Anti-Müllerian Hormone Type II Receptor Expression in Endometrial Cancer Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Tissue Microarrays (TMAs)
2.3. Immunohistochemistry (IHC)
2.4. Other Definitions
2.5. Statistical Analysis
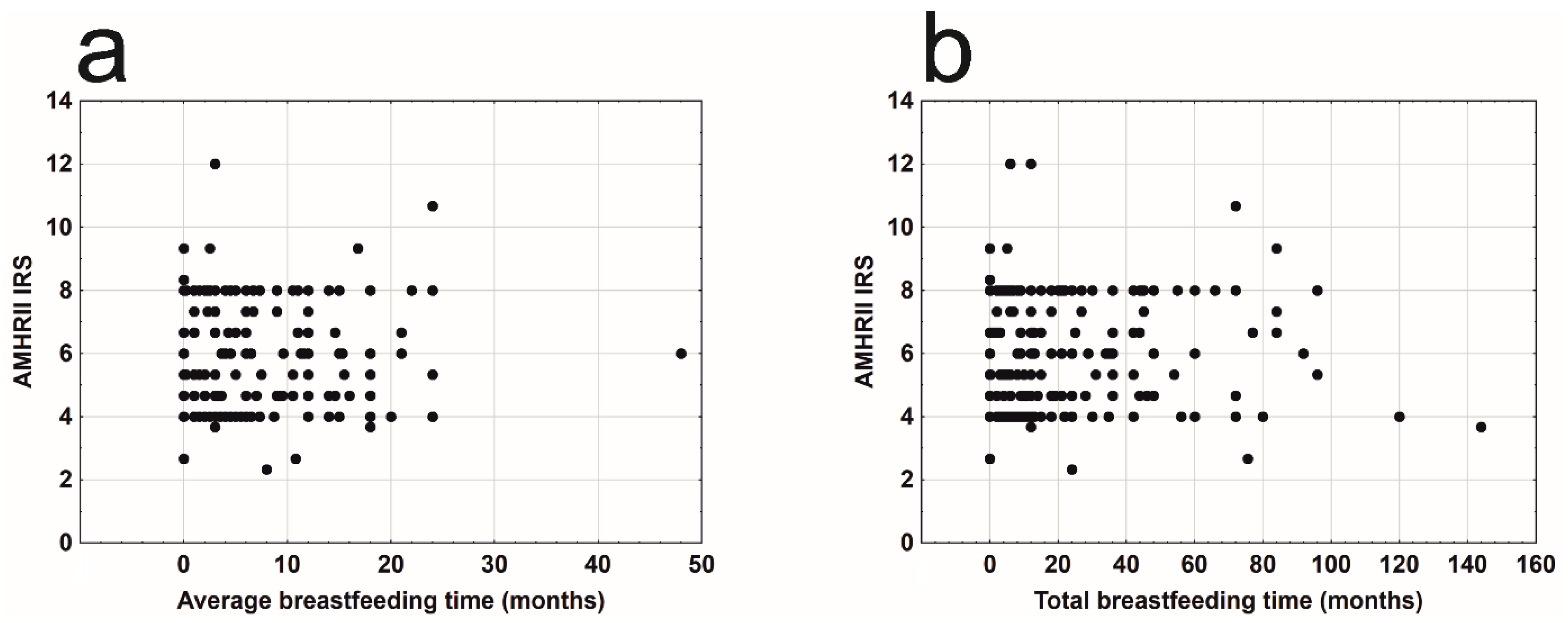
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- MacLaughlin, D.T.; Teixeira, J.; Donahoe, P.K. Perspective: Reproductive tract development—New discoveries and future directions. Endocrinology 2001, 142, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Josso, N. WOMEN IN REPRODUCTIVE SCIENCE: Anti-Müllerian hormone: A look back and ahead. Reproduction 2019, 158, F81–F89. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; MacLaughlin, D.T.; Donahoe, P.K. Müllerian inhibiting substance/anti-Müllerian hormone: A novel treatment for gynecologic tumors. Obstet. Gynecol. Sci. 2014, 57, 343–357. [Google Scholar] [CrossRef]
- Visser, J.A. AMH signaling: From receptor to target gene. Mol. Cell. Endocrinol. 2003, 211, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Belville, C.; Jamin, S.P.; Picard, J.-Y.; Josso, N.; Di Clemente, N. Role of type I receptors for anti-Müllerian hormone in the SMAT-1 Sertoli cell line. Oncogene 2005, 24, 4984–4992. [Google Scholar] [CrossRef] [PubMed]
- Goder, V.; Spiess, M. Topogenesis of membrane proteins: Determinants and dynamics. FEBS Lett. 2001, 504, 87–93. [Google Scholar] [CrossRef]
