Perivascular Inflammation in Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Inflammatory Mediators and their Effects on Vascular Remodeling
2.1. Cytokines
2.1.1. IL-1β
| Model | Severity | Inflammation | Notes | Refs |
|---|---|---|---|---|
| Chronic hypoxic mouse | Mild | -Early macrophage infiltration -Requires eicosanoids -Aggravated by IL-6 | -Reversible | [18] |
| Sugen-hypoxia mouse | Mild-moderate | -No significant pulmonary infiltration seen | -Slower to reverse than hypoxia alone | [19] |
| Monocrotaline rat | Severe | -Severe inflammation of lungs | -Also significant extrapulmonary inflammation | [20] |
| Sugen-hypoxia rat | Severe | -Closest approximation of human disease in rodents -Most immune lineages seen in lung vascular lesions | -Irreversible, plexiform angiopathy | [21] |
2.1.2. IL-6
2.1.3. IL-18
Chemokines and Their Receptors
2.2. Inflammatory Mediators
2.2.1. Leukotriene B4 (LTB4)
2.2.2. Macrophage Migration Inhibitory Factor (MIF)
2.2.3. Hypoxia-Induced Mitogenic Factor (HIMF)
2.2.4. High Mobility Group Box-1 (HMGB1)
2.2.5. Complement
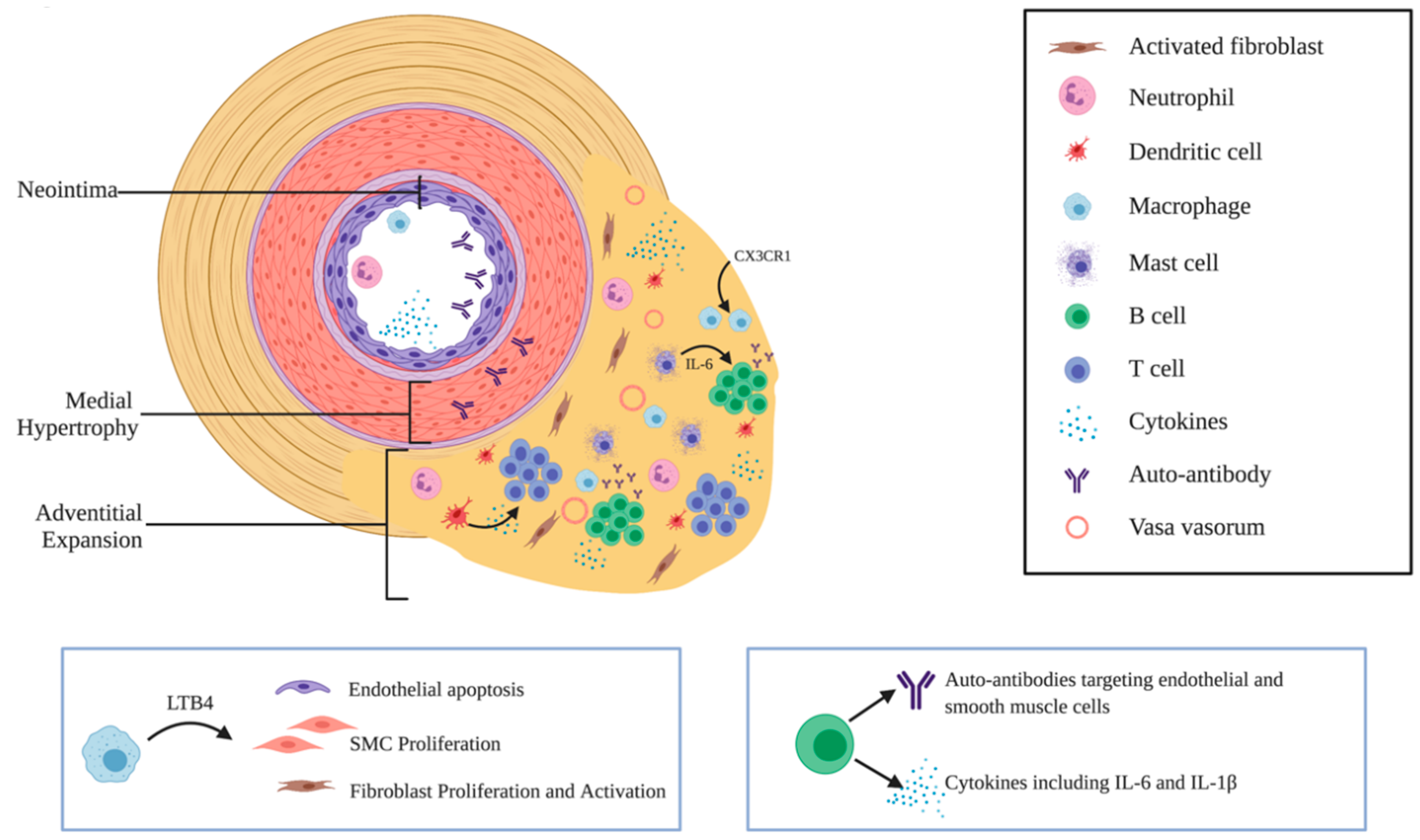
2.3. Immune Cells in PH and Their Roles in Vascular Remodeling
2.3.1. Macrophages
2.3.2. Dendritic Cells
2.3.3. Mast Cells
2.3.4. T Cells
2.3.5. B Cells and Autoantibodies in PAH
2.3.6. Neutrophils
2.4. Phenotype Changes of Pulmonary Vascular Cells
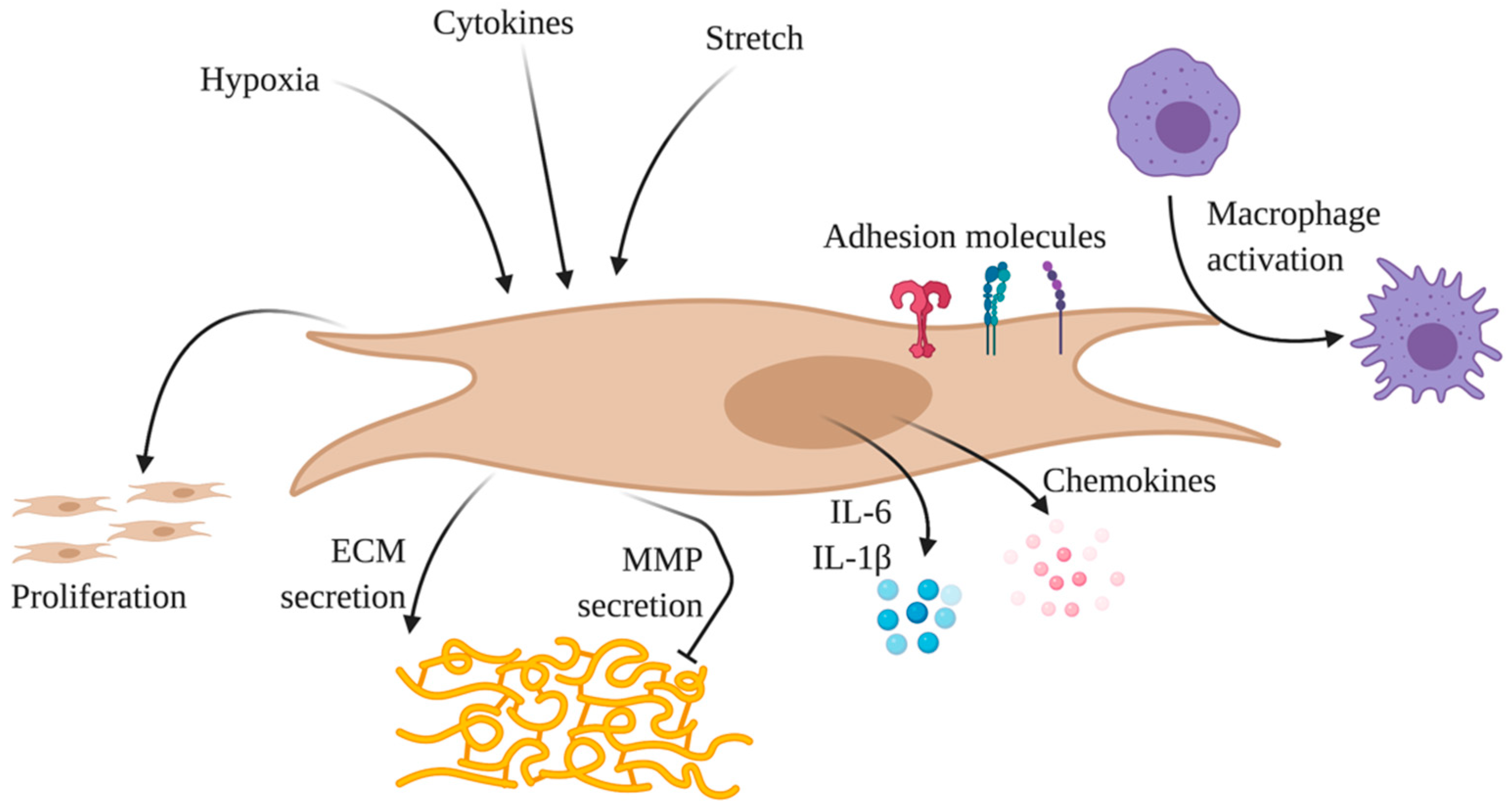
2.4.1. Endothelial Cells
2.4.2. Smooth Muscle Cells
2.4.3. Fibroblasts
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Savai, R.; Pullamsetti, S.S.; Kolbe, J.; Bieniek, E.; Voswinckel, R.; Fink, L.; Scheed, A.; Ritter, C.; Dahal, B.K.; Vater, A.; et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 897–908. [Google Scholar] [CrossRef]
- Goldenberg, N.M.; Rabinovitch, M.; Steinberg, B.E. Inflammatory Basis of Pulmonary Arterial Hypertension: Implications for Perioperative and Critical Care Medicine. Anesthesiology 2019, 131, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Frid, M.G.; Yeager, M.; Li, M.; Riddle, S.; McKinsey, T.; El Kasmi, K.C. Targeting the adventitial microenvironment in pulmonary hypertension: A potential approach to therapy that considers epigenetic change. Pulm. Circ. 2012, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Monti, G.; Brenot, F.; Sitbon, O.; Portier, A.; Grangeot-Keros, L.; Duroux, P.; Galanaud, P.; Simonneau, G.; Emilie, D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1995, 151, 1628–1631. [Google Scholar] [CrossRef] [PubMed]
- Soon, E.; Holmes, A.M.; Treacy, C.M.; Doughty, N.J.; Southgate, L.; Machado, R.D.; Trembath, R.C.; Jennings, S.; Barker, L.; Nicklin, P.; et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010, 122, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Cero, F.T.; Hillestad, V.; Sjaastad, I.; Yndestad, A.; Aukrust, P.; Ranheim, T.; Lunde, I.G.; Olsen, M.B.; Lien, E.; Zhang, L.; et al. Absence of the inflammasome adaptor ASC reduces hypoxia-induced pulmonary hypertension in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L378–L387. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Tuder, R.M.; Bridges, J.; Arend, W.P. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am. J. Respir. Cell Mol. Biol. 1994, 11, 664–675. [Google Scholar] [CrossRef]
- Parpaleix, A.; Amsellem, V.; Houssaini, A.; Abid, S.; Breau, M.; Marcos, E.; Sawaki, D.; Delcroix, M.; Quarck, R.; Maillard, A.; et al. Role of interleukin-1 receptor 1/MyD88 signalling in the development and progression of pulmonary hypertension. Eur. Respir. J. 2016, 48, 470–483. [Google Scholar] [CrossRef]
- Itoh, A.; Nishihira, J.; Makita, H.; Miyamoto, K.; Yamaguchi, E.; Nishimura, M. Effects of IL-1beta, TNF-alpha, and macrophage migration inhibitory factor on prostacyclin synthesis in rat pulmonary artery smooth muscle cells. Respirology 2003, 8, 467–472. [Google Scholar] [CrossRef]
- Yang, P.-S.; Kim, D.-H.; Lee, Y.; Lee, S.-E.; Kang, W.; Chang, H.-J.; Shin, J.-S. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir. Res. 2014, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Trankle, C.R.; Canada, J.M.; Kadariya, D.; Markley, R.; De Chazal, H.M.; Pinson, J.; Fox, A.; Van Tassell, B.W.; Abbate, A.; Grinnan, D. IL-1 Blockade Reduces Inflammation in Pulmonary Arterial Hypertension and Right Ventricular Failure: A Single-Arm, Open-Label, Phase IB/II Pilot Study. Am. J. Respir. Crit. Care Med. 2019, 199, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.C.; Hassoun, P.M. Pulmonary arterial hypertension in connective tissue diseases. Heart Fail. Clin. 2012, 8, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.; Sitbon, O.; Jaïs, X.; Simonneau, G.; Humbert, M. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest 2006, 130, 182–189. [Google Scholar] [CrossRef]
