Cytotoxicity and Mitochondrial Dysregulation Caused by α-Synuclein in Dictyostelium discoideum
Abstract
1. Introduction
2. Materials and Methods
2.1. Dictyostelium Strains, Culture Conditions and Development
2.2. Plasmid Construction
2.3. Transformation
2.4. Calculation of Construct Copy Numbers
2.4.1. Quantitative Southern Blotting
2.4.2. Quantitative PCR
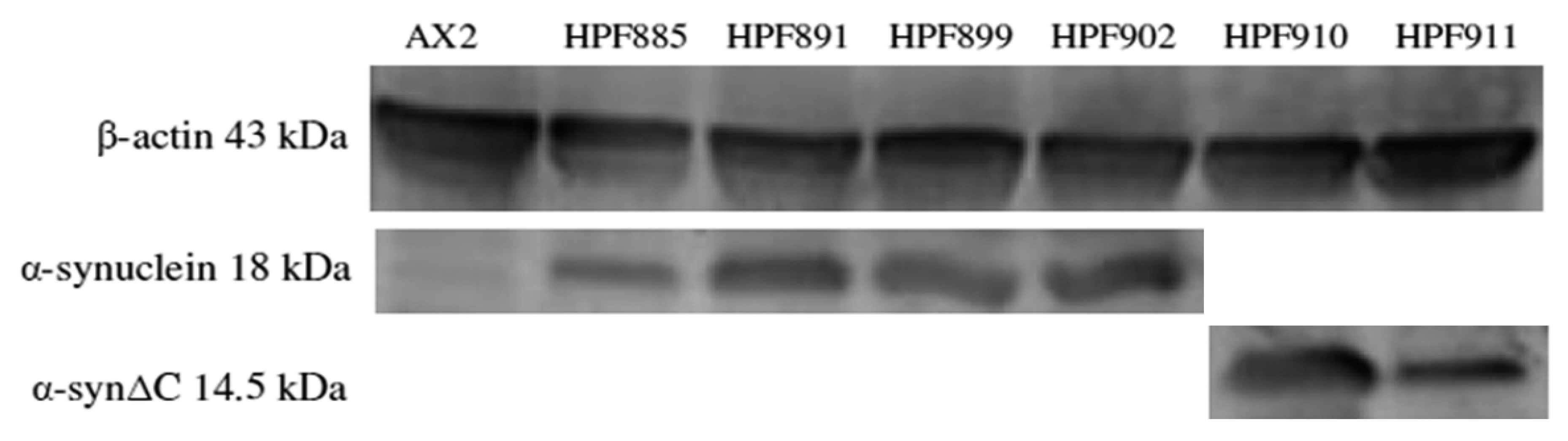
2.5. Western Blotting
2.6. Growth in Axenic Medium
2.7. Growth on Bacterial Lawns