- Park, S.H.; Chung, Y.J.; Song, J.Y.; Kim, S.I.; Pépin, D.; MacLaughlin, D.T.; Donahoe, P.K.; Kim, J.H. Müllerian inhibiting substance inhibits an ovarian cancer cell line via β-catenin interacting protein deregulation of the Wnt signal pathway. Int. J. Oncol. 2017, 50, 1022–1028. [Google Scholar] [CrossRef][Green Version]
- Barbie, T.U.; Barbie, D.A.; MacLaughlin, D.T.; Maheswaran, S.; Donahoe, P.K. Mullerian Inhibiting Substance inhibits cervical cancer cell growth via a pathway involving p130 and p107. Proc. Natl. Acad. Sci. USA 2003, 100, 15601–15606. [Google Scholar] [CrossRef]
- Namkung, J.; Song, J.Y.; Jo, H.H.; Kim, M.R.; Lew, Y.O.; Donahoe, P.K.; MacLaughlin, D.T.; Kim, J.H. Müllerian Inhibiting Substance Induces Apoptosis of Human Endometrial Stromal Cells in Endometriosis. J. Clin. Endocrinol. Metab. 2012, 97, 3224–3230. [Google Scholar] [CrossRef]
- Segev, D.L.; Ha, T.U.; Tran, T.T.; Kenneally, M.; Harkin, P.; Jung, M.; MacLaughlin, D.T.; Donahoe, P.K.; Maheswaran, S. Müllerian Inhibiting Substance Inhibits Breast Cancer Cell Growth through an NFκB-mediated Pathway. J. Biol. Chem. 2000, 275, 28371–28379. [Google Scholar] [CrossRef]
- Hoshiya, Y.; Gupta, V.; Segev, R.L.; Hoshiya, M.; Carey, J.L.; Sasur, L.M.; Tran, T.T.; Ha, T.U.; Maheswaran, S. Mullerian Inhibiting Substance induces NFkB signaling in breast and prostate cancer cells. Mol. Cell. Endocrinol. 2003, 211, 43–49. [Google Scholar] [CrossRef]
- Parry, R.L.; Chin, T.W.; Epstein, J.; Hudson, P.L.; Powell, D.M.; Donahoe, P.K. Recombinant human mullerian inhibiting substance inhibits human ocular melanoma cell lines in vitro and in vivo. Cancer Res. 1992, 52, 1182–1186. [Google Scholar] [PubMed]