- Colvin, K.L.; Yeager, M.E. Animal Models of Pulmonary Hypertension: Matching Disease Mechanisms to Etiology of the Human Disease. J. Pulm. Respir. Med. 2014, 4. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.; Saleem, S.J.; Mizuno, S.; Syed, A.A.; Bogaard, H.J.; Abbate, A.; Taraseviciene-Stewart, L.; Sung, Y.; Kraskauskas, D.; Farkas, D.; et al. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L977–L991. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Meyrick, B.; Galiè, N.; Mooi, W.J.; McMurtry, I.F. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L1013–L1032. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Tuder, R.M.; Wade, K.; Höper, M.; Lepley, R.A.; Goulet, J.L.; Koller, B.H.; Fitzpatrick, F. Inhibition of 5-lipoxygenase-activating protein (FLAP) reduces pulmonary vascular reactivity and pulmonary hypertension in hypoxic rats. J. Clin. Invest. 1996, 97, 2491–2498. [Google Scholar] [CrossRef]
- Vitali, S.H.; Hansmann, G.; Rose, C.; Fernandez-Gonzalez, A.; Scheid, A.; Mitsialis, S.A.; Kourembanas, S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: Long-term follow-up. Pulm. Circ. 2014, 4, 619–629. [Google Scholar] [CrossRef]
- Wilson, D.W.; Segall, H.J.; Pan, L.C.; Dunston, S.K. Progressive inflammatory and structural changes in the pulmonary vasculature of monocrotaline-treated rats. Microvasc. Res. 1989, 38, 57–80. [Google Scholar] [CrossRef]
- Abe, K.; Toba, M.; Alzoubi, A.; Ito, M.; Fagan, K.A.; Cool, C.D.; Voelkel, N.F.; McMurtry, I.F.; Oka, M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 2010, 121, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Kumar, A.; Yuan, N.; Gewitz, M.H.; Mathew, R. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis 1999, 1, 126–132. [Google Scholar] [PubMed]
- Prins, K.W.; Archer, S.L.; Pritzker, M.; Rose, L.; Weir, E.K.; Sharma, A.; Thenappan, T. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J. Heart Lung Transplant. 2018, 37, 376–384. [Google Scholar] [CrossRef]
- Heresi, G.A.; Minai, O.A.; Tonelli, A.R.; Hammel, J.P.; Farha, S.; Parambil, J.G.; Dweik, R.A. Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm. Circ. 2013, 3, 916–925. [Google Scholar] [CrossRef]
- Steiner, M.K.; Syrkina, O.L.; Kolliputi, N.; Mark, E.J.; Hales, C.A.; Waxman, A.B. Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res. 2009, 104, 236–244. [Google Scholar] [CrossRef]
- Golembeski, S.M.; West, J.; Tada, Y.; Fagan, K.A. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest 2005, 128, 572S–573S. [Google Scholar] [CrossRef]
- Savale, L.; Tu, L.; Rideau, D.; Izziki, M.; Maitre, B.; Adnot, S.; Eddahibi, S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir. Res. 2009, 10, 6. [Google Scholar] [CrossRef]
- Tamura, Y.; Phan, C.; Tu, L.; Le Hiress, M.; Thuillet, R.; Jutant, E.-M.; Fadel, E.; Savale, L.; Huertas, A.; Humbert, M.; et al. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J. Clin. Invest. 2018, 128, 1956–1970. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sánchez, J.; Harlow, L.; Church, C.; Gaine, S.; Knightbridge, E.; Bunclark, K.; Gor, D.; Bedding, A.; Morrell, N.; Corris, P.; et al. Clinical trial protocol for TRANSFORM-UK: A therapeutic open-label study of tocilizumab in the treatment of pulmonary arterial hypertension. Pulm. Circ. 2018, 8. [Google Scholar] [CrossRef]
- Davies, R.J.; Holmes, A.M.; Deighton, J.; Long, L.; Yang, X.; Barker, L.; Walker, C.; Budd, D.C.; Upton, P.D.; Morrell, N.W. BMP type II receptor deficiency confers resistance to growth inhibition by TGF-β in pulmonary artery smooth muscle cells: Role of proinflammatory cytokines. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L604–L615. [Google Scholar] [CrossRef]
- Simpson, C.E.; Chen, J.Y.; Damico, R.L.; Hassoun, P.M.; Martin, L.J.; Yang, J.; Nies, M.; Griffiths, M.; Vaidya, R.D.; Brandal, S.; et al. Cellular sources of interleukin-6 and associations with clinical phenotypes and outcomes in pulmonary arterial hypertension. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Breitling, S.; Hui, Z.; Zabini, D.; Hu, Y.; Hoffmann, J.; Goldenberg, N.M.; Tabuchi, A.; Buelow, R.; Dos Santos, C.; Kuebler, W.M. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L710–L721. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.B.; Chabon, J.; Kumar, R.; Kolosionek, E.; Gebreab, L.; Debella, E.; Edwards, M.; Diener, K.; Shade, T.; Bifeng, G.; et al. Protective role of IL-6 in vascular remodeling in Schistosoma pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 49, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Caramori, G.; Perros, F.; Meng, C.; Gambaryan, N.; Dorfmüller, P.; Montani, D.; Casolari, P.; Zhu, J.; Dimopoulos, K.; et al. Nuclear factor κ-B is activated in the pulmonary vessels of patients with end-stage idiopathic pulmonary arterial hypertension. PLoS ONE 2013, 8, e75415. [Google Scholar] [CrossRef] [PubMed]
- Farkas, D.; Thompson, A.A.R.; Bhagwani, A.R.; Hultman, S.; Ji, H.; Kotha, N.; Farr, G.; Arnold, N.D.; Braithwaite, A.; Casbolt, H.; et al. Toll-like Receptor 3 is a Therapeutic Target for Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 199, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Vengethasamy, L.; Hautefort, A.; Tielemans, B.; Belge, C.; Perros, F.; Verleden, S.; Fadel, E.; Van Raemdonck, D.; Delcroix, M.; Quarck, R. BMPRII influences the response of pulmonary microvascular endothelial cells to inflammatory mediators. Pflugers Arch. 2016, 468, 1969–1983. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Seeger, W.; Savai, R. Classical IL-6 signaling: A promising therapeutic target for pulmonary arterial hypertension. J. Clin. Invest. 2018, 128, 1720–1723. [Google Scholar] [CrossRef]
