicchitta, C.V.; et al. Translocation of Sickle Cell Erythrocyte MicroRNAs into Plasmodium falciparum Inhibits Parasite Translation and Contributes to Malaria Resistance. Cell Host Microbe 2012, 12, 187–199. [Google Scholar] [CrossRef]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; Navarin, N.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nat. Cell Biol. 2020, 584, 425–429. [Google Scholar] [CrossRef]
- Zamai, L. The Yin and Yang of ACE/ACE2 Pathways: The Rationale for the Use of Renin-Angiotensin System Inhibitors in COVID-19 Patients. Cells 2020, 9, 1704. [Google Scholar] [CrossRef]
- Patel, S.K.; Juno, J.A.; Lee, W.S.; Wragg, K.M.; Hogarth, P.M.; Kent, S.J.; Burrell, M. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: Implications for COVID-19 pathogenesis and consequences. medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.10.06.20207514v2 (accessed on 12 October 2020). [CrossRef]
- Wilson, D.S.; Wilson, E.O. Evolution “for the good of the group”. Am. Sci. 2008, 96, 380–389. [Google Scholar] [CrossRef]
- Losa, G.A. The fractal geometry of life. Riv. Biol. 2009, 102, 29–60. [Google Scholar] [PubMed]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.P.; Joyce, G.F. The Origins of the RNA World. Cold Spring Harb. Perspect. Biol. 2010, 4, a003608. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Shekhawat, G.; Purohit, S. Analysis of similarities between Viroid, Prokaryote and Eukaryote genomes to revisit theories of origin of Viroids. J. Cell Mol. Biol. 2007, 6, 9–18. [Google Scholar]
- Dreher, T.W. Role of tRNA-like structures in controlling plant virus replication. Virus Res. 2009, 139, 217–229. [Google Scholar] [CrossRef]
- Dreher, T.W. Viral tRNAs and tRNA-like structures. Wiley Interdiscip. Rev. RNA 2010, 1, 402–414. [Google Scholar] [CrossRef]
- Krupovic, M.; Koonin, E.V. Multiple origins of viral capsid proteins from cellular ancestors. Proc. Natl. Acad. Sci. USA 2017, 114, E2401–E2410. [Google Scholar] [CrossRef]
- Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origin of viruses: Primordial replicators recruiting capsids from hosts. Nat. Rev. Genet. 2019, 17, 449–458. [Google Scholar] [CrossRef]
- Kazlauskas, D.; Varsani, A.; Koonin, E.V.; Krupovic, M. Multiple origins of prokaryotic and eukaryotic single-stranded DNA viruses from bacterial and archaeal plasmids. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Knisbacher, B.A.; Levanon, E.Y. DNA Editing of LTR Retrotransposons Reveals the Impact of APOBECs on Vertebrate Genomes. Mol. Biol. Evol. 2015, 33, 554–567. [Google Scholar] [CrossRef]
- Levanon, K.; Eisenberg, E.; Rechavi, G.; Levanon, E.Y.; Rose, S.; Flipse, S.M.; Van Der Sanden, M.C.A.; Radstake, M.; De Winde, J.H.; Osseweijer, P.; et al. Letter from the editor: Adenosine-to-inosine RNA editing in Alu repeats in the human genome. EMBO Rep. 2005, 6, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Deininger, P.L. Alu elements: Know the SINEs. Genome Biol. 2011, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; McConnell, M.J.; Marchetto, M.C.; Coufal, N.G.; Gage, F.H. LINE-1 retrotransposons: Mediators of somatic variation in neuronal genomes? Trends Neurosci. 2010, 33, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Bodea, G.O.; McKelvey, E.G.Z.; Faulkner, G.J. Retrotransposon-induced mosaicism in the neural genome. Open Biol. 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Pastuzyn, E.D.; Day, C.E.; Kearns, R.B.; Kyrke-Smith, M.; Taibi, A.V.; McCormick, J.; Yoder, N.; Belnap, D.M.; Erlendsson, S.; Morado, D.R.; et al. The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell 2018, 172, 275–288.e18. [Google Scholar] [CrossRef]
- Nadassy, K. Standard atomic volumes in double-stranded DNA and packing in protein-DNA interfaces. Nucleic Acids Res. 2001, 29, 3362–3376. [Google Scholar] [CrossRef]
- Liang, J.; Dill, K.A. Are proteins well-packed? Biophys. J. 2001, 81, 751–766. [Google Scholar] [CrossRef]
 : transcription factor;
: transcription factor;  : repressor;
: repressor;  : increase;
: increase;  : decrease.
: decrease.
 : transcription factor;
: transcription factor;  : repressor;
: repressor;  : increase;
: increase;  : decrease.