- Pieretti-Vanmarcke, R.; Donahoe, P.K.; Pearsall, L.A.; Dinulescu, D.M.; Connolly, D.C.; Halpern, E.F.; Seiden, M.V.; MacLaughlin, D.T. Mullerian Inhibiting Substance enhances subclinical doses of chemotherapeutic agents to inhibit human and mouse ovarian cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 17426–17431. [Google Scholar] [CrossRef]
- Siegel, R.; Miller, K.D.; Jemal, A. Cancer statistics 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, Y.O.; Lee, M.K.; Chung, Y.J.; Jeung, I.C.; Kim, M.R.; Kim, J.H. Müllerian inhibiting substance/anti-Müllerian hormone type II receptor protein and mRNA expression in the healthy and cancerous endometria. Oncol. Lett. 2018, 17, 532–538. [Google Scholar] [CrossRef]
- Deshayes, E.; Ladjohounlou, R.; Le Fur, P.; Pichard, A.; Lozza, C.; Boudousq, V.; Sevestre, S.; Jarlier, M.; Kashani, R.; Koch, J.; et al. Radiolabeled Antibodies Against Müllerian-Inhibiting Substance Receptor, Type II: New Tools for a Theranostic Approach in Ovarian Cancer. J. Nucl. Med. 2018, 59, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Bakkum-Gamez, J.N.; Aletti, G.D.; Lewis, K.A.; Keeney, G.L.; Thomas, B.M.; Navarro-Teulon, I.; Cliby, W.A. Müllerian inhibiting substance type II receptor (MISIIR): A novel, tissue-specific target expressed by gynecologic cancers. Gynecol. Oncol. 2008, 108, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Green, A.K.; Wenham, R.M.; Mutch, D.M.; Davidson, B.A.; Miller, D.S. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecol. Oncol. Res. Pract. 2017, 4, 19. [Google Scholar] [CrossRef]