- Courboulin, A.; Tremblay, V.L.; Barrier, M.; Meloche, J.; Jacob, M.H.; Chapolard, M.; Bisserier, M.; Paulin, R.; Lambert, C.; Provencher, S.; et al. Krüppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir. Res. 2011, 12, 128. [Google Scholar] [CrossRef]
- Hashimoto-Kataoka, T.; Hosen, N.; Sonobe, T.; Arita, Y.; Yasui, T.; Masaki, T.; Minami, M.; Inagaki, T.; Miyagawa, S.; Sawa, Y.; et al. Interleukin-6/interleukin-21 signaling axis is critical in the pathogenesis of pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA 2015, 112, E2677–E2686. [Google Scholar] [CrossRef]
- Brock, M.; Trenkmann, M.; Gay, R.E.; Michel, B.A.; Gay, S.; Fischler, M.; Ulrich, S.; Speich, R.; Huber, L.C. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ. Res. 2009, 104, 1184–1191. [Google Scholar] [CrossRef]
- Colvin, K.L.; Cripe, P.J.; Ivy, D.D.; Stenmark, K.R.; Yeager, M.E. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am. J. Respir. Crit. Care Med. 2013, 188, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Ross, D.J.; Strieter, R.M.; Fishbein, M.C.; Ardehali, A.; Belperio, J.A. Type I immune response cytokine-chemokine cascade is associated with pulmonary arterial hypertension. J. Heart Lung Transplant. 2012, 31, 865–873. [Google Scholar] [CrossRef]
- Bruns, D.R.; Buttrick, P.M.; Walker, L.A. Genetic ablation of interleukin-18 does not attenuate hypobaric hypoxia-induced right ventricular hypertrophy. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L542–L550. [Google Scholar] [CrossRef]
- Tielemans, B.; Stoian, L.; Gijsbers, R.; Michiels, A.; Wagenaar, A.; Farre Marti, R.; Belge, C.; Delcroix, M.; Quarck, R. Cytokines trigger disruption of endothelium barrier function and p38 MAP kinase activation in BMPR2-silenced human lung microvascular endothelial cells. Pulm. Circ. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.-O.; Yndestad, A.; Sjaastad, I.; Løberg, E.M.; Goverud, I.L.; Halvorsen, B.; Jia, J.; Andreassen, A.K.; Husberg, C.; Jonasson, S.; et al. Lack of CCR7 induces pulmonary hypertension involving perivascular leukocyte infiltration and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L50–L59. [Google Scholar] [CrossRef]
- Perros, F.; Dorfmüller, P.; Montani, D.; Hammad, H.; Waelput, W.; Girerd, B.; Raymond, N.; Mercier, O.; Mussot, S.; Cohen-Kaminsky, S.; et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 185, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.M.; Olle, S.; Fuge, J.; Welte, T.; Hoeper, M.M.; Lerch, C.; Maegel, L.; Haller, H.; Jonigk, D.; Schiffer, L. CXCL13 in idiopathic pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir. Res. 2016, 17, 21. [Google Scholar] [CrossRef]
- Amsellem, V.; Lipskaia, L.; Abid, S.; Poupel, L.; Houssaini, A.; Quarck, R.; Marcos, E.; Mouraret, N.; Parpaleix, A.; Bobe, R.; et al. CCR5 as a treatment target in pulmonary arterial hypertension. Circulation 2014, 130, 880–891. [Google Scholar] [CrossRef]
- Abid, S.; Marcos, E.; Parpaleix, A.; Amsellem, V.; Breau, M.; Houssaini, A.; Vienney, N.; Lefevre, M.; Derumeaux, G.; Evans, S.; et al. CCR2/CCR5-mediated macrophage-smooth muscle cell crosstalk in pulmonary hypertension. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Jiang, X.; Tamosiuniene, R.; Sung, Y.K.; Qian, J.; Dhillon, G.; Gera, L.; Farkas, L.; Rabinovitch, M.; Zamanian, R.T.; et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci. Transl. Med. 2013, 5, 200ra117. [Google Scholar] [CrossRef]
- Qian, J.; Tian, W.; Jiang, X.; Tamosiuniene, R.; Sung, Y.K.; Shuffle, E.M.; Tu, A.B.; Valenzuela, A.; Jiang, S.; Zamanian, R.T.; et al. Leukotriene B4 Activates Pulmonary Artery Adventitial Fibroblasts in Pulmonary Hypertension. Hypertension 2015, 66, 1227–1239. [Google Scholar] [CrossRef][Green Version]
- Tian, W.; Jiang, X.; Sung, Y.K.; Qian, J.; Yuan, K.; Nicolls, M.R. Leukotrienes in pulmonary arterial hypertension. Immunol. Res. 2014, 58, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Melao, A. Eiger BioPharmaceuticals Discontinues Ubenimex for PAH Clinical Trial. Available online: https://pulmonaryhypertensionnews.com/2018/01/17/eiger-biopharmaceuticals-discontinues-ubenimex-pah-clinical-trial/ (accessed on 10 September 2020).
- Tian, W.; Jiang, X.; Sung, Y.K.; Shuffle, E.; Wu, T.-H.; Kao, P.N.; Tu, A.B.; Dorfmüller, P.; Cao, A.; Wang, L.; et al. Phenotypically Silent Bone Morphogenetic Protein Receptor 2 Mutations Predispose Rats to Inflammation-Induced Pulmonary Arterial Hypertension by Enhancing the Risk for Neointimal Transformation. Circulation 2019, 140, 1409–1425. [Google Scholar] [CrossRef]
- Newman, J.H.; Wheeler, L.; Lane, K.B.; Loyd, E.; Gaddipati, R.; Phillips, J.A.; Loyd, J.E. Mutation in the Gene for Bone Morphogenetic Protein Receptor II as a Cause of Primary Pulmonary Hypertension in a Large Kindred. N. Engl. J. Med. 2001, 345, 319–324. [Google Scholar] [CrossRef]
- Marshall, J.D.; Sauler, M.; Tonelli, A.; Rao, Y.; Bucala, R.; Lee, P.J.; Fares, W.H. Complexity of macrophage migration inhibitory factor (MIF) and other angiogenic biomarkers profiling in pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 730–733. [Google Scholar] [CrossRef]
