Potential Drug Candidates to Treat TRPC6 Channel Deficiencies in the Pathophysiology of Alzheimer’s Disease and Brain Ischemia
Abstract
:1. Introduction
2. TRPC Channels and Their Regulation in Cells
3. Role of TRPC6 in the Formation of Excitatory Synapses
4. Hypo- and Hyperactivation of TRPC6 Channels in Different Pathogenetic Forms of AD
5. Cerebral Ischemia as a Risk Factor for AD Development
6. Role of TRPC6 in the Development of Ischemia
7. Available Drug Candidates to Modulate TRPC6 Activity
7.1. TRPC6 Activators
7.1.1. Endogenous Ligands and Analogs
DAG
Lysophosphatidylcholine
20-Hydroxy-5Z,8Z,11Z,14Z-Eicosatetraenoic Acid
10R,17R-Dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-Hexaenoic Acid
7.1.2. Hyperforin and Other Phytochemicals
Hyperforin
Resveratrol
7.1.3. Synthetic Compounds
Flufenamic Acid
Piperazines
Bis-{2-[(2E)-4-Hydroxy-4-Oxobut-2-Enoyloxy]-N,N-Diethylethanaminium} Butandioate
N-[4-[2-[(6-Aminoquinazolin-4-yl)Amino]ethyl]phenyl]acetamide
7.1.4. Other TRPC6 Agonists
Pyrazolopyrimidines
GSK1702934A and OptoBI-1
3-(6,7-Dimethoxy-3,3-Dimethyl-3,4-Dihydroisoquinolin-1-yl)-2H-Chromen-2-One
7.2. TRPC6 Inhibitors
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forabosco, P.; Ramasamy, A.; Trabzuni, D.; Walker, R.; Smith, C.; Bras, J.; Levine, A.P.; Hardy, J.; Pocock, J.M.; Guerreiro, R.; et al. Insights into TREM2 biology by network analysis of human brain gene expression data. Neurobiol. Aging 2013, 34, 2699–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, J.; Wang, H.; Meng, X.; Tan, C.; Wang, J.; Wang, C.; Tan, L. Association between Stroke and Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Chi, N.-F.; Chien, L.-N.; Ku, H.-L.; Hu, C.-J.; Chiou, H.-Y. Alzheimer disease and risk of stroke: A population-based cohort study. Neurology 2013, 80, 705–711. [Google Scholar] [CrossRef]
- Tolppanen, A.; Lavikainen, P.; Solomon, A.; Kivipelto, M.; Esoininen, H.; Hartikainen, S. Incidence of stroke in people with Alzheimer disease: A national register-based approach. Neurology 2013, 80, 353–358. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Lu, T.-J.; Chen, X.-J.; Zhou, Y.; Chen, Q.; Feng, X.-Y.; Xu, L.; Duan, W.-H.; Xiong, Z.-Q. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 2008, 39, 3042–3048. [Google Scholar] [CrossRef]
- Grossberg, G.T.; Thomas, S.J. Memantine: A review of studies into its safety and efficacy in treating Alzheimer’s disease and other dementias. Clin. Interv. Aging 2009, 4, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.S.; Dagerman, K.S.; Higgins, J.P.; McShane, R. Lack of Evidence for the Efficacy of Memantine in Mild Alzheimer Disease. Arch. Neurol. 2011, 68, 991. [Google Scholar] [CrossRef]
- Hoyte, L.; Barber, P.A.; Buchan, A.M.; Hill, M.D. The rise and fall of NMDA antagonists for ischemic stroke. Curr. Mol. Med. 2004, 4, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.D. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology 2008, 55, 363–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Du, W.; Zhou, K.; Tai, Y.; Yao, H.; Jia, Y.; Ding, Y.; Wang, Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat. Neurosci. 2008, 11, 741–743. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Wu, L.; Pchitskaya, E.; Zakharova, O.; Tacer, K.F.; Bezprozvanny, I. Store-Operated Calcium Channel Complex in Postsynaptic Spines: A New Therapeutic Target for Alzheimer’s Disease Treatment. J. Neurosci. 2016, 36, 11837–11850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Pan, J.; Pan, L.; Zhang, N. TRPC6 inhibited NMDA current in cultured hippocampal neurons. Neuromol. Med. 2013, 15, 389–395. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Du, W.; Jia, C.; Yao, H.; Wang, Y. TRPC6 inhibited NMDA receptor activities and protected neurons from ischemic excitotoxicity. J. Neurochem. 2012, 123, 1010–1018. [Google Scholar] [CrossRef]
- Tai, Y.; Feng, S.; Ge, R.; Du, W.; Zhang, X.; He, Z.; Wang, Y. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J. Cell Sci. 2008, 121, 2301–2307. [Google Scholar] [CrossRef] [Green Version]
- Guilbert, A.; Dhennin-Duthille, I.; El Hiani, Y.; Haren, N.; Khorsi, H.; Sevestre, H.; Ahidouch, A.; Ouadid-Ahidouch, H. Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer 2008, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, G.A.; Coletto, L.A.; Sciorati, C.; Bozzolo, E.P.; Manunta, P.; Rovere-Querini, P.; Manfredi, A.A. Ion Channels and Transporters in Inflammation: Special Focus on TRP Channels and TRPC6. Cells 2018, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Santín, S.; Ars, E.; Rossetti, S.; Salido, E.; Silva, I.; García-Maset, R.; Giménez, I.; Ruíz, P.; Mendizábal, S.; Nieto, J.L.; et al. TRPC6 mutational analysis in a large cohort of patients with focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2009, 24, 3089–3096. [Google Scholar] [CrossRef] [Green Version]
- Popugaeva, E.; Chernyuk, D.; Zhang, H.; Postnikova, T.Y.; Pats, K.; Fedorova, E.; Poroikov, V.; Zaitsev, A.V.; Bezprozvanny, I.; Cherniuk, D.; et al. Derivatives of Piperazines as Potential Therapeutic Agents for Alzheimer’s Disease. Mol. Pharmacol. 2019, 95, 337–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lessard, C.B.; Lussier, M.P.; Cayouette, S.; Bourque, G.; Boulay, G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cell. Signal. 2005, 17, 437–445. [Google Scholar] [CrossRef]
- Liu, L.; Gu, L.; Chen, M.; Zheng, Y.; Xiong, X.; Zhu, S. Novel Targets for Stroke Therapy: Special Focus on TRPC Channels and TRPC6. Front. Aging Neurosci. 2020, 12, 70. [Google Scholar] [CrossRef] [Green Version]
- Chernyuk, D.; Zernov, N.; Kabirova, M.; Bezprozvanny, I.; Popugaeva, E. Antagonist of neuronal store-operated calcium entry exerts beneficial effects in neurons expressing PSEN1ΔE9 mutant linked to familial Alzheimer disease. Neuroscience 2019, 410, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Z.; Hatcher, J.T.; Chen, Q.-H.; Chen, L.; Wurster, R.D.; Chan, S.L.; Cheng, Z. Deletion of TRPC6 Attenuates NMDA Receptor-Mediated Ca(2+) Entry and Ca(2+)-Induced Neurotoxicity Following Cerebral Ischemia and Oxygen-Glucose Deprivation. Front. Neurosci. 2017, 11, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Lu, M.; He, X.; Ma, L.; Birnbaumer, L.; Liao, Y. TRPC3/6/7 Knockdown Protects the Brain from Cerebral Ischemia Injury via Astrocyte Apoptosis Inhibition and Effects on NF-small ka, CyrillicB Translocation. Mol. Neurobiol. 2017, 54, 7555–7566. [Google Scholar] [CrossRef]
- Wang, J.; Lu, R.; Yang, J.; Li, H.; He, Z.; Jing, N.; Wang, X.; Wang, Y. TRPC6 specifically interacts with APP to inhibit its cleavage by gamma-secretase and reduce Abeta production. Nat. Commun. 2015, 6, 8876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Sukumaran, P.; Bandyopadhyay, B.C.; Singh, B.B. Physiological Function and Characterization of TRPCs in Neurons. Cells 2014, 3, 455–475. [Google Scholar] [CrossRef] [Green Version]