- Gowkielewicz, M.; Lipka, A.; Piotrowska, A.; Szadurska-Noga, M.; Nowakowski, J.J.; Dziegiel, P.; Majewski, M.K.; Jozwik, M.; Majewska, M. Anti-Müllerian Hormone Expression in Endometrial Cancer Tissue. Int. J. Mol. Sci. 2019, 20, 1325. [Google Scholar] [CrossRef]
- Remmele, W. Hans-Egon Stegner Recommendation for uniform definition of an immunoreactive Score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- CareExcellence, N.I. Menopause; National Collaborating Centre for Women’s and Children’s Health (UK): London, UK, 2015. [Google Scholar]
- Shaw, E.; Farris, M.; McNeil, J.; Friedenreich, C.M. Obesity and Endometrial Cancer. Recent Results Cancer Res. 2016, 208, 107–136. [Google Scholar] [CrossRef]
- Chia, V.M.; Newcomb, P.A.; Hampton, J.M.; Trentham-Dietz, A. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int. J. Gynecol. Cancer 2007, 17, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Schwartzbaum, J.; Hulka, B.S.; Fowler, W.C.; Kaufman, D.G.; Hoberman, D. The influence of exogenous estrogen use on survival after diagnosis of endometrial cancer. Am. J. Epidemiol. 1987, 126, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.-T.; Wang, Y.-L.; Ma, X.-X. Age at menarche and endometrial cancer risk: A dose-response meta-analysis of prospective studies. Sci. Rep. 2015, 5, 14051. [Google Scholar] [CrossRef]