- Le Hiress, M.; Tu, L.; Ricard, N.; Phan, C.; Thuillet, R.; Fadel, E.; Dorfmüller, P.; Montani, D.; de Man, F.; Humbert, M.; et al. Proinflammatory Signature of the Dysfunctional Endothelium in Pulmonary Hypertension. Role of the Macrophage Migration Inhibitory Factor/CD74 Complex. Am. J. Respir. Crit. Care Med. 2015, 192, 983–997. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, M.; Xu, M.; Liu, L.-L.; Luo, Y.; Xu, D.-Q.; Wang, Y.-X.; Liu, M.-L.; Liu, Y.; Dong, H.-Y.; et al. Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediat. Inflamm. 2012, 2012, 840737. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Angelini, D.J.; Su, Q.; Yamaji-Kegan, K.; Fan, C.; Skinner, J.T.; Champion, H.C.; Crow, M.T.; Johns, R.A. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) induces the vascular and hemodynamic changes of pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L582–L593. [Google Scholar] [CrossRef] [PubMed]
- Johns, R.A.; Takimoto, E.; Meuchel, L.W.; Elsaigh, E.; Zhang, A.; Heller, N.M.; Semenza, G.L.; Yamaji-Kegan, K. Hypoxia-Inducible Factor 1α Is a Critical Downstream Mediator for Hypoxia-Induced Mitogenic Factor (FIZZ1/RELMα)-Induced Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Yoshida, M. HMGB proteins and transcriptional regulation. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2010, 1799, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Volchuk, A.; Ye, A.; Chi, L.; Steinberg, B.E.; Goldenberg, N.M. Indirect regulation of HMGB1 release by gasdermin D. Nat. Commun. 2020, 11, 4561. [Google Scholar] [CrossRef]
- Bauer, E.M.; Shapiro, R.; Zheng, H.; Ahmad, F.; Ishizawar, D.; Comhair, S.A.; Erzurum, S.C.; Billiar, T.R.; Bauer, P.M. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol. Med. 2012, 18, 1509–1518. [Google Scholar] [CrossRef]
- Goldenberg, N.M.; Hu, Y.; Hu, X.; Volchuk, A.; Zhao, Y.D.; Kucherenko, M.M.; Knosalla, C.; de Perrot, M.; Tracey, K.J.; Al-Abed, Y.; et al. Therapeutic Targeting of High-Mobility Group Box-1 in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2019, 199, 1566–1569. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, C.; Gomez-Arroyo, J.; Van Raemdonck, K.; Meuchel, L.W.; Skinner, J.T.; Everett, A.D.; Fang, X.; Macdonald, A.A.; Yamaji-Kegan, K.; et al. HIMF (Hypoxia-Induced Mitogenic Factor) Signaling Mediates the HMGB1 (High Mobility Group Box 1)-Dependent Endothelial and Smooth Muscle Cell Crosstalk in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2505–2519. [Google Scholar] [CrossRef]
- Sadamura-Takenaka, Y.; Ito, T.; Noma, S.; Oyama, Y.; Yamada, S.; Kawahara, K.-I.; Inoue, H.; Maruyama, I. HMGB1 Promotes the Development of Pulmonary Arterial Hypertension in Rats. PLoS ONE 2014, 9, e102482. [Google Scholar] [CrossRef]
- Jia, D.; He, Y.; Zhu, Q.; Liu, H.; Zuo, C.; Chen, G.; Yu, Y.; Lu, A. RAGE-mediated extracellular matrix proteins accumulation exacerbates HySu-induced pulmonary hypertension. Cardiovasc. Res. 2017, 113, 586–597. [Google Scholar] [CrossRef]
- Zabini, D.; Crnkovic, S.; Xu, H.; Tscherner, M.; Ghanim, B.; Klepetko, W.; Olschewski, A.; Kwapiszewska, G.; Marsh, L.M. High-mobility group box-1 induces vascular remodelling processes via c-Jun activation. J. Cell. Mol. Med. 2015, 19, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Cleary, S.J.; Kwaan, N.; Tian, J.J.; Calabrese, D.R.; Mallavia, B.; Magnen, M.; Greenland, J.R.; Urisman, A.; Singer, J.P.; Hays, S.R.; et al. Complement activation on endothelium initiates antibody-mediated acute lung injury. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Frid, M.G.; McKeon, B.A.; Thurman, J.M.; Maron, B.A.; Li, M.; Zhang, H.; Kumar, S.; Sullivan, T.; Laskowsky, J.; Fini, M.A.; et al. Immunoglobulin-driven Complement Activation Regulates Proinflammatory Remodeling in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Salam, V.B.; Paul, G.A.; Ali, J.O.; Gibbs, S.R.; Rahman, D.; Taylor, G.W.; Wilkins, M.R.; Edwards, R.J. Identification of plasma protein biomarkers associated with idiopathic pulmonary arterial hypertension. Proteomics 2006, 6, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Li, N.; Liu, Z.; Xiong, C.; Ni, X.; Pu, Y.; Hui, R.; He, J.; Pu, J. Potential diagnostic biomarkers in serum of idiopathic pulmonary arterial hypertension. Respir. Med. 2009, 103, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.M.; Zheng, H.; Comhair, S.; Erzurum, S.; Billiar, T.R.; Bauer, P.M. Complement C3 deficiency attenuates chronic hypoxia-induced pulmonary hypertension in mice. PLoS ONE 2011, 6, e28578. [Google Scholar] [CrossRef]
- Zipfel, P.F.; Wiech, T.; Rudnick, R.; Afonso, S.; Person, F.; Skerka, C. Complement Inhibitors in Clinical Trials for Glomerular Diseases. Front. Immunol. 2019, 10, 2166. [Google Scholar] [CrossRef]
- Vergadi, E.; Chang, M.S.; Lee, C.; Liang, O.D.; Liu, X.; Fernandez-Gonzalez, A.; Mitsialis, S.A.; Kourembanas, S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 2011, 123, 1986–1995. [Google Scholar] [CrossRef]
- Frid, M.G.; Brunetti, J.A.; Burke, D.L.; Carpenter, T.C.; Davie, N.J.; Reeves, J.T.; Roedersheimer, M.T.; Van Rooijen, N.; Stenmark, K.R. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am. J. Pathol. 2006, 168, 659–669. [Google Scholar] [CrossRef]