- Montell, C. The TRP superfamily of cation channels. Sci. Signal. 2005, 2005, re3. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, A.; Gudermann, T. TRPC6: Physiological function and pathophysiological relevance. Handb. Exp. Pharmacol. 2014, 222, 157–188. [Google Scholar] [PubMed]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [Green Version]
- Roos, J.; Digregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.-H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.J.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Liu, J.; Popugaeva, E.; Xu, N.-J.; Feske, S.; White, C.L.; Bezprozvanny, I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 2014, 82, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Popugaeva, E.; Pchitskaya, E.; Speshilova, A.B.; Alexandrov, S.; Zhang, H.; Vlasova, O.L.; Bezprozvanny, I. STIM2 protects hippocampal mushroom spines from amyloid synaptotoxicity. Mol. Neurodegener. 2015, 10, 37. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, Y.; Li, X.; Wu, L.; Wang, Y. TRPC6 expression in neurons is differentially regulated by NR2A- and NR2B-containing NMDA receptors. J. Neurochem. 2017, 143, 282–293. [Google Scholar] [CrossRef]
- Tu, H.; Nelson, O.; Bezprozvanny, A.; Wang, Z.; Lee, S.; Hao, Y.; Serneels, L.; de Strooper, B.; Yu, G.; Bezprozvanny, I. Presenilins form ER calcium leak channels, a function disrupted by mutations linked to familial Alzheimer’s disease. Cell 2006, 126, 981–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zatti, G.; Burgo, A.; Giacomello, M.; Barbiero, L.; Ghidoni, R.; Sinigaglia, G.; Florean, C.; Bagnoli, S.; Binetti, G.; Sorbi, S.; et al. Presenilin mutations linked to familial Alzheimer’s disease reduce endoplasmic reticulum and Golgi apparatus calcium levels. Cell Calcium 2006, 39, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, S.; Herreman, A.; De Strooper, B.; Bezprozvanny, I. Role of presenilins in neuronal calcium homeostasis. J. Neurosci. 2010, 30, 8566–8580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, J.; Sun, S.; Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling, excitability, and synaptic plasticity defects in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 561–580. [Google Scholar] [CrossRef] [Green Version]
- Tong, B.C.; Lee, C.S.; Cheng, W.; Lai, K.; Foskett, J.K.; Cheung, K. Familial Alzheimer’s disease-associated presenilin 1 mutants promote gamma-secretase cleavage of STIM1 to impair store-operated Ca2+ entry. Sci. Signal. 2016, 9, ra89. [Google Scholar] [CrossRef] [Green Version]
- Bojarski, L.; Pomorski, P.; Szybinska, A.; Drab, M.; Skibinska-Kijek, A.; Gruszczynska-Biegala, J.; Kuznicki, J. Presenilin-dependent expression of STIM proteins and dysregulation of capacitative Ca2+ entry in familial Alzheimer’s disease. Biochim. Biophys. Acta 2009, 1793, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Popugaeva, E.; Pchitskaya, E.; Bezprozvanny, I. Dysregulation of neuronal calcium homeostasis in Alzheimer’s disease—A therapeutic opportunity? Biochem. Biophys. Res. Commun. 2017, 483, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef]
- Popugaeva, E.; Chernyuk, D.; Bezprozvanny, I. Correction of calcium dysregulation as potential approach for treating Alzheimer’s disease. Curr. Alzheimer Res. 2019, in press. [Google Scholar]
- Ryazantseva, M.A.; Goncharova, A.; Skobeleva, K.; Erokhin, M.; Methner, A.; Georgiev, P.; Kaznacheyeva, E.V. Presenilin-1 Delta E9 Mutant Induces STIM1-Driven Store-Operated Calcium Channel Hyperactivation in Hippocampal Neurons. Mol. Neurobiol. 2017, 55, 4667–4680. [Google Scholar] [CrossRef]
- Yoo, A.S.; Cheng, I.; Chung, S.; Grenfell, T.Z.; Lee, H.; Pack-Chung, E.; Handler, M.; Shen, J.; Xia, W.; Tesco, G.; et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron 2000, 27, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Le, W. Pathological role of hypoxia in Alzheimer’s disease. Exp. Neurol. 2010, 223, 299–303. [Google Scholar] [CrossRef]
- Vijayan, M.; Reddy, P.H. Stroke, Vascular Dementia, and Alzheimer’s Disease: Molecular Links. J. Alzheimers Dis. 2016, 54, 427–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olichney, J.M.; Hansen, L.A.; Galasko, D.; Saitoh, T.; Hofstetter, C.R.; Katzman, R.; Thal, L.J. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology 1996, 47, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, D.R.; Cohen, D.L.; Hedera, P.; Friedland, R.P.; Kalaria, R.N. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am. J. Pathol. 1996, 148, 2083–2095. [Google Scholar]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska, K.; Tymianski, M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef]
- Wang, X.; Teng, L.; Li, A.; Ge, J.; Laties, A.M.; Zhang, X. TRPC6 channel protects retinal ganglion cells in a rat model of retinal ischemia/reperfusion-induced cell death. Investig. Opthalmol. Vis. Sci. 2010, 51, 5751–5758. [Google Scholar] [CrossRef] [Green Version]
- Nakuluri, K.; Nishad, R.; Mukhi, D.; Kumar, S.; Nakka, V.P.; Kolligundla, L.P.; Narne, P.; Natuva, S.S.K.; Phanithi, P.B.; Pasupulati, A.K. Cerebral ischemia induces TRPC6 via HIF1alpha/ZEB2 axis in the glomerular podocytes and contributes to proteinuria. Sci. Rep. 2019, 9, 17897. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, J.; Tai, Y.; Wang, Y. TRPC channels promote cerebellar granule neuron survival. Nat. Neurosci. 2007, 10, 559–567. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Cui, K.; Li, N.; Zheng, Z.-Y.; Wang, Y.; Yuan, X.-B. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 2005, 434, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, L.; Marosi, M.; Mazzitelli, J.; Latifi, S.; Sano, Y.; Galvan, L.; Kawaguchi, R.; Holley, S.; Levine, M.S.; Coppola, G.; et al. CREB controls cortical circuit plasticity and functional recovery after stroke. Nat. Commun. 2018, 9, 2250. [Google Scholar] [CrossRef] [PubMed]
- McCullough, L.D.; Tarabishy, S.; Liu, L.; Benashski, S.; Xu, Y.; Ribar, T.; Means, A.; Li, J. Inhibition of calcium/calmodulin-dependent protein kinase kinase beta and calcium/calmodulin-dependent protein kinase IV is detrimental in cerebral ischemia. Stroke 2013, 44, 2559–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; Huang, J.; Yao, H.; Zhou, K.; Duan, B.; Wang, Y. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J. Clin. Investig. 2010, 120, 3480–3492. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Xue, R.; Gu, J.; Xiao, Y.; Zhong, H.; Pan, X.; Ran, R. Neuroprotective effect of calcitriol on ischemic/reperfusion injury through the NR3A/CREB pathways in the rat hippocampus. Mol. Med. Rep. 2013, 8, 1708–1714. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Chen, F.; Zhang, J.; Wang, T.; Wei, X.; Wu, J.; Feng, Y.; Dai, Z.; Wu, Q. Neuroprotective effect of resveratrol on ischemia/reperfusion injury in rats through TRPC6/CREB pathways. J. Mol. Neurosci. 2013, 50, 504–513. [Google Scholar] [CrossRef]