- van Dongen, G.A.M.S.; Visser, G.W.; Hooge, M.N.L.-D.; De Vries, E.G.; Perk, L.R. Immuno-PET: A Navigator in Monoclonal Antibody Development and Applications. Oncologist 2007, 12, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Chekol, R.; Solomon, V.R.; Alizadeh, E.; Bernhard, W.; Fisher, D.; Hill, W.; Barreto, K.; DeCoteau, J.F.; Parada, A.C.; Geyer, C.R.; et al. 89Zr-nimotuzumab for immunoPET imaging of epidermal growth factor receptor I. Oncotarget 2018, 9, 17117–17132. [Google Scholar] [CrossRef]
- Chung, C.H.; Ely, K.; McGavran, L.; Varella-Garcia, M.; Parker, J.; Parker, N.; Jarrett, C.; Carter, J.; Murphy, B.A.; Netterville, J.; et al. Increased Epidermal Growth Factor Receptor Gene Copy Number Is Associated With Poor Prognosis in Head and Neck Squamous Cell Carcinomas. J. Clin. Oncol. 2006, 24, 4170–4176. [Google Scholar] [CrossRef]
- Bensch, F.; Smeenk, M.M.; Van Es, S.C.; De Jong, J.R.; Schröder, C.P.; Oosting, S.F.; Lub-de Hooge, M.N.; van der Houven, C.W.M.; Brouwers, A.H.; Boellaard, R.; et al. Comparative biodistribution analysis across four different 89Zr-monoclonal antibody tracers—The first step towards an imaging warehouse. Theranostics 2018, 8, 4295–4304. [Google Scholar] [CrossRef]
- Ravishankar, S.; Mangray, S.; Kurkchubasche, A.; Yakirevich, E.; Young, R.H. Unusual Sertoli Cell Tumor Associated with Sex Cord Tumor With Annular Tubules in Peutz-Jeghers Syndrome. Int. J. Surg. Pathol. 2015, 24, 269–273. [Google Scholar] [CrossRef]
- Reyes-Muñoz, E.; Sathyapalan, T.; Rossetti, P.; Shah, M.; Long, M.; Buscema, M.; Valenti, G.; La Rosa, V.L.; Cianci, S.; Vitale, S.G. Polycystic Ovary Syndrome: Implication for Drug Metabolism on Assisted Reproductive Techniques—A Literature Review. Adv. Ther. 2018, 35, 1805–1815. [Google Scholar] [CrossRef]