- Marsh, L.M.; Jandl, K.; Grünig, G.; Foris, V.; Bashir, M.; Ghanim, B.; Klepetko, W.; Olschewski, H.; Olschewski, A.; Kwapiszewska, G. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1701214. [Google Scholar] [CrossRef]
- Žaloudíková, M.; Vytášek, R.; Vajnerová, O.; Hniličková, O.; Vízek, M.; Hampl, V.; Herget, J. Depletion of alveolar macrophages attenuates hypoxic pulmonary hypertension but not hypoxia-induced increase in serum concentration of MCP-1. Physiol. Res. 2016, 65, 763–768. [Google Scholar] [CrossRef]
- Talati, M.; Gladson, S.; Blackwell, T.; Shay, S.; Hamid, R.; West, J. Macrophages are part of cause, not consequence, in PAH. FASEB J. 2016, 30, 774.12. [Google Scholar] [CrossRef]
- Bryant, A.J.; Fu, C.; Brown, G.; Biswas, A.; Shenoy, V.; Katovich, M.; Scott, E. Macrophage Depletion Results in Worsened Secondary Pulmonary Hypertension and Improved Pulmonary Fibrosis. In D52. MOLECULAR INSIGHT INTO PULMONARY HYPERTENSION; American Thoracic Society: New York, NY, USA, 2016; p. A7274. [Google Scholar]
- Pugliese, S.C.; Kumar, S.; Janssen, W.J.; Graham, B.B.; Frid, M.G.; Riddle, S.R.; El Kasmi, K.C.; Stenmark, K.R. A Time- and Compartment-Specific Activation of Lung Macrophages in Hypoxic Pulmonary Hypertension. J. Immunol. 2017, 198, 4802–4812. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Amsellem, V.; Abid, S.; Poupel, L.; Parpaleix, A.; Rodero, M.; Gary-Bobo, G.; Latiri, M.; Dubois-Rande, J.-L.; Lipskaia, L.; Combadiere, C.; et al. Roles for the CX3CL1/CX3CR1 and CCL2/CCR2 Chemokine Systems in Hypoxic Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2017, 56, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, P.; Hildebrandt, T.; Bernlöhr, C.; Lee, D.; Khang, G.; Doods, H.; Wu, D. Inhibition of kinin B1 receptors attenuates pulmonary hypertension and vascular remodeling. Hypertension 2015, 66, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Tso, C.; Rye, K.-A.; Barter, P. Phenotypic and functional changes in blood monocytes following adherence to endothelium. PLoS ONE 2012, 7, e37091. [Google Scholar] [CrossRef]
- Bohgaki, M.; Kitaguchi, H. Conversion of cultured monocytes/macrophages into endothelial-like cells through direct contact with endothelial cells. Int. J. Hematol. 2007, 86, 42–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gerasimovskaya, E.; Kratzer, A.; Sidiakova, A.; Salys, J.; Zamora, M.; Taraseviciene-Stewart, L. Interplay of macrophages and T cells in the lung vasculature. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L1014–L1022. [Google Scholar] [CrossRef]
- Yeager, M.E.; Nguyen, C.M.; Belchenko, D.D.; Colvin, K.L.; Takatsuki, S.; Ivy, D.D.; Stenmark, K.R. Circulating myeloid-derived suppressor cells are increased and activated in pulmonary hypertension. Chest 2012, 141, 944–952. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, M.; Crinier, A.; Vély, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Price, J.D.; Tarbell, K.V. The Role of Dendritic Cell Subsets and Innate Immunity in the Pathogenesis of Type 1 Diabetes and Other Autoimmune Diseases. Front. Immunol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yan, H.; Zhu, W.; Cui, Y.; Chen, J.; Wang, X.; Li, S.; Zhu, J. Impairment of monocyte-derived dendritic cells in idiopathic pulmonary arterial hypertension. J. Clin. Immunol. 2009, 29, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Hautefort, A.; Girerd, B.; Montani, D.; Cohen-Kaminsky, S.; Price, L.; Lambrecht, B.N.; Humbert, M.; Perros, F. T-helper 17 cell polarization in pulmonary arterial hypertension. Chest 2015, 147, 1610–1620. [Google Scholar] [CrossRef]
- Perros, F.; Dorfmüller, P.; Souza, R.; Durand-Gasselin, I.; Mussot, S.; Mazmanian, M.; Hervé, P.; Emilie, D.; Simonneau, G.; Humbert, M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur. Respir. J. 2007, 29, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Yin, J.; Kukucka, M.; Yin, N.; Saarikko, I.; Sterner-Kock, A.; Fujii, H.; Leong-Poi, H.; Kuppe, H.; Schermuly, R.T.; et al. Mast cells promote lung vascular remodelling in pulmonary hypertension. Eur. Respir. J. 2011, 37, 1400–1410. [Google Scholar] [CrossRef]
- Dahal, B.K.; Kosanovic, D.; Kaulen, C.; Cornitescu, T.; Savai, R.; Hoffmann, J.; Reiss, I.; Ghofrani, H.A.; Weissmann, N.; Kuebler, W.M.; et al. Involvement of mast cells in monocrotaline-induced pulmonary hypertension in rats. Respir. Res. 2011, 12, 60. [Google Scholar] [CrossRef]
- Montani, D.; Perros, F.; Gambaryan, N.; Girerd, B.; Dorfmuller, P.; Price, L.C.; Huertas, A.; Hammad, H.; Lambrecht, B.; Simonneau, G.; et al. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2011, 184, 116–123. [Google Scholar] [CrossRef]
- Farha, S.; Dweik, R.; Rahaghi, F.; Benza, R.; Hassoun, P.; Frantz, R.; Torres, F.; Quinn, D.A.; Comhair, S.; Erzurum, S.; et al. Imatinib in pulmonary arterial hypertension: C-Kit inhibition. Pulm. Circ. 2014, 4, 452–455. [Google Scholar] [CrossRef]