- Omura, T.; Tanaka, Y.; Miyata, N.; Koizumi, C.; Sakurai, T.; Fukasawa, M.; Hachiuma, K.; Minagawa, T.; Susumu, T.; Yoshida, S.; et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke 2006, 37, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Renic, M.; Klaus, J.A.; Omura, T.; Kawashima, N.; Onishi, M.; Miyata, N.; Koehler, R.C.; Harder, D.R.; Roman, R.J. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. Br. J. Pharmacol. 2008, 29, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Shaik, J.S.B.; Poloyac, S.M.; Kochanek, P.M.; Alexander, H.; Tudorascu, D.L.; Clark, R.S.; Manole, M.D. 20-Hydroxyeicosatetraenoic Acid Inhibition by HET0016 Offers Neuroprotection, Decreases Edema, and Increases Cortical Cerebral Blood Flow in a Pediatric Asphyxial Cardiac Arrest Model in Rats. Br. J. Pharmacol. 2015, 35, 1757–1763. [Google Scholar] [CrossRef] [Green Version]
- Urban, N.; Hill, K.; Wang, L.; Kuebler, W.M.; Schaefer, M. Novel pharmacological TRPC inhibitors block hypoxia-induced vasoconstriction. Cell Calcium 2012, 51, 194–206. [Google Scholar] [CrossRef]
- Maier, T.; Follmann, M.; Hessler, G.; Kleemann, H.-W.; Hachtel, S.; Fuchs, B.; Weissmann, N.; Linz, W.; Schmidt, T.; Löhn, M.; et al. Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br. J. Pharmacol. 2015, 172, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.; Wang, L.; Kwiek, S.; Rademann, J.; Kuebler, W.M.; Schaefer, M. Identification and Validation of Larixyl Acetate as a Potent TRPC6 Inhibitor. Mol. Pharmacol. 2015, 89, 197–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Xiao, H.; Zhang, Y.; Zeng, X.; Huang, M.; Chen, X.; Birnbaumer, L.; Liao, Y. Transient receptor potential channel 6 knockdown prevents apoptosis of renal tubular epithelial cells upon oxidative stress via autophagy activation. Cell Death Dis. 2018, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Taylor-Nguyen, N.N.; Riley, A.M.; Herring, B.P.; White, F.A.; Obukhov, A.G. The TRPC6 inhibitor, larixyl acetate, is effective in protecting against traumatic brain injury-induced systemic endothelial dysfunction. J. Neuroinflamm. 2019, 16, 21. [Google Scholar] [CrossRef] [Green Version]
- Cross, J.L.; Meloni, B.P.; Bakker, A.J.; Lee, S.; Knuckey, N.W. Modes of Neuronal Calcium Entry and Homeostasis following Cerebral Ischemia. Stroke Res. Treat. 2010, 2010, 316862. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.J.; Tymianski, M. Targeting NMDA receptors in stroke: New hope in neuroprotection. Mol. Brain 2018, 11, 15. [Google Scholar] [CrossRef]
- Basora, N.; Boulay, G.; Bilodeau, L.; Payet, M.D.; Rousseau, E. 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J. Biol. Chem. 2003, 278, 31709–31716. [Google Scholar] [CrossRef] [Green Version]
- Aires, V.; Hichami, A.; Boulay, G.; Khan, N.A. Activation of TRPC6 calcium channels by diacylglycerol (DAG)-containing arachidonic acid: A comparative study with DAG-containing docosahexaenoic acid. Biochim. 2007, 89, 926–937. [Google Scholar] [CrossRef]
- Belayev, L.; Khoutorova, L.; Atkins, K.D.; Eady, T.N.; Hong, S.; Lu, Y.; Obenaus, A.; Bazan, N.G. Docosahexaenoic Acid therapy of experimental ischemic stroke. Transl. Stroke Res. 2010, 2, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Zhang, J.; Chen, F.; Lin, Y. Neuroprotectin D1 attenuates brain damage induced by transient middle cerebral artery occlusion in rats through TRPC6/CREB pathways. Mol. Med. Rep. 2013, 8, 543–550. [Google Scholar] [CrossRef]
- Guinamard, R.; Simard, C.; Del Negro, C. Flufenamic acid as an ion channel modulator. Pharmacol. Ther. 2013, 138, 272–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, C.; Ding, M.; Zhu, Y.; Lu, Y.; Du, J.; Miller, M.; Tian, J.; Zhu, J.; Xu, J.; Wen, M.; et al. Pyrazolopyrimidines as Potent Stimulators for Transient Receptor Potential Canonical 3/6/7 Channels. J. Med. Chem. 2017, 60, 4680–4692. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, S.; Hatano, M.; Takada, Y.; Hino, K.; Kawamura, T.; Tanikawa, J.; Nakagawa, H.; Hase, H.; Nakao, A.; Hirano, M.; et al. Screening of Transient Receptor Potential Canonical Channel Activators Identifies Novel Neurotrophic Piperazine Compounds. Mol. Pharmacol. 2016, 89, 348–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiapko, O.; Shrestha, N.; Lindinger, S.; De La Cruz, G.G.; Graziani, A.; Klec, C.; Butorac, C.; Graier, W.F.; Kubista, H.; Freichel, M.; et al. Lipid-independent control of endothelial and neuronal TRPC3 channels by light. Chem. Sci. 2019, 10, 2837–2842. [Google Scholar] [CrossRef] [Green Version]
- Häfner, S.; Urban, N.; Schaefer, M. Discovery and characterization of a positive allosteric modulator of transient receptor potential canonical 6 (TRPC6) channels. Cell Calcium 2019, 78, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Tong, L.; Xi, M.; Yang, H.; Dong, H.; Wen, A. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J. Ethnopharmacol. 2012, 144, 768–774. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, J.; Liu, G.; Chen, F.; Lin, Y. Neuroprotection by (−)-epigallocatechin-3-gallate in a rat model of stroke is mediated through inhibition of endoplasmic reticulum stress. Mol. Med. Rep. 2014, 9, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Sysoev, Y.I.; Uzuegbunam, B.C.; Okovityi, S.V. Attenuation of neurological deficit by a novel ethanolamine derivative in rats after brain trauma. J. Exp. Pharmacol. 2019, 11, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Sysoev, Y.I.; Popugaeva, E.A.; Chernyuk, D.P.; Titovich, I.A.; Zagladkina, E.V.; Bolotova, V.C.; Bezprozvanny, I.; Okovityi, S.V. Mechanism of action of the new ethanolamine derivative bis{2-[(2E)-4-hydroxy-4-oxobut-2-enoyloxy]-N,N-diethylethanaminium}butanedioate. Eksperimental’naya Klin. Farmakol. 2019, 82, 3–10. [Google Scholar]
- Harteneck, C.; Klose, C.; Krautwurst, D. Synthetic modulators of TRP channel activity. Adv. Exp. Med. Biol. 2011, 704, 87–106. [Google Scholar]
- Tu, P.; Kunert-Keil, C.; Lucke, S.; Brinkmeier, H.; Bouron, A. Diacylglycerol analogues activate second messenger-operated calcium channels exhibiting TRPC-like properties in cortical neurons. J. Neurochem. 2009, 108, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Montecinos-Oliva, C.; Schuller, A.; Parodi, J.; Melo, F.; Inestrosa, N.C. Effects of tetrahydrohyperforin in mouse hippocampal slices: Neuroprotection, long-term potentiation and TRPC channels. Curr. Med. Chem. 2014, 21, 3494–3506. [Google Scholar] [CrossRef] [PubMed]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, S.; Yamamoto, S.; Hayasaka, T.; Konishi, Y.; Yamaguchi-Okada, M.; Goto-Inoue, N.; Sugiura, Y.; Setou, M.; Namba, H. Imaging mass spectrometry revealed the production of lyso-phosphatidylcholine in the injured ischemic rat brain. Neuroscience 2010, 168, 219–225. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Posada-Duque, R.; Cortes, N.C.; Arias-Londoño, J.D.; Cardona-Gómez, G.P. Changes in the hippocampal and peripheral phospholipid profiles are associated with neurodegeneration hallmarks in a long-term global cerebral ischemia model: Attenuation by Linalool. Neuropharmacology 2018, 135, 555–571. [Google Scholar] [CrossRef]