- Renaud, E.J.; MacLaughlin, D.T.; Oliva, E.; Rueda, B.R.; Donahoe, P.K. Endometrial cancer is a receptor-mediated target for Mullerian Inhibiting Substance. Proc. Natl. Acad. Sci. USA 2005, 102, 111–116. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta Gene Regul. Mech. 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Ao, Z.; Prasad, K.V.; Wu, R.; Scholssman, S.F. IEX -1L, an apoptosis inhibitor involved in NF- kappaB – Mediated Cell Survival. Science 1998, 281, 998–1001. [Google Scholar] [CrossRef]
- Ge, W.; Clendenen, T.V.; Afanasyeva, Y.; Koenig, K.L.; Agnoli, C.; Brinton, L.A.; Dorgan, J.F.; Eliassen, A.H.; Falk, R.T.; Hallmans, G.; et al. Circulating anti-Müllerian hormone and breast cancer risk: A study in ten prospective cohorts. Int. J. Cancer 2018, 142, 2215–2226. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, Z. Anti-Mullerian hormone and breast cancer risk—Is the correlation possibly associated with the PCOS? Int. J. Cancer 2019, 144, 211. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Kim, C.; Karvonen-Gutierrez, C.; Kong, S.; Arends, V.; Steffes, M.; McConnell, D.S.; Randolph, J.F.; Harlow, S.D. Antimüllerian hormone among women with and without type 1 diabetes: The Epidemiology of Diabetes Interventions and Complications Study and the Michigan Bone Health and Metabolism Study. Fertil. Steril. 2016, 106, 1446–1452. [Google Scholar] [CrossRef]