- Farha, S.; Sharp, J.; Asosingh, K.; Park, M.; Comhair, S.A.A.; Tang, W.H.W.; Thomas, J.; Farver, C.; Hsieh, F.; Loyd, J.E.; et al. Mast cell number, phenotype, and function in human pulmonary arterial hypertension. Pulm. Circ. 2012, 2, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, B.; van Loon, R.L.E.; Mohaupt, S.; Wijnberg, H.; Dickinson, M.G.; Boersma, B.; Takens, J.; van Albada, M.; Berger, R.M.F. Mast cell inhibition improves pulmonary vascular remodeling in pulmonary hypertension. Chest 2012, 141, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Akers, I.A.; Parsons, M.; Hill, M.R.; Hollenberg, M.D.; Sanjar, S.; Laurent, G.J.; McAnulty, R.J. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L193–L201. [Google Scholar] [CrossRef] [PubMed]
- Kosanovic, D.; Dahal, B.K.; Peters, D.M.; Seimetz, M.; Wygrecka, M.; Hoffmann, K.; Antel, J.; Reiss, I.; Ghofrani, H.A.; Weissmann, N.; et al. Histological characterization of mast cell chymase in patients with pulmonary hypertension and chronic obstructive pulmonary disease. Pulm. Circ. 2014, 4, 128–136. [Google Scholar] [CrossRef]
- Bulfone-Paus, S.; Bahri, R. Mast Cells as Regulators of T Cell Responses. Front. Immunol. 2015, 6, 394. [Google Scholar] [CrossRef]
- Kwapiszewska, G.; Markart, P.; Dahal, B.K.; Kojonazarov, B.; Marsh, L.M.; Schermuly, R.T.; Taube, C.; Meinhardt, A.; Ghofrani, H.A.; Steinhoff, M.; et al. PAR-2 inhibition reverses experimental pulmonary hypertension. Circ. Res. 2012, 110, 1179–1191. [Google Scholar] [CrossRef]
- Sismanopoulos, N.; Delivanis, D.A.; Alysandratos, K.D.; Angelidou, A.; Vasiadi, M.; Therianou, A.; Theoharides, T.C. IL-9 induces VEGF secretion from human mast cells and IL-9/IL-9 receptor genes are overexpressed in atopic dermatitis. PLoS ONE 2012, 7, e33271. [Google Scholar] [CrossRef]
- Hu, Y.; Zabini, D.; Gu, W.; Goldenberg, N.M.; Breitling, S.; Kabir, G.; Connelly, K.A.; Kuebler, W.M. The Role of the Human Immune System in Chronic Hypoxic Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Cannon, J.L.; te Riet, J.; Holmes, A.; Kawakami, Y.; Kawakami, T.; Cambi, A.; Lidke, D.S. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef]
- Cuttica, M.J.; Langenickel, T.; Noguchi, A.; Machado, R.F.; Gladwin, M.T.; Boehm, M. Perivascular T-cell infiltration leads to sustained pulmonary artery remodeling after endothelial cell damage. Am. J. Respir. Cell Mol. Biol. 2011, 45, 62–71. [Google Scholar] [CrossRef]
- Taraseviciene-Stewart, L.; Nicolls, M.R.; Kraskauskas, D.; Scerbavicius, R.; Burns, N.; Cool, C.; Wood, K.; Parr, J.E.; Boackle, S.A.; Voelkel, N.F. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am. J. Respir. Crit. Care Med. 2007, 175, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Tamosiuniene, R.; Tian, W.; Dhillon, G.; Wang, L.; Sung, Y.K.; Gera, L.; Patterson, A.J.; Agrawal, R.; Rabinovitch, M.; Ambler, K.; et al. Regulatory T Cells Limit Vascular Endothelial Injury and Prevent Pulmonary Hypertension. Circ. Res. 2011, 109, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Tamosiuniene, R.; Manouvakhova, O.; Mesange, P.; Saito, T.; Qian, J.; Sanyal, M.; Lin, Y.-C.; Nguyen, L.P.; Luria, A.; Tu, A.B.; et al. Dominant Role for Regulatory T Cells in Protecting Females Against Pulmonary Hypertension. Circ. Res. 2018, 122, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Nicolls, M.R.; Taraseviciene, L.; Speich, R.; Voelkel, N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration 2008, 75, 272–280. [Google Scholar] [CrossRef]
- Huertas, A.; Tu, L.; Gambaryan, N.; Girerd, B.; Perros, F.; Montani, D.; Fabre, D.; Fadel, E.; Eddahibi, S.; Cohen-Kaminsky, S.; et al. Leptin and regulatory T-lymphocytes in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2012, 40, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, A.; Lim, W.; Park, S.; Cho, M.S.; Koo, H.; Moon, B.-I.; Sung, S.H. Zonal difference and prognostic significance of foxp3 regulatory T cell infiltration in breast cancer. J. Breast Cancer 2014, 17, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Nicolls, M.; Badesch, D.; Chung, L.; Domsic, R.; Medsger, T. Pinckney Safety and Efficacy of B-cell Depletion with Rituximab for the Treatment of Systemic Sclerosis-associated Pulmonary Arterial Hypertension in a Multi-center NIH Clinical Trial. Arthritis Rheumatol. 2019, 71. [Google Scholar]
- De Bourcy, C.F.A.; Dekker, C.L.; Davis, M.M.; Nicolls, M.R.; Quake, S.R. Dynamics of the human antibody repertoire after B cell depletion in systemic sclerosis. Sci. Immunol. 2017, 2, eaan8289. [Google Scholar] [CrossRef]
- Hennigan, S.; Channick, R.N.; Silverman, G.J. Rituximab treatment of pulmonary arterial hypertension associated with systemic lupus erythematosus: A case report. Lupus 2008, 17, 754–756. [Google Scholar] [CrossRef]
- Kuebler, W.M.; Bonnet, S.; Tabuchi, A. Inflammation and autoimmunity in pulmonary hypertension: Is there a role for endothelial adhesion molecules? (2017 Grover Conference Series). Pulm. Circ. 2018, 8. [Google Scholar] [CrossRef]
- Mouthon, L.; Guillevin, L.; Humbert, M. Pulmonary arterial hypertension: An autoimmune disease? Eur. Respir. J. 2005, 26, 986–988. [Google Scholar] [CrossRef]
- Wang, J.; Qian, J.; Wang, Y.; Zhao, J.; Wang, Q.; Tian, Z.; Li, M.; Zeng, X. Serological biomarkers as risk factors of SLE-associated pulmonary arterial hypertension: A systematic review and meta-analysis. Lupus 2017, 26, 1390–1400. [Google Scholar] [CrossRef]