- Jickling, G.C.; Montaner, J. Lysophosphatidylcholine to stratify risk of ischemic stroke in TIA. Neurology 2014, 84, 17–18. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, P.; Colles, S.M.; Damron, D.S.; Graham, L.M. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arter. Thromb. Vasc. Biol. 2003, 23, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, P.; Rosenbaum, M.A.; Birnbaumer, L.; Graham, L.M. Integration of TRPC6 and NADPH oxidase activation in lysophosphatidylcholine-induced TRPC5 externalization. Am. J. Physiol. Physiol. 2017, 313, C541–C555. [Google Scholar] [CrossRef]
- Cloutier, M.; Campbell, S.; Basora, N.; Proteau, S.; Payet, M.D.; Rousseau, E. 20-HETE inotropic effects involve the activation of a nonselective cationic current in airway smooth muscle. Am. J. Physiol. Cell. Mol. Physiol. 2003, 285, L560–L568. [Google Scholar] [CrossRef]
- Lu, L.; Wang, M.; Wei, X.; Li, W. Corrigendum: 20-HETE Inhibition by HET0016 Decreases the Blood-Brain Barrier Permeability and Brain Edema After Traumatic Brain Injury. Front. Aging Neurosci. 2018, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Östman, J.; Bubb, K.J.; Panayiotou, C.; Priestley, J.V.; Baker, M.D.; Ahluwalia, A. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. J. Biol. Chem. 2012, 287, 13868–13876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asatryan, A.; Bazan, N.G. Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. J. Biol. Chem. 2017, 292, 12390–12397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Zhang, Y.; Shu, H.; Xie, B.; Tao, Y.; Yuan, Y.; Shang, Y.; Yuan, S.; Zhang, J. Hyperforin Promotes Post-stroke Neuroangiogenesis via Astrocytic IL-6-Mediated Negative Immune Regulation in the Ischemic Brain. Front. Cell. Neurosci. 2019, 13, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Mdzinarishvili, A.; Kiewert, C.; Abbruscato, T.; Bickel, U.; Van Der Schyf, C.J.; Klein, J. NMDA receptor-antagonistic properties of hyperforin, a constituent of St. John’s Wort. J. Pharmacol. Sci. 2006, 102, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Dinamarca, M.C.; Cerpa, W.; Garrido, J.; Hancke, J.L.; Inestrosa, N.C. Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer’s amyloid-beta-deposits. Mol. Psychiatry 2006, 11, 1032–1048. [Google Scholar] [CrossRef]
- Leuner, K.; Li, W.; Amaral, M.D.; Rudolph, S.; Calfa, G.; Schuwald, A.M.; Harteneck, C.; Inoue, T.; Pozzo-Miller, L. Hyperforin modulates dendritic spine morphology in hippocampal pyramidal neurons by activating Ca(2+) -permeable TRPC6 channels. Hippocampus 2012, 23, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, P.; Liu, H.; Yao, H.; Yao, S.; Yuan, S.; Zhang, J.-C. Hyperforin improves post-stroke social isolation-induced exaggeration of PSD and PSA via TGF-β. Int. J. Mol. Med. 2018, 43, 413–425. [Google Scholar] [CrossRef]
- Ma, L.; Pan, X.; Zhou, F.; Liu, K.; Wang, L. Hyperforin protects against acute cerebral ischemic injury through inhibition of interleukin-17A-mediated microglial activation. Brain Res. 2018, 1678, 254–261. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, C.; Chen, J.; Zhang, Y.; Yuan, S.; Lin, Y. Hyperforin promotes post-stroke functional recovery through interleukin (IL)−17A-mediated angiogenesis. Brain Res. 2016, 1646, 504–513. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, J.-C.; Fu, J.; Chen, F.; Wang, J.; Wu, Z.-L.; Yuan, S.-Y. Hyperforin attenuates brain damage induced by transient middle cerebral artery occlusion (mcao) in rats via inhibition of TRPC6 channels degradation. Br. J. Pharmacol. 2012, 33, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Cerpa, W.; Hancke, J.L.; Morazzoni, P.; Bombardelli, E.; Riva, A.; Marin, P.P.; Inestrosa, N.C. The hyperforin derivative IDN5706 occludes spatial memory impairments and neuropathological changes in a double transgenic Alzheimer’s mouse model. Curr. Alzheimer Res. 2010, 7, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Tuor, U.I.; Thompson, R.; Institoris, A.; Kulynych, A.; Zhang, X.; Kinniburgh, D.W.; Bari, F.; Busija, D.W.; Barber, P.A. Protection against recurrent stroke with resveratrol: Endothelial protection. PLoS ONE 2012, 7, e47792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, S.V.; Dave, K.R.; Saul, I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2-Related Factor 2. Stroke 2015, 46, 1626–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Li, N.; Gao, D.; Zhen, H.; Zhang, X.; Li, F. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J. Vasc. Surg. 2008, 48, 709–714. [Google Scholar] [CrossRef] [Green Version]
- Della-Morte, D.; Dave, K.R.; DeFazio, R.A.; Bao, Y.C.; Raval, A.P.; Perez-Pinzon, M.A. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 2009, 159, 993–1002. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Xu, J.; Rottinghaus, G.E.; Simonyi, A.; Lubahn, D.; Sun, G.Y.; Sun, A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002, 958, 439–447. [Google Scholar] [CrossRef]
- Drygalski, K.; Fereniec, E.; Koryciński, K.; Chomentowski, A.; Kiełczewska, A.; Odrzygóźdź, C.; Modzelewska, B. Resveratrol and Alzheimer’s disease. From molecular pathophysiology to clinical trials. Exp. Gerontol. 2018, 113, 36–47. [Google Scholar] [CrossRef]
- Jia, J.; Verma, S.; Nakayama, S.; Quillinan, N.; Grafe, M.R.; Hurn, P.D.; Herson, P.S. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J. Cereb. Blood Flow Metab. 2011, 31, 2160–2168. [Google Scholar] [CrossRef] [Green Version]
- Khansari, P.S.; Halliwell, R.F. Mechanisms Underlying Neuroprotection by the NSAID Mefenamic Acid in an Experimental Model of Stroke. Front. Neurosci. 2019, 13, 64. [Google Scholar] [CrossRef] [Green Version]
- Titovich, I.A.; Sysoev, Y.I.; Bolotova, V.C.; Okovityi, S.V. Neurotropic activity of a new aminoethanol derivative under conditions of experimental brain ischemia. Exp. Clin. Pharmacol. (Rus) 2017, 80, 3–6. [Google Scholar]