- Dewailly, D.; Gronier, H.; Poncelet, E.; Robin, G.; Leroy, M.; Pigny, P.; Duhamel, A.; Catteau-Jonard, S. Diagnosis of polycystic ovary syndrome (PCOS): Revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum. Reprod. 2011, 26, 3123–3129. [Google Scholar] [CrossRef]
- De Kat, A.C.; Gremmels, H.; Verhaar, M.C.; Broekmans, F.J.M.; Yarde, F. Early Vascular Damage in Young Women with DM-1 and Its Relation to Anti-Müllerian Hormone: A Cross-Sectional Study. Int. J. Endocrinol. 2016, 2016. [Google Scholar] [CrossRef]
- Getz, G.; Gabriel, S.B.; Cibulskis, K.; Lander, E.; Sivachenko, A.; Sougnez, C.; Lawrence, M.; Kandoth, C.; Dooling, D.; Fulton, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
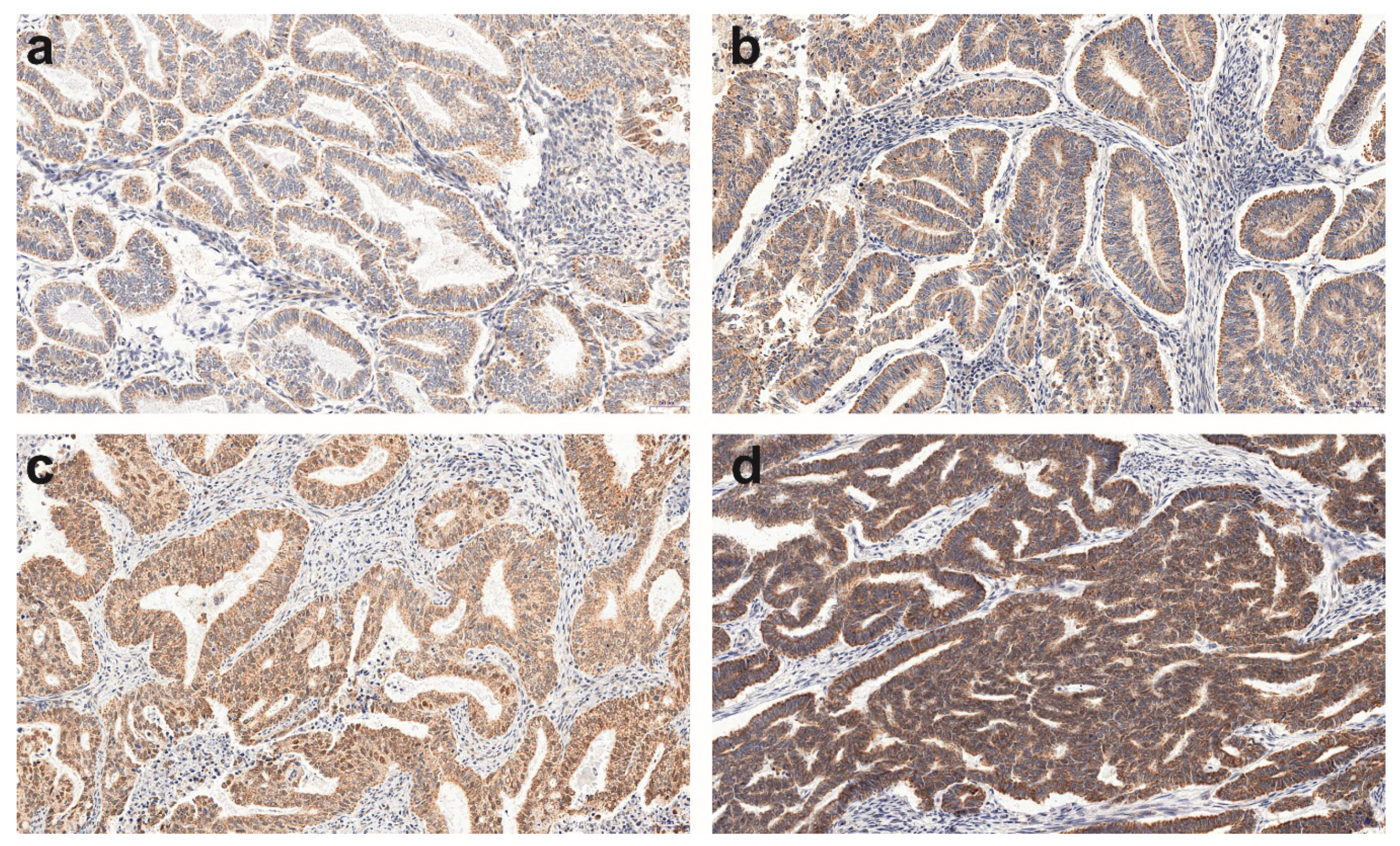
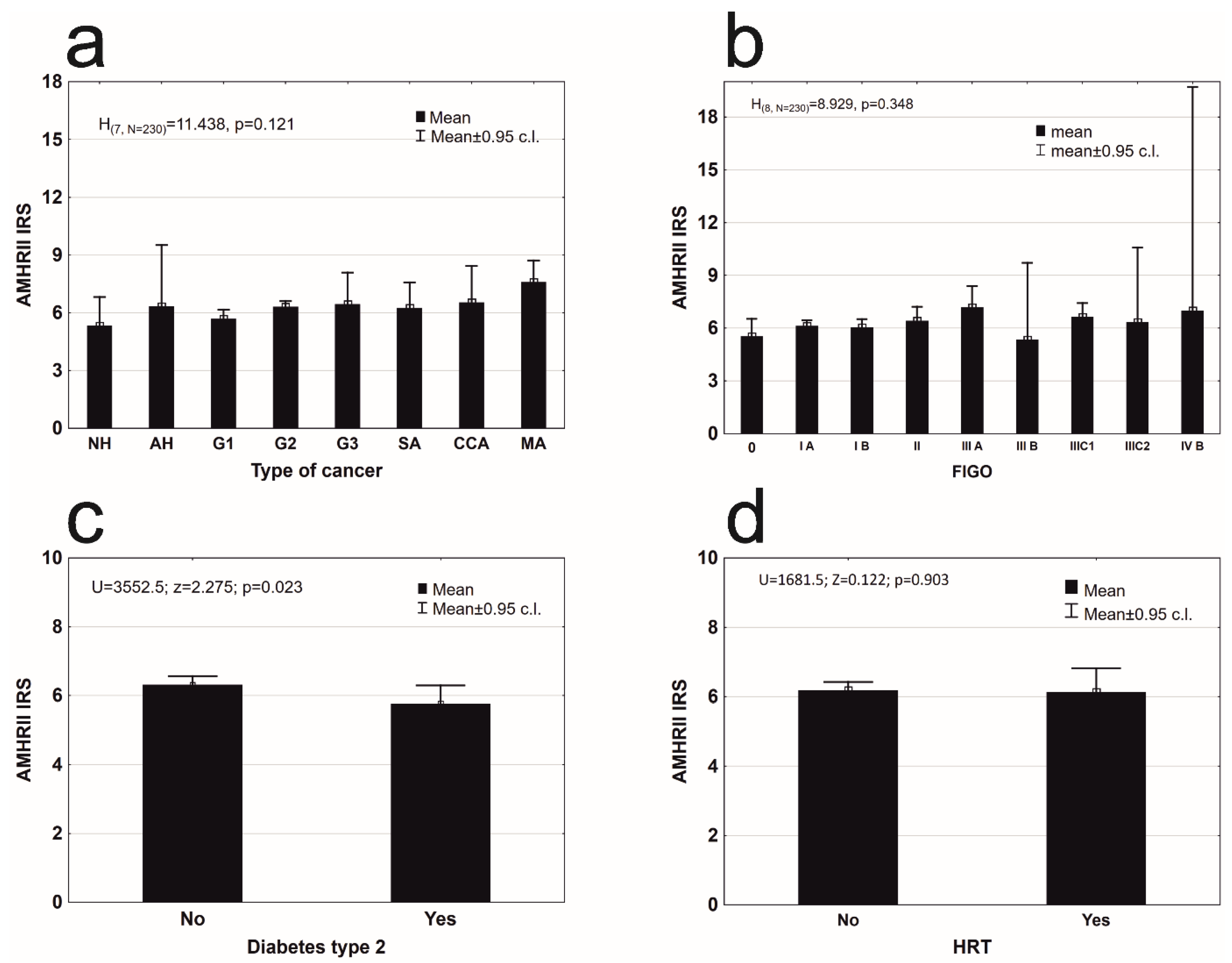
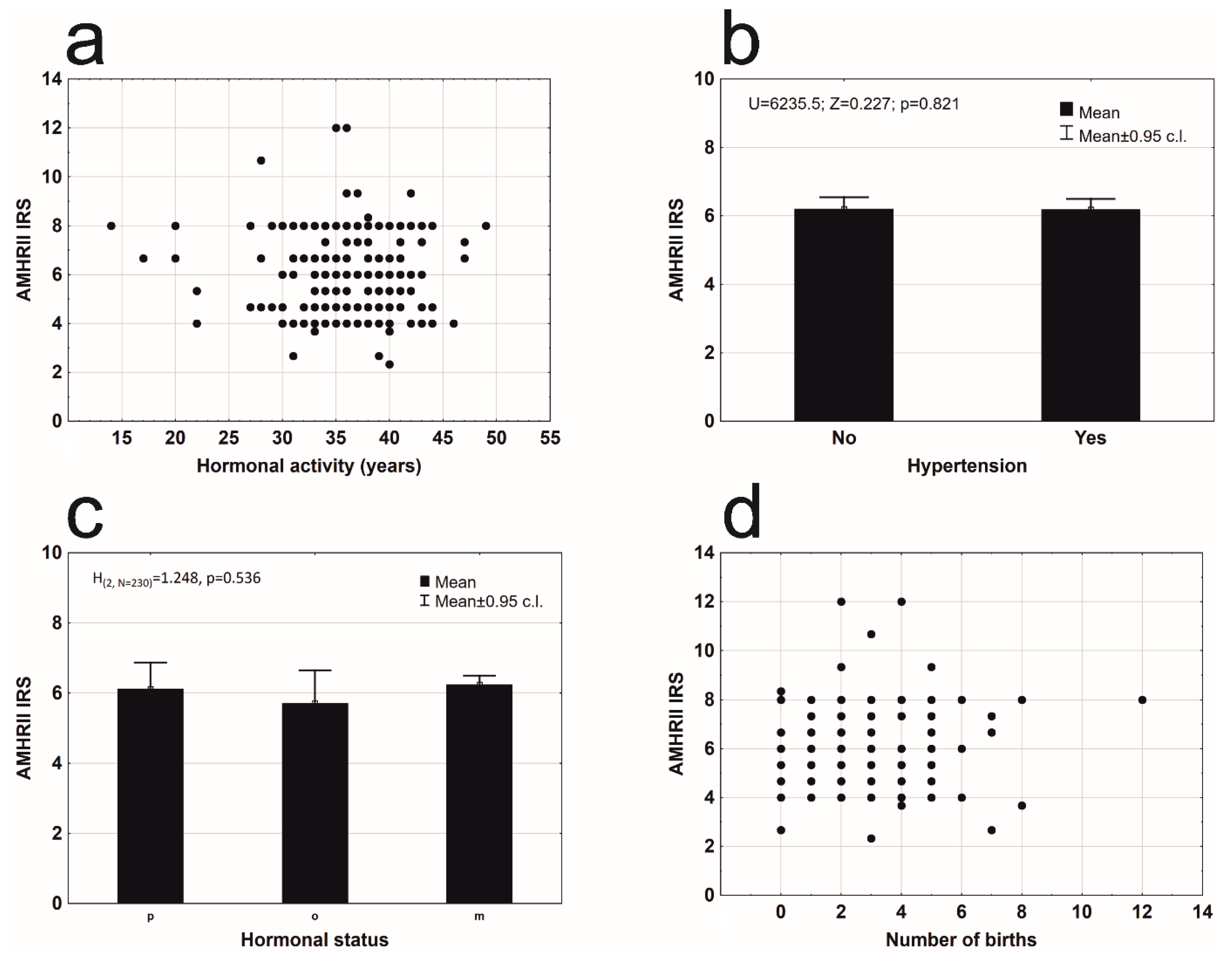
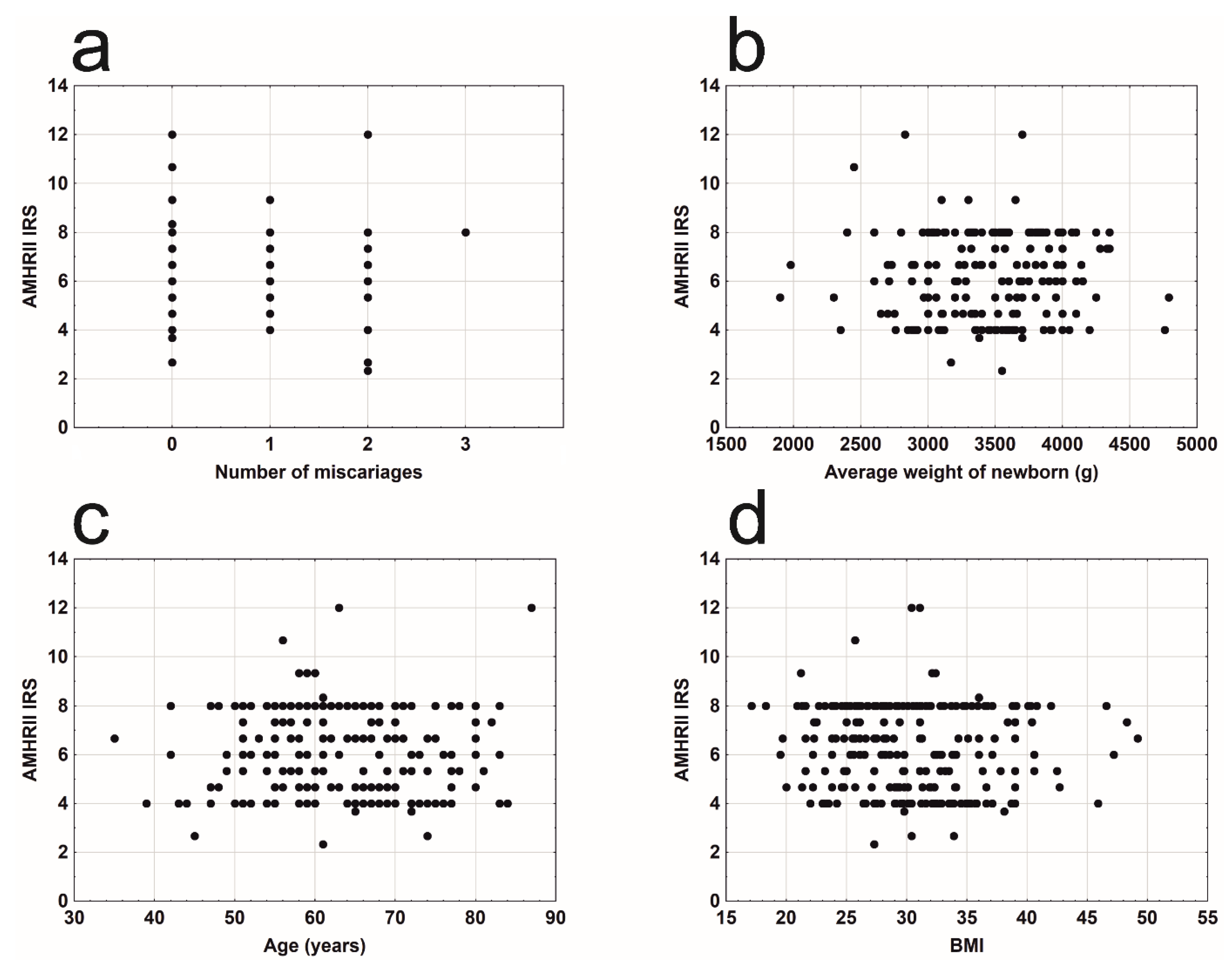
| Histopathological Type of Endometrial Lesion | Patients Number | Mean ± SD | 95% C.l. | Q1–Q2–Q3 | Min–Max | |
|---|---|---|---|---|---|---|
| precancerous state (PCS) | Nonatypical endometrial hyperplasia (NH) | 8 | 5.33 ± 1.782 | 3.84–6.82 | 4.0–4.33–7.0 | 4.00–8.00 |
| Atypical hyperplasia (AH) | 4 | 6.33 ± 2.000 | 3.15–9.52 | 4.67–6.67–8.0 | 4.00–8.00 | |
| type 1 according Bokhman’s | Endometrioid adenocarcinoma G1 (G1) | 49 | 5.69 ± 1.645 | 5.21–6.16 | 4.0–5.33–7.33 | 2.67–8.00 |
| Endometrioid adenocarcinoma G2 (G2) | 146 | 6.32 ± 1.801 | 6.03–6.62 | 4.67–6.67–8.0 | 2.33–12.00 | |
| Endometrioid adenocarcinoma G3 (G3) | 6 | 6.44 ± 1.559 | 4.81–8.08 | 4.67–6.67–8.0 | 4.67–8.00 | |
| type 2 according Bokhman’s | Serous adenocarcinoma (SA) | 8 | 6.25 ± 1.591 | 4.92–7.58 | 4.0–4.33–7.0 | 4.00–8.00 |