: decrease.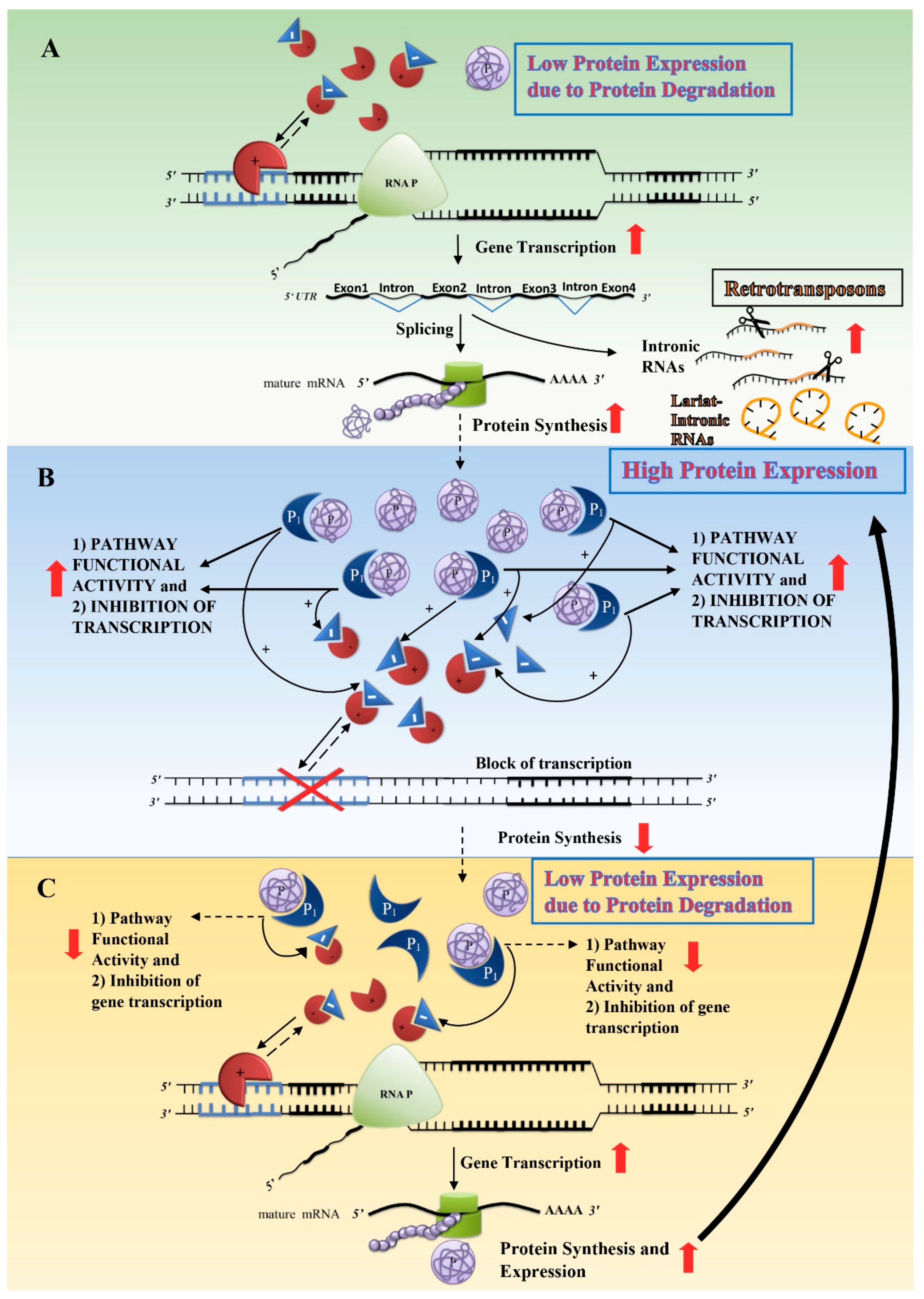
 : transcription factor;
: transcription factor;  : repressor;
: repressor;  : increase;
: increase;  : decrease; DBR1: lariat debranching enzyme.
: decrease; DBR1: lariat debranching enzyme.
 : transcription factor;
: transcription factor;  : repressor;
: repressor;  : increase;
: increase;  : decrease; DBR1: lariat debranching enzyme.