- Poloyac, S.M.; Zhang, Y.; Bies, R.R.; Kochanek, P.M.; Graham, S.H. Protective effect of the 20-HETE inhibitor het0016 on brain damage after temporary focal ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 1551–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Wang, B.; Lee, J.-H.; Armstrong, J.S.; Kulikowicz, E.; Bhalala, U.S.; Martin, L.J.; Koehler, R.C.; Yang, Z.-J. Additive Neuroprotection of a 20-HETE Inhibitor with Delayed Therapeutic Hypothermia after Hypoxia-Ischemia in Neonatal Piglets. Dev. Neurosci. 2015, 37, 376–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.-J.; Carter, E.L.; Kibler, K.K.; Kwansa, H.; Crafa, D.; Martin, L.J.; Roman, R.J.; Harder, D.R.; Koehler, R.C. Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor. J. Neurochem. 2012, 121, 168–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khansari, P.S.; Halliwell, R.F. Evidence for neuroprotection by the fenamate NSAID, mefenamic acid. Neurochem. Int. 2009, 55, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.J.D.; Rivers-Auty, J.; Schilling, T.; Spencer, N.G.; Watremez, W.; Fasolino, V.; Booth, S.J.; White, C.S.; Baldwin, A.G.; Freeman, S.; et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 2016, 7, 12504. [Google Scholar] [CrossRef] [Green Version]
- Chauvet, S.; Jarvis, L.; Chevallet, M.; Shrestha, N.; Groschner, K.; Bouron, A. Pharmacological Characterization of the Native Store-Operated Calcium Channels of Cortical Neurons from Embryonic Mouse Brain. Front. Pharmacol. 2016, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Leuner, K.; Kazanski, V.; Muller, M.; Essin, K.; Henke, B.; Gollasch, M.; Harteneck, C.; Müller, W.E. Hyperforin—A key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J. 2007, 21, 4101–4111. [Google Scholar] [CrossRef] [Green Version]
- Leuner, K.; Heiser, J.H.; Derksen, S.; Mladenov, M.I.; Fehske, C.J.; Schubert, R.; Gollasch, M.; Schneider, G.; Harteneck, C.; Chatterjee, S.S.; et al. Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators—Identification of a novel pharmacophore. Mol. Pharmacol. 2009, 77, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.; Wonnemann, M.; Müller, W.E. Hyperforin, a major antidepressant constituent of St. John’s Wort, inhibits serotonin uptake by elevating free intracellular Na+1. J. Pharmacol. Exp. Ther. 1999, 290, 1363–1368. [Google Scholar]
- Pochwat, B.; Szewczyk, B.; Kotarska, K.; Rafało-Ulińska, A.; Siwiec, M.; Sowa, J.E.; Tokarski, K.; Siwek, A.; Bouron, A.; Friedland, K.; et al. Hyperforin Potentiates Antidepressant-Like Activity of Lanicemine in Mice. Front. Mol. Neurosci. 2018, 11, 456. [Google Scholar] [CrossRef]
- Gibon, J.; Deloulme, J.C.; Chevallier, T.; Ladevèze, E.; Abrous, D.N.; Bouron, A. The antidepressant hyperforin increases the phosphorylation of CREB and the expression of TrkB in a tissue-specific manner. Int. J. Neuropsychopharmacol. 2013, 16, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Inestrosa, N.C.; Tapia-Rojas, C.; Griffith, T.N.; Carvajal, F.J.; Benito, M.J.; Rivera-Dictter, A.; Alvarez, A.R.; Serrano, F.G.; Hancke, J.L.; Burgos, P.V.; et al. Tetrahydrohyperforin prevents cognitive deficit, Abeta deposition, tau phosphorylation and synaptotoxicity in the APPswe/PSEN1DeltaE9 model of Alzheimer’s disease: A possible effect on APP processing. Transl. Psychiatry 2011, 1, e20. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Essin, K.; Hill, K.; Beschmann, H.; Rubant, S.; Schempp, C.M.; Gollasch, M.; Boehncke, W.H.; Harteneck, C.; Müller, W.E.; et al. Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation. J. Biol. Chem. 2008, 283, 33942–33954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, L.B.; Goodwin, B.; Jones, S.A.; Wisely, G.B.; Serabjit-Singh, C.J.; Willson, T.M.; Collins, J.L.; Kliewer, S.A. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 7500–7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, C.P.; Maimone, T.J. Total Synthesis of Hyperforin. J. Am. Chem. Soc. 2015, 137, 10516–10519. [Google Scholar] [CrossRef] [PubMed]
- Woelk, H.; Burkard, G.; Grünwald, J. Benefits and risks of the hypericum extract LI 160: Drug monitoring study with 3250 patients. J. Geriatr. Psychiatry Neurol. 1994, 7, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, D.D.; Arbo, M.D.; Valente, M.J.; Bastos, M.D.L.; Carmo, H. Hepatotoxicity of piperazine designer drugs: Comparison of different in vitro models. Toxicol. Vitr. 2015, 29, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Agrawal, M.; Doré, S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem. Neurosci. 2013, 4, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Albani, D.; Polito, L.; Forloni, G. Sirtuins as novel targets for Alzheimer’s disease and other neurodegenerative disorders: Experimental and genetic evidence. J. Alzheimers Dis. 2010, 19, 11–26. [Google Scholar] [CrossRef]
- Anekonda, T.S. Resveratrol—A boon for treating Alzheimer’s disease? Brain Res. Rev. 2006, 52, 316–326. [Google Scholar] [CrossRef]
- Rasouri, S.; Lagouge, M.; Auwerx, J. SIRT1/PGC-1: A neuroprotective axis? Med. Sci (Paris) 2007, 23, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Piver, B.; Berthou, F.; Dreano, Y.; Lucas, D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol. Lett. 2001, 125, 83–91. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Garland, L.L.; Hsu, C.-H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, R.; Okada, T.; Onoue, H.; Hara, Y.; Shimizu, S.; Naitoh, S.; Ito, Y.; Mori, Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α 1-adrenoceptor-activated ca 2+-permeable cation channel. Circ. Res. 2001, 88, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Foster, R.R.; Zadeh, M.A.; Welsh, G.I.; Satchell, S.C.; Ye, Y.; Mathieson, P.W.; Bates, D.O.; Saleem, M.A. Flufenamic acid is a tool for investigating TRPC6-mediated calcium signalling in human conditionally immortalised podocytes and HEK293 cells. Cell Calcium 2009, 45, 384–390. [Google Scholar] [CrossRef]
- Macianskiene, R.; Gwanyanya, A.; Sipido, K.; Vereecke, J.; Mubagwa, D.K. Induction of a novel cation current in cardiac ventricular myocytes by flufenamic acid and related drugs. Br. J. Pharmacol. 2010, 161, 416–429. [Google Scholar] [CrossRef] [Green Version]
- Klose, C.; Straub, I.; Riehle, M.; Ranta, F.; Krautwurst, D.; Ullrich, S.; Meyerhof, W.; Harteneck, C. Fenamates as TRP channel blockers: Mefenamic acid selectively blocks TRPM3. Br. J. Pharmacol. 2011, 162, 1757–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Tian, J.; Zhu, Y.; Wang, C.; Xiao, R.; Herz, J.M.; Wood, J.D.; Zhu, M.X. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflug. Arch. 2009, 459, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Kochetkov, K.V.; Kazachenko, V.N.; Marinov, B.S. Dose-dependent potentiation and inhibition of single Ca2+-activated K+ channels by flufenamic acid. Membr. cell Boil. 2000, 14, 285–298. [Google Scholar]
- Barrier, M.; Burlet, S.; Estrella, C.; Melnyk, P.; Sergeant, N.; Buee, L.; Verwaerde, P. Sulfate Salts of N-(3-(4-(3-(diisobutylamino)propyl)piperazin-1-yl)propyl)-1H-benzo [d]imidazol-2 Amine, Preparation Thereof and Use of the Same. U.S. Patent 9562018B2, 7 February 2017. [Google Scholar]
- U.S. National Library of Medicine. A Study to Assess Tolerability, Safety, Pharmacokinetics and Effect of AZP2006 in Patients with PS. 2019. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04008355?term=azp2006&draw=2&rank=1 (accessed on 1 August 2020).