| Clear cell adenocarcinoma (CCA) | 4 | 6.83 ± 1.575 | 4.33–9.34 | 5.67–7.33–8.0 | 4.67–8.00 | |
| Mixed adenocarcinoma (MA) | 5 | 7.60 ± 0.894 | 6.49–8.71 | 8.0–8.0–8.0 | 6.00–8.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gowkielewicz, M.; Lipka, A.; Majewska, M.; Piotrowska, A.; Szadurska-Noga, M.; Nowakowski, J.J.; Wiszpolska, M.; Dzięgiel, P.; Wasniewski, T.; Majewski, M.K.; et al. Anti-Müllerian Hormone Type II Receptor Expression in Endometrial Cancer Tissue. Cells 2020, 9, 2312. https://doi.org/10.3390/cells9102312
Gowkielewicz M, Lipka A, Majewska M, Piotrowska A, Szadurska-Noga M, Nowakowski JJ, Wiszpolska M, Dzięgiel P, Wasniewski T, Majewski MK, et al. Anti-Müllerian Hormone Type II Receptor Expression in Endometrial Cancer Tissue. Cells. 2020; 9(10):2312. https://doi.org/10.3390/cells9102312
Chicago/Turabian StyleGowkielewicz, Marek, Aleksandra Lipka, Marta Majewska, Aleksandra Piotrowska, Marta Szadurska-Noga, Jacek J. Nowakowski, Marta Wiszpolska, Piotr Dzięgiel, Tomasz Wasniewski, Mariusz Krzysztof Majewski, and et al. 2020. "Anti-Müllerian Hormone Type II Receptor Expression in Endometrial Cancer Tissue" Cells 9, no. 10: 2312. https://doi.org/10.3390/cells9102312
APA StyleGowkielewicz, M., Lipka, A., Majewska, M., Piotrowska, A., Szadurska-Noga, M., Nowakowski, J. J., Wiszpolska, M., Dzięgiel, P., Wasniewski, T., Majewski, M. K., & Jozwik, M. (2020). Anti-Müllerian Hormone Type II Receptor Expression in Endometrial Cancer Tissue. Cells, 9(10), 2312. https://doi.org/10.3390/cells9102312