- Hinchcliff, M.; Khanna, S.; Hsu, V.M.; Lee, J.; Almagor, O.; Chang, R.W.; Steen, V.; Chung, L.; PHAROS Investigators. Survival in systemic sclerosis-pulmonary arterial hypertension by serum autoantibody status in the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) Registry. Semin. Arthritis Rheum. 2015, 45, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zuily, S.; Domingues, V.; Suty-Selton, C.; Eschwège, V.; Bertoletti, L.; Chaouat, A.; Chabot, F.; Regnault, V.; Horn, E.M.; Erkan, D.; et al. Antiphospholipid antibodies can identify lupus patients at risk of pulmonary hypertension: A systematic review and meta-analysis. Autoimmun. Rev. 2017, 16, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Tamby, M.C.; Camoin, L.; Guilpain, P.; Broussard, C.; Bussone, G.; Yaïci, A.; Hotellier, F.; Simonneau, G.; Guillevin, L.; et al. Identification of target antigens of antifibroblast antibodies in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Bussone, G.; Tamby, M.C.; Calzas, C.; Kherbeck, N.; Sahbatou, Y.; Sanson, C.; Ghazal, K.; Dib, H.; Weksler, B.B.; Broussard, C.; et al. IgG from patients with pulmonary arterial hypertension and/or systemic sclerosis binds to vascular smooth muscle cells and induces cell contraction. Ann. Rheum. Dis. 2012, 71, 596–605. [Google Scholar] [CrossRef]
- Becker, M.O.; Kill, A.; Kutsche, M.; Guenther, J.; Rose, A.; Tabeling, C.; Witzenrath, M.; Kühl, A.A.; Heidecke, H.; Ghofrani, H.A.; et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am. J. Respir. Crit. Care Med. 2014, 190, 808–817. [Google Scholar] [CrossRef]
- Rose, F.; Hattar, K.; Gakisch, S.; Grimminger, F.; Olschewski, H.; Seeger, W.; Tschuschner, A.; Schermuly, R.T.; Weissmann, N.; Hanze, J.; et al. Increased neutrophil mediator release in patients with pulmonary hypertension--suppression by inhaled iloprost. Thromb. Haemost. 2003, 90, 1141–1149. [Google Scholar] [CrossRef]
- Von Nussbaum, F.; Li, V.M.; Meibom, D.; Anlauf, S.; Bechem, M.; Delbeck, M.; Gerisch, M.; Harrenga, A.; Karthaus, D.; Lang, D.; et al. Potent and Selective Human Neutrophil Elastase Inhibitors with Novel Equatorial Ring Topology: In vivo Efficacy of the Polar Pyrimidopyridazine BAY-8040 in a Pulmonary Arterial Hypertension Rat Model. ChemMedChem 2016, 11, 199–206. [Google Scholar] [CrossRef]
- Cowan, K.N.; Heilbut, A.; Humpl, T.; Lam, C.; Ito, S.; Rabinovitch, M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat. Med. 2000, 6, 698–702. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Mortaz, E.; Omar, H.R.; Camporesi, E.M.; Sweiss, N. Association of Neutrophil to Lymphocyte Ratio and Pulmonary Hypertension in Sarcoidosis Patients. Tanaffos 2016, 15, 44–47. [Google Scholar] [PubMed]
- Yanartas, M.; Kalkan, M.E.; Arslan, A.; Tas, S.G.; Koksal, C.; Bekiroglu, N.; Yildizeli, B. Neutrophil/Lymphocyte Ratio Can Predict Postoperative Mortality in Patients with Chronic Thromboembolic Pulmonary Hypertension. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 229–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Özpelit, E.; Akdeniz, B.; Özpelit, M.E.; Tas, S.; Bozkurt, S.; Tertemiz, K.C.; Sevinç, C.; Badak, Ö. Prognostic value of neutrophil-to-lymphocyte ratio in pulmonary arterial hypertension. J. Int. Med. Res. 2015, 43, 661–671. [Google Scholar] [CrossRef]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Aldabbous, L.; Abdul-Salam, V.; McKinnon, T.; Duluc, L.; Pepke-Zaba, J.; Southwood, M.; Ainscough, A.J.; Hadinnapola, C.; Wilkins, M.R.; Toshner, M.; et al. Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2078–2087. [Google Scholar] [CrossRef]
- Foo, S.Y.; Zhang, V.; Lalwani, A.; Lynch, J.P.; Zhuang, A.; Lam, C.E.; Foster, P.S.; King, C.; Steptoe, R.J.; Mazzone, S.B.; et al. Regulatory T cells prevent inducible BALT formation by dampening neutrophilic inflammation. J. Immunol. 2015, 194, 4567–4576. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.H.E.; You, X.-M.; Ciura, S.; Husain, M.; Rabinovitch, M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 2002, 105, 516–521. [Google Scholar] [CrossRef]
- Alkhouri, H.; Poppinga, W.J.; Tania, N.P.; Ammit, A.; Schuliga, M. Regulation of pulmonary inflammation by mesenchymal cells. Pulm. Pharmacol. Ther. 2014, 29, 156–165. [Google Scholar] [CrossRef]
- Kuebler, W.M.; Nicolls, M.R.; Olschewski, A.; Abe, K.; Rabinovitch, M.; Stewart, D.J.; Chan, S.Y.; Morrell, N.W.; Archer, S.L.; Spiekerkoetter, E. A Pro-Con debate: Current Controversies in PAH Pathogenesis at the American Thoracic Society International Meeting in 2017. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 136, 2486. [Google Scholar] [CrossRef]
- Ye, J.-X.; Wang, S.-S.; Ge, M.; Wang, D.-J. Suppression of endothelial PGC-1α is associated with hypoxia-induced endothelial dysfunction and provides a new therapeutic target in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L1233–L1242. [Google Scholar] [CrossRef] [PubMed]
- Cassano, S.; Pucino, V.; La Rocca, C.; Procaccini, C.; De Rosa, V.; Marone, G.; Matarese, G. Leptin modulates autophagy in human CD4+CD25- conventional T cells. Metab. Clin. Exp. 2014, 63, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Budhiraja, R.; Tuder, R.M.; Hassoun, P.M. Endothelial dysfunction in pulmonary hypertension. Circulation 2004, 109, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, N.M.; Kuebler, W.M. Endothelial Cell Regulation of Pulmonary Vascular Tone, Inflammation, and Coagulation. Compr. Physiol. 2015, 5, 531–559. [Google Scholar] [CrossRef]