- Titovich, I.A.; Radko, S.V.; Lisitsky, D.S.; Okovity, S.V.; Bolotov, V.T.; Belsky, A.V.; Mikhailova, M.V.; Sysoev, I.Y. The study of a novel diethylaminoethanol derivative cognitive function in laboratory animals. J. Biomed (Ru) 2017, 3, 102–110. [Google Scholar]
- Xu, X.; Lozinskaya, I.; Costell, M.; Lin, Z.; Ball, J.A.; Bernard, R.; Behm, D.J.; Marino, J.P.; Schnackenberg, C.G. Characterization of small molecule TRPC3 and TRPC6 agonist and antagonists. Biophys. J. 2013, 104, 454a. [Google Scholar] [CrossRef] [Green Version]
- Inoue, R.; Jensen, L.J.; Jian, Z.; Shi, J.; Hai, L.; Lurie, A.I.; Henriksen, F.H.; Salomonsson, M.; Morita, H.; Kawarabayashi, Y.; et al. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ. Res. 2009, 104, 1399–1409. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Reed, T.; Sultana, R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic Res. 2011, 45, 59–72. [Google Scholar] [CrossRef]
- Jin, Z.; Berthiaume, J.M.; Li, Q.; Henry, F.; Huang, Z.; Sadhukhan, S.; Gao, P.; Tochtrop, G.P.; Puchowicz, M.A.; Zhang, G. Catabolism of (2E)-4-hydroxy-2-nonenal via omega- and omega-1-oxidation stimulated by ketogenic diet. J. Biol. Chem. 2014, 289, 32327–32338. [Google Scholar] [CrossRef] [Green Version]
- van Ierssel, S.H.; Conraads, V.M.; van Craenenbroeck, E.M.; Liu, Y.; Maas, A.I.; Parizel, P.M.; Hoymans, V.Y.; Vrints, C.J.; Jorens, P.G. Endothelial dysfunction in acute brain injury and the development of cerebral ischemia. J. Neurosci. Res. 2015, 93, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Clementi, E.; Meldolesi, J. Pharmacological and functional properties of voltagemi independent Ca2+ channels. Cell Calcium 1996, 19, 269–279. [Google Scholar] [CrossRef]
- Tesfai, Y.; Brereton, H.M.; Barritt, G.J. A diacylglycerol-activated Ca2+ channel in PC12 cells (an adrenal chromaffin cell line) correlates with expression of the TRP-6 (transient receptor potential) protein. Biochem. J. 2001, 358, 717. [Google Scholar] [CrossRef]
- Zhang, S.L.; Kozak, J.A.; Jiang, W.; Yeromin, A.V.; Chen, J.; Yu, Y.; Penna, A.; Shen, W.; Chi, V.; Cahalan, M.D. Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3. J. Biol. Chem. 2008, 283, 17662–17671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilius, B.; Flockerzi, V. Mammalian transient receptor potential (TRP) cation channels. In Handbook of Experimental Pharmacology; Springer: Heidelberg, Germany, 2014; Volume 223. [Google Scholar]
- Wu, J.; Ryskamp, D.A.; Liang, X.; Egorova, P.; Zakharova, O.; Hung, G.; Bezprozvanny, I. Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington’s Disease Mouse Model. J. Neurosci. 2016, 36, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Tobe, M.; Yoshiaki Isobe, H.T.; Nagasaki, T.; Takahashi, H.; Fukazawa, T.; Hayashi, H. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation. Bioorg. Med. Chem. 2003, 11, 383–391. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.X.; Ding, M. Nuclear transcription factor kappa B (NF-kB) mediates ROS and PKC-induced decrease in TRPC6 protein expression in human glomerular mesangial cells (HMCs). Faseb. J. 2012, 26, 687. [Google Scholar]
- Tang, Q.; Guo, W.; Zheng, L.; Wu, J.-X.; Liu, M.; Zhou, X.; Zhang, X.; Chen, L. Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res. 2018, 28, 746–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| No. | Compound Name and Structural Formula | Experimental Model | Mode of Administration | Effect(s) | Reference(s) |
|---|---|---|---|---|---|
| TRPC6 Activators | |||||
| Endogenous ligands | |||||
| 1 | Neuroprotectin D1 10R,17R-Dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid 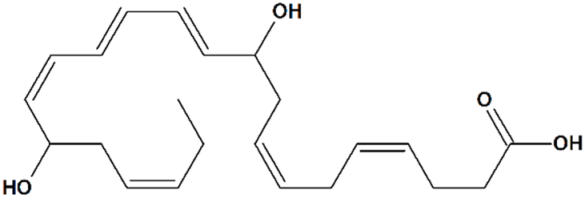 | Rat transient MCAO | Icv injection at 2 h after reperfusion | reduced infarct volume reduced sensory and motor deficits | [80] |
| Phytochemicals | |||||
| 2 | Hyperforin (1R,5S,6R,7S)-4-Hydroxy-6-methyl-1,3,7-tris(3-methylbut-2-en-1-yl)-6-(4-methylpent-3-en-1-yl)-5-(2-methylpropanoyl) bicyclo[3.3.1]non-3-ene-2,9-dione 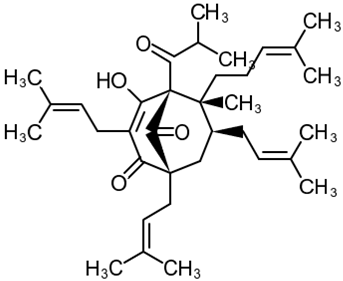 | Lipopolysaccharide stimulation in 28 d post-MCAO isolated mouse astrocytes | Co-incubation for 16 h | increased viability | [103] |
| Lipopolysaccharide stimulation in 28 d post-MCAO isolated mouse cortical neurons | increased viability | [103] | |||
| NMDA toxicity in rat hippocampal slices | Co-incubation for 30 min | reduced edema | [104] | ||
| Male Sprague-Dawley rats injected with fibrillary Aβ | Intrahippocampal co-injection of Aβ with the drug for 14 days | amyloid deposits disaggregation reduced spatial memory deficit | [105] | ||
| Rat hippocampal slice cultures | Co-incubation for 24 h | increased proportion of mature stubby spines | [106] | ||
| Hippocampal cultures from PS1-M146VKI and APPKI transgenic mice | Incubation for 16 h | increased percentage of mushroom spines in TRPC6-dependent manner increased neuronal SOCE in postsynaptic spines | [14] | ||
| Hippocampal cultures treated with synthetic Aβ42 peptides | Co-incubation for 16 h | increased percentage of mushroom spines increased neuronal SOCE in postsynaptic spines | [21] | ||