: decrease; DBR1: lariat debranching enzyme.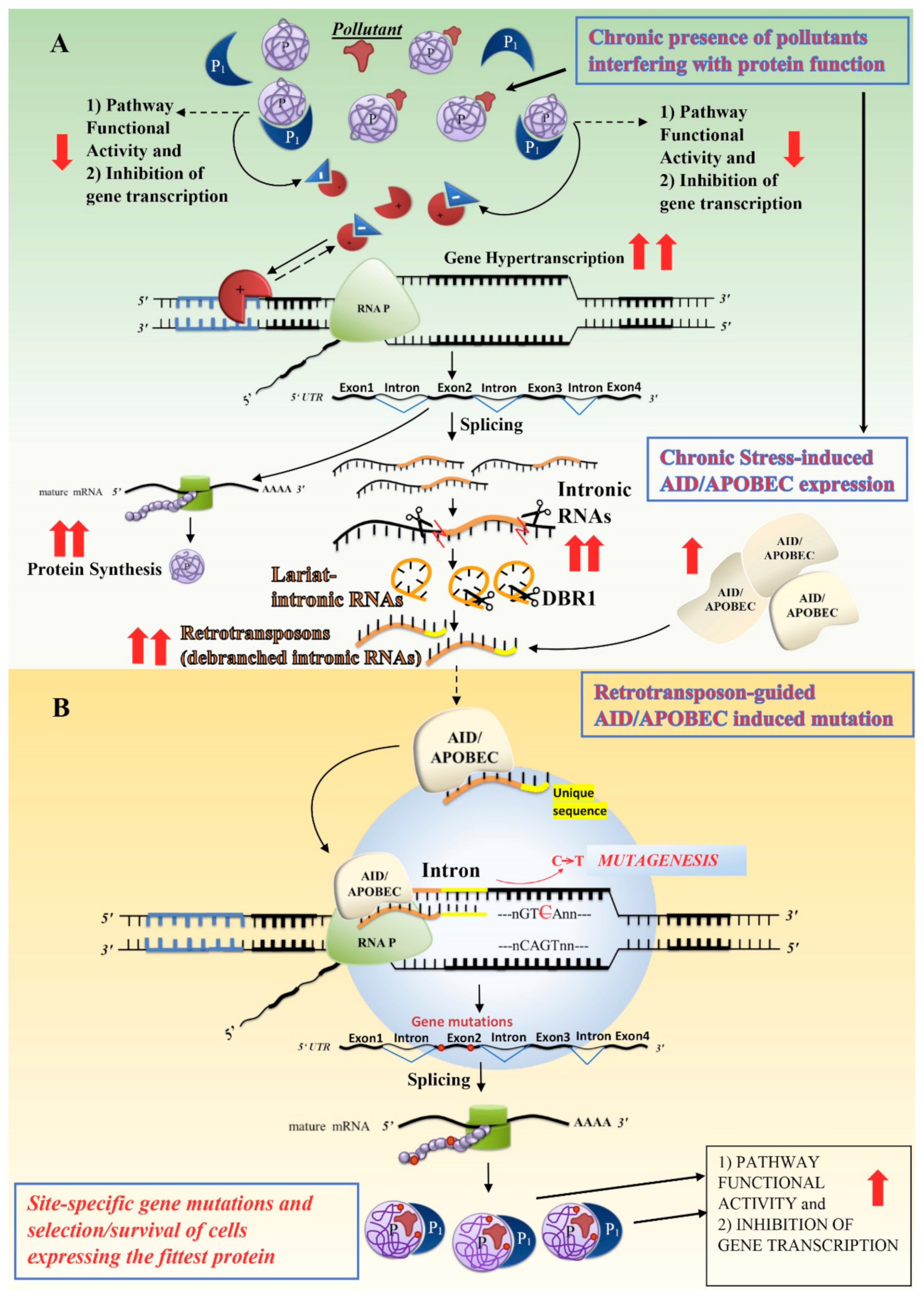
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamai, L. Unveiling Human Non-Random Genome Editing Mechanisms Activated in Response to Chronic Environmental Changes: I. Where Might These Mechanisms Come from and What Might They Have Led To? Cells 2020, 9, 2362. https://doi.org/10.3390/cells9112362
Zamai L. Unveiling Human Non-Random Genome Editing Mechanisms Activated in Response to Chronic Environmental Changes: I. Where Might These Mechanisms Come from and What Might They Have Led To? Cells. 2020; 9(11):2362. https://doi.org/10.3390/cells9112362
Chicago/Turabian StyleZamai, Loris. 2020. "Unveiling Human Non-Random Genome Editing Mechanisms Activated in Response to Chronic Environmental Changes: I. Where Might These Mechanisms Come from and What Might They Have Led To?" Cells 9, no. 11: 2362. https://doi.org/10.3390/cells9112362
APA StyleZamai, L. (2020). Unveiling Human Non-Random Genome Editing Mechanisms Activated in Response to Chronic Environmental Changes: I. Where Might These Mechanisms Come from and What Might They Have Led To? Cells, 9(11), 2362. https://doi.org/10.3390/cells9112362