- Soon, E.; Crosby, A.; Southwood, M.; Yang, P.; Tajsic, T.; Toshner, M.; Appleby, S.; Shanahan, C.M.; Bloch, K.D.; Pepke-Zaba, J.; et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2015, 192, 859–872. [Google Scholar] [CrossRef]
- Song, Y.; Coleman, L.; Shi, J.; Beppu, H.; Sato, K.; Walsh, K.; Loscalzo, J.; Zhang, Y.-Y. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H677–H690. [Google Scholar] [CrossRef]
- Sakamaki, F.; Kyotani, S.; Nagaya, N.; Sato, N.; Oya, H.; Satoh, T.; Nakanishi, N. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation 2000, 102, 2720–2725. [Google Scholar] [CrossRef]
- Sanchez, O.; Marcos, E.; Perros, F.; Fadel, E.; Tu, L.; Humbert, M.; Dartevelle, P.; Simonneau, G.; Adnot, S.; Eddahibi, S. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2007, 176, 1041–1047. [Google Scholar] [CrossRef]
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef]
- Otsuki, S.; Sawada, H.; Yodoya, N.; Shinohara, T.; Kato, T.; Ohashi, H.; Zhang, E.; Imanaka-Yoshida, K.; Shimpo, H.; Maruyama, K.; et al. Potential contribution of phenotypically modulated smooth muscle cells and related inflammation in the development of experimental obstructive pulmonary vasculopathy in rats. PLoS ONE 2015, 10, e0118655. [Google Scholar] [CrossRef]
- Yuan, K.; Shamskhou, E.A.; Orcholski, M.E.; Nathan, A.; Reddy, S.; Honda, H.; Mani, V.; Zeng, Y.; Ozen, M.O.; Wang, L.; et al. Loss of Endothelium-Derived Wnt5a Is Associated With Reduced Pericyte Recruitment and Small Vessel Loss in Pulmonary Arterial Hypertension. Circulation 2019, 139, 1710–1724. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Tu, L.; Le Hiress, M.; Huertas, A.; Phan, C.; Thuillet, R.; Sattler, C.; Fadel, E.; Seferian, A.; Montani, D.; et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 2014, 129, 1586–1597. [Google Scholar] [CrossRef]
- Dreymueller, D.; Martin, C.; Schumacher, J.; Groth, E.; Boehm, J.K.; Reiss, L.K.; Uhlig, S.; Ludwig, A. Smooth muscle cells relay acute pulmonary inflammation via distinct ADAM17/ErbB axes. J. Immunol. 2014, 192, 722–731. [Google Scholar] [CrossRef]
- Novoyatleva, T.; Kojonazarov, B.; Owczarek, A.; Veeroju, S.; Rai, N.; Henneke, I.; Böhm, M.; Grimminger, F.; Ghofrani, H.A.; Seeger, W.; et al. Evidence for the Fucoidan/P-Selectin Axis as a Therapeutic Target in Hypoxia-induced Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2019, 199, 1407–1420. [Google Scholar] [CrossRef]
- Horita, H.; Furgeson, S.B.; Ostriker, A.; Olszewski, K.A.; Sullivan, T.; Villegas, L.R.; Levine, M.; Parr, J.E.; Cool, C.D.; Nemenoff, R.A.; et al. Selective inactivation of PTEN in smooth muscle cells synergizes with hypoxia to induce severe pulmonary hypertension. J. Am. Heart Assoc. 2013, 2, e000188. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Yeager, M.E.; El Kasmi, K.C.; Nozik-Grayck, E.; Gerasimovskaya, E.V.; Li, M.; Riddle, S.R.; Frid, M.G. The adventitia: Essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 2013, 75, 23–47. [Google Scholar] [CrossRef]
- Kitamura, H.; Cambier, S.; Somanath, S.; Barker, T.; Minagawa, S.; Markovics, J.; Goodsell, A.; Publicover, J.; Reichardt, L.; Jablons, D.; et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Invest. 2011, 121, 2863–2875. [Google Scholar] [CrossRef]
- Buckley, C.D. Why does chronic inflammation persist: An unexpected role for fibroblasts. Immunol. Lett. 2011, 138, 12–14. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Pugliese, S.C.; Riddle, S.R.; Poth, J.M.; Anderson, A.L.; Frid, M.G.; Li, M.; Pullamsetti, S.S.; Savai, R.; Nagel, M.A.; et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J. Immunol. 2014, 193, 597–609. [Google Scholar] [CrossRef]
- Li, M.; Riddle, S.R.; Frid, M.G.; El Kasmi, K.C.; McKinsey, T.A.; Sokol, R.J.; Strassheim, D.; Meyrick, B.; Yeager, M.E.; Flockton, A.R.; et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J. Immunol. 2011, 187, 2711–2722. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Michelakis, E.D. The metabolic theory of pulmonary arterial hypertension. Circ. Res. 2014, 115, 148–164. [Google Scholar] [CrossRef]
- Boucherat, O.; Vitry, G.; Trinh, I.; Paulin, R.; Provencher, S.; Bonnet, S. The cancer theory of pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 285–299. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chi, L.; Kuebler, W.M.; Goldenberg, N.M. Perivascular Inflammation in Pulmonary Arterial Hypertension. Cells 2020, 9, 2338. https://doi.org/10.3390/cells9112338
Hu Y, Chi L, Kuebler WM, Goldenberg NM. Perivascular Inflammation in Pulmonary Arterial Hypertension. Cells. 2020; 9(11):2338. https://doi.org/10.3390/cells9112338
Chicago/Turabian StyleHu, Yijie, Leon Chi, Wolfgang M Kuebler, and Neil M Goldenberg. 2020. "Perivascular Inflammation in Pulmonary Arterial Hypertension" Cells 9, no. 11: 2338. https://doi.org/10.3390/cells9112338
APA StyleHu, Y., Chi, L., Kuebler, W. M., & Goldenberg, N. M. (2020). Perivascular Inflammation in Pulmonary Arterial Hypertension. Cells, 9(11), 2338. https://doi.org/10.3390/cells9112338