| Mouse transient MCAO | Intranasal administration q.d. for 7 d starting at day 7 post-MCAO | increased hippocampal neurogenesis improved post-stroke depression and anxiety reduced memory deficit | [107] | ||
| Icv injections at 1, 24, and 48 h after MCAO | reduced microglial activation reduced infarct volume reduced neurological deficit | [108] | |||
| Icv injections q.d. for 14 d starting at day 14 post-MCAO | increased angiogenesis reduced motor deficit | [109] | |||
| increased angiogenesis increased subventricular neurogenesis reduced motor deficit | [103] | ||||
| Mouse permanent MCAO | Ip injection before ischemia onset | no effect on infarct volume or brain edema | [104] | ||
| Mouse water intoxication | |||||
| Rat transient MCAO | Icv injection at 6, 12, or 24 h after reperfusion | prevented neuronal apoptosis reduced infarct volume reduced neurological deficit | [110] | ||
| 3 | Tetrahydrohyperforin (1S,5S,7S,8R)-4,9-dihydroxy-1-(1-hydroxy-2-methylpropyl)-8-methyl-3,5,7-tris(3-methylbut-2-enyl)-8-(4-methylpent-3-enyl)bicyclo[3.3.1]non-3-en-2-one 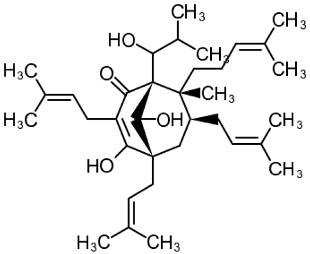 | APPSEN1ΔE9 mice | Ip injections for 4 weeks | reduced memory deficit reduced amyloid deposition attenuated neuroinflammation and oxidative stress | [111] |
| 4 | Resveratrol 5-[(E)-2-(4-Hydroxyphenyl)ethenyl] benzene-1,3-diol 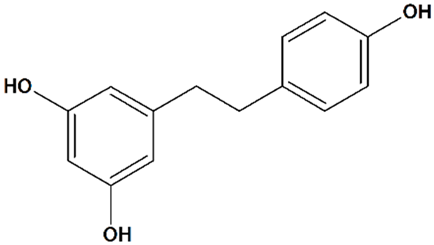 | Oxygen/glucose deprivation in isolated rat brain endothelial cells | Preincubation for 3 d | increased viability | [112] |
| Mouse transient MCAO | Ip injection at 48 h before MCAO | reduced infarct volume no effect on cerebral blood flow | [113] | ||
| Oral gavage q.d. for 7 d starting from 24 h after MCAO, or for 5 d starting from 72 h after MCAO | increased vascular density in the basal ganglia region and cortex reduced infarct volume reduced neurological deficit | [114] | |||
| Rat transient MCAO | Ip injections q.d. for 7 d before MCAO | reduced infarct volume reduced neurological deficit | [66] | ||
| Rat recurrent transient MCAO | Oral gavage q.d. for 3 d between strokes | reduced infarct volume following an initial and recurrent stroke reduced blood–brain barrier disruption following a recurrent stroke reduced brain edema following a recurrent stroke reduced astrogliosis following a recurrent stroke no effect on cerebral blood flow during or after recurrent stroke | [112] | ||
| Rat asphyxial cardiac arrest | Ip injection at 48 h before cardiac arrest | enhanced ATP synthesis efficiency in hippocampal mitochondria prevented hippocampal neuronal apoptosis | [115] | ||
| Gerbil transient bilateral common carotid artery occlusion | Ip injections during occlusion or at reperfusion + at 24 h after reperfusion | reduced hippocampal microglial activation prevented hippocampal-delayed neuronal death | [116] | ||
| Clinical trials in patients | diverse | affected neuroinflammation, Aβ deposition, and adaptive immunity in patients with mild to moderate Alzheimer’s disease | for a review, see [117] | ||
| Synthetic compounds | |||||
| 5 | Flufenamic acid 2-[[3-(Trifluoromethyl)phenyl]amino]benzoic acid 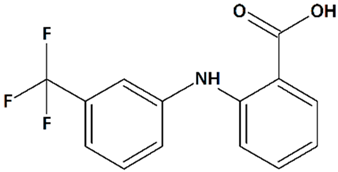 | Oxygen/glucose deprivation in isolated mouse embryonic cortical neurons | Incubation from 15 min before oxygen/glucose deprivation until 24 h of reoxygenation | increased viability of male neurons no effect on female neurons | [118] |
| Glutamate toxicity in isolated rat embryonic hippocampal neurons | Co-incubation for 10 min | increased viability | [119] | ||
| 6 | PPZ1 [4-(5-Chloro-2-methylphenyl)piperazin-1-yl]-3-fluorophenylmethanone 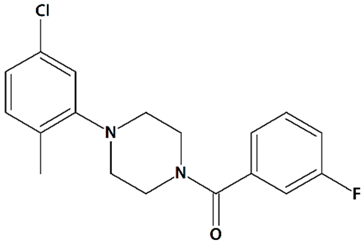 | Serum deprivation in isolated rat cerebellar granule neurons | Incubation for 24 h before and 24 h after serum deprivation | increased neurite outgrowth increased cell viability | [83] |
| 7 | PPZ2 2-[4-(2,3-Dimethylphenyl)piperazin-1-yl]-N-(2-ethoxyphenyl)acetamide 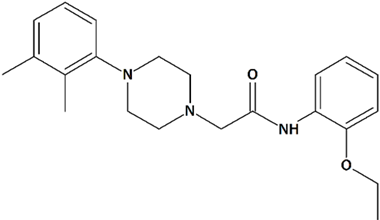 | ||||
| 8 | 51164 N-(2-chlorophenyl)-2-(4-phenylpiperazin-1-yl)acetamide 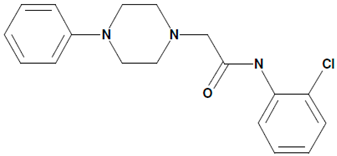 | Aβ42-induced toxicity in primary hippocampal neurons | Co-incubation for 16 h | restored mushroom spines percentage induced neuronal SOCE in postsynaptic spines | [21] |
| 6 month-old 5xFAD mouse hippocampal slices | 30 min incubation | restored LTP induction | |||
| 9 | FDES Bis-{2-[(2E)-4-hydroxy-4-oxobut-2-enoyloxy]-N,N-diethylethanaminium} butandioate 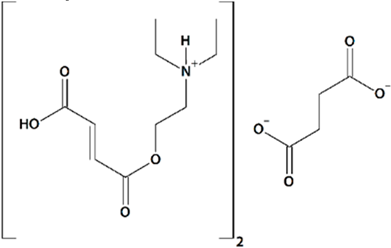 | Aβ42-induced toxicity in primary hippocampal neurons | Incubation for 16 h | restored mushroom spines percentage induced neuronal SOCE in postsynaptic spines | [89] |
| Rat transient MCAO | Ip injections at 1 h after reperfusion + q.d. for 7 d after reperfusion | improved spatial memory retention no effect on mortality | |||
| Rat permanent bilateral common carotid artery ligation | Oral gavage at 30 min before MCAO + q.d. for 21 d after reperfusion | reduced mortality reduced motor deficit reduced aggressiveness reduced emotional lability increased exploratory behavior | [120] | ||
| 10 | NSN21778 N-[4-[2-[(6-aminoquinazolin-4-yl)amino]ethyl]phenyl]acetamide  | Primary hippocampal cell culture models from PS1-M146V and APPKI mice | Incubation for 16 h | increased percentage of mushroom spines in TRPC6-dependent manner increased neuronal SOCE in postsynaptic spines | [14] |
| Hippocampal brain slices from PS1-M146V and APPKI mice | Pretreatment for 30 min | recovered LTP induction | |||
| TRPC6 inhibitors | |||||
| 11 | HET0016 N-Hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine 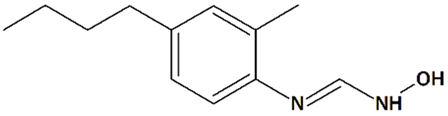 | Rat pediatric asphyxial cardiac arrest | Iv injection at reperfusion | increased cortical CBF reduced brain edema | [69] |
| Iv injection at reperfusion + ip injections every 6 h for 24 h after reperfusion | reduced neurological deficit reduced neurodegeneration | ||||
| Rat transient MCAO | Iv injection immediately before reperfusion | reduced infarct volume | [68] | ||
| Ip injections q.d. for 3 d before and 3 d after MCAO | increased CBF reduced infarct volume | [121] | |||
| Piglet neonatal hypoxia/ ischemia | 5 min-infusion at 5 min after reperfusion + hypothermia at 3 h after reperfusion | increased neuronal viability in the putamen, cortex, and thalamus prevention of seizures | [122] | ||
| Iv injection at 5 min after reperfusion | increased neuronal viability in the putamen reduced neurological deficit no effect on cerebral blood flow (CBF) | [123] | |||
| 12 | TS-011 N-(3-Chloro-4-morpholin-4-yl)phenyl-N′-hydroxyimidoformamide 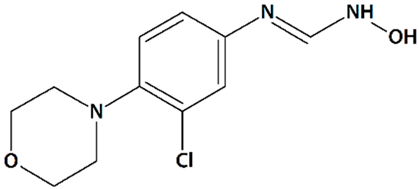 | Rat transient MCAO | Iv injection at 30 min before MCAO + 1 or 2 h-infusion during MCAO | reduced cortical, subcortical, and total infarct volumes reduced the delayed drop in CBF no effect on volume at risk | [68] |
| Iv injection at 20 min after MCAO + 2 h-infusion at reperfusion | reduced cortical and total infarct volumes no effect on volume at risk no effect on CBF | ||||
| 1 h-infusion at reperfusion, 1, 2, or 4 h after reperfusion | reduced infarct volumes | [67] | |||
| 1 h-infusion at reperfusion + iv injections q.d. for 7 d | reduced infarct volumes reduced sensory and motor deficits | ||||
| Crab-eating macaque thrombotic internal carotid artery occlusion | Iv injection + 24 h-infusion after embolization | reduced infarct volume (when co-administered with tissue plasminogen activator) reduced neurological deficit | |||
| 13 | Mefenamic acid 2-(2,3-Dimethylphenyl)aminobenzoic acid 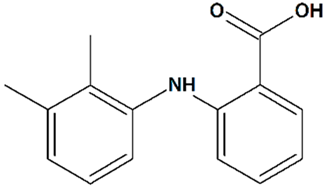 | Glutamate toxicity in isolated rat embryonic hippocampal neurons | Co-incubation for 10 min | increased cell viability | [124] |
| 3xTg mice AD model | Administration by osmotic minipump over 28 days | reduced cognitive deficit | [125] | ||
| Rat transient MCAO | Iv injection before MCAO | no effect on infarct and penumbra volumes and brain edema | [124] | ||
| Iv injections at 1 h before + at 1, 2, and 3 h after MCAO | reduced infarct volume reduced brain edema | ||||
| Icv 24 h-infusion starting at 1 h before MCAO | [119] | ||||
| 14 | Meclofenamic acid 2-(2,6-Dichloro-3-methylanilino)benzoic acid 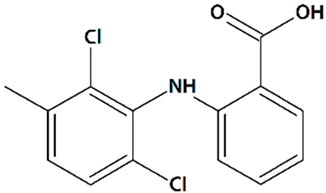 | Glutamate toxicity in isolated rat embryonic hippocampal neurons | Co-incubation for 10 min | increased viability | [119] |
| 15 | Niflumic acid 2-{[3-(Trifluoromethyl)phenyl]amino}pyridine-3-carboxylic acid 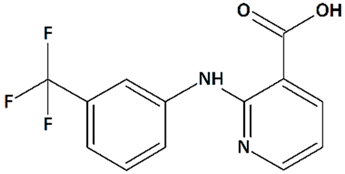 | ||||
| 16 | SAR7334 4-[[(1R,2R)-2-[(3R)-3-Amino-1-piperidinyl]-2,3-dihydro-1H-inden-1-yl]oxy]-3-chlorobenzonitrile dihydrochloride 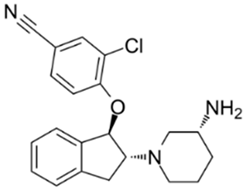 | Primary cortical neurons | Treatment with 1 μM SAR7334 at the time of imaging | no effect on neuronal SOCE | [126] |
| 17 | EVP4593 4-N- [2- (4-phenoxyphenyl)ethyl]quinazoline-4,6-diamine 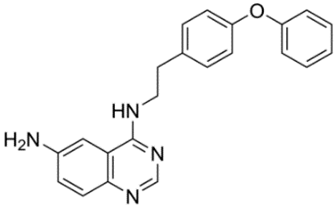 | PSEN1ΔE9-hyperexpressing primary hippocampal neurons | Co-incubation for 16 h | reduced TRPC6-dependent neuronal SOCE in postsynaptic spines increased mushroom spines percentages | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prikhodko, V.; Chernyuk, D.; Sysoev, Y.; Zernov, N.; Okovityi, S.; Popugaeva, E. Potential Drug Candidates to Treat TRPC6 Channel Deficiencies in the Pathophysiology of Alzheimer’s Disease and Brain Ischemia. Cells 2020, 9, 2351. https://doi.org/10.3390/cells9112351
Prikhodko V, Chernyuk D, Sysoev Y, Zernov N, Okovityi S, Popugaeva E. Potential Drug Candidates to Treat TRPC6 Channel Deficiencies in the Pathophysiology of Alzheimer’s Disease and Brain Ischemia. Cells. 2020; 9(11):2351. https://doi.org/10.3390/cells9112351
Chicago/Turabian StylePrikhodko, Veronika, Daria Chernyuk, Yurii Sysoev, Nikita Zernov, Sergey Okovityi, and Elena Popugaeva. 2020. "Potential Drug Candidates to Treat TRPC6 Channel Deficiencies in the Pathophysiology of Alzheimer’s Disease and Brain Ischemia" Cells 9, no. 11: 2351. https://doi.org/10.3390/cells9112351
APA StylePrikhodko, V., Chernyuk, D., Sysoev, Y., Zernov, N., Okovityi, S., & Popugaeva, E. (2020). Potential Drug Candidates to Treat TRPC6 Channel Deficiencies in the Pathophysiology of Alzheimer’s Disease and Brain Ischemia. Cells, 9(11), 2351. https://doi.org/10.3390/cells9112351