2.8. Phagocytosis Assay
2.9. Pinocytosis Assay
2.10. Phototaxis and Thermotaxis Assays
2.11. Immunofluorescence
2.12. Seahorse Respirometry
3. Results
3.1. Human α-Synuclein Can Be Expressed in D. discoideum
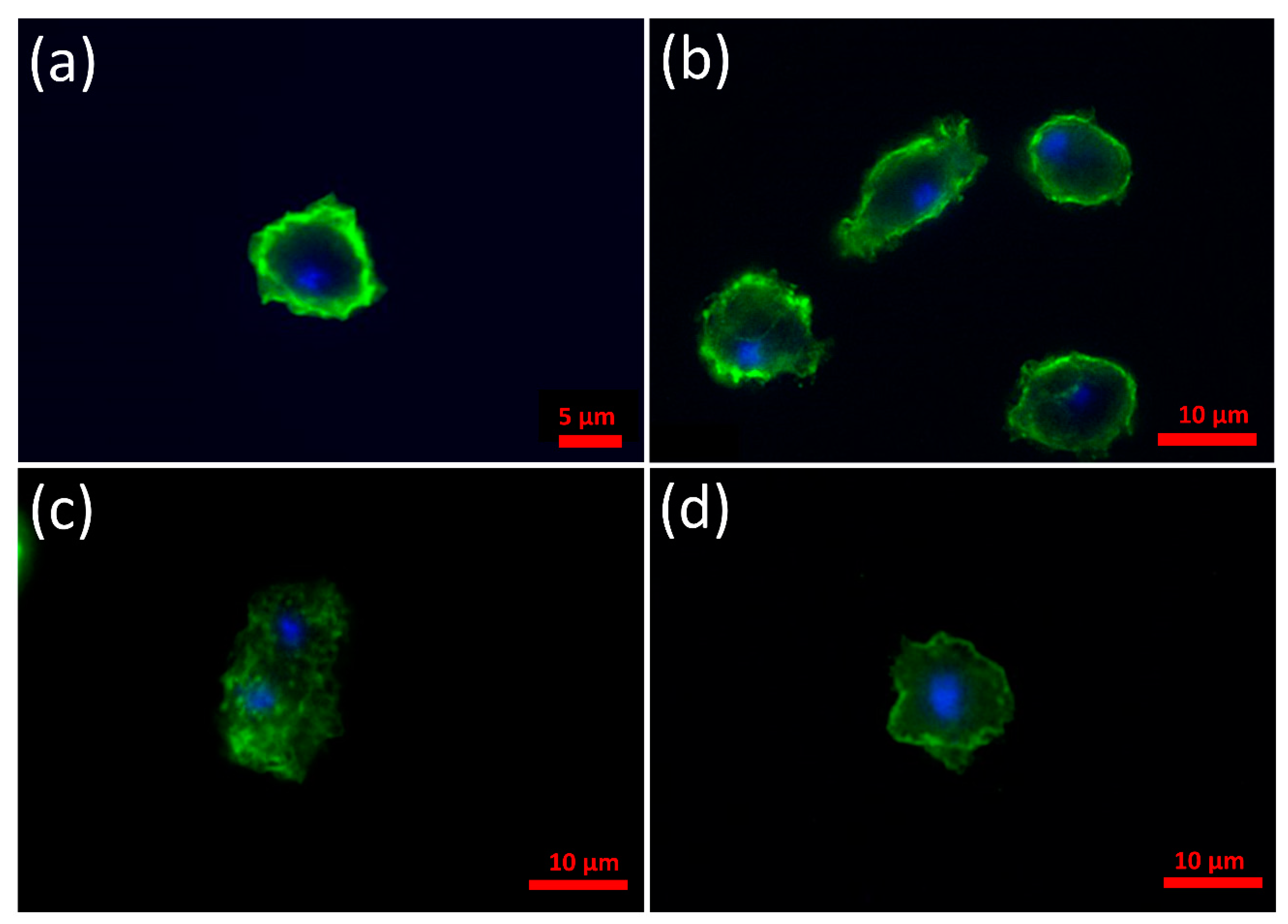
3.2. α-Synuclein Localizes to the Cell Cortex in D. discoideum and the C-Terminus Is Necessary and Sufficient for This to Occur
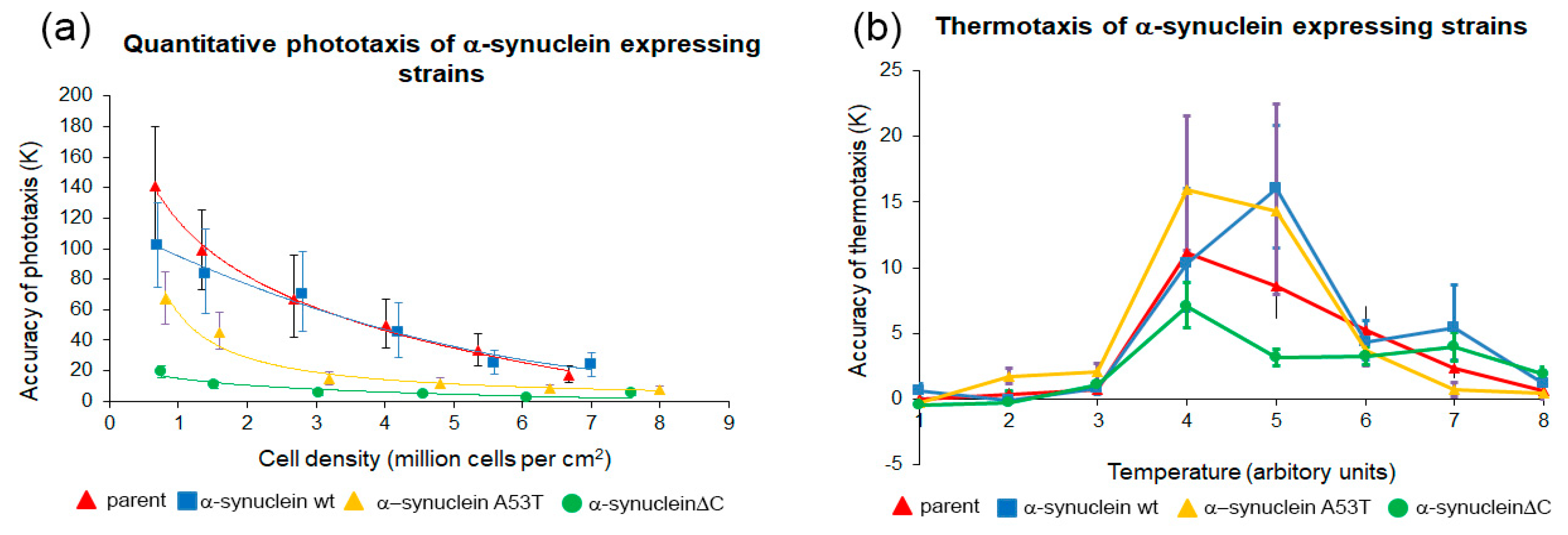
3.3. Mutant α-Synuclein Negatively Affects Phototaxis and Thermotaxis
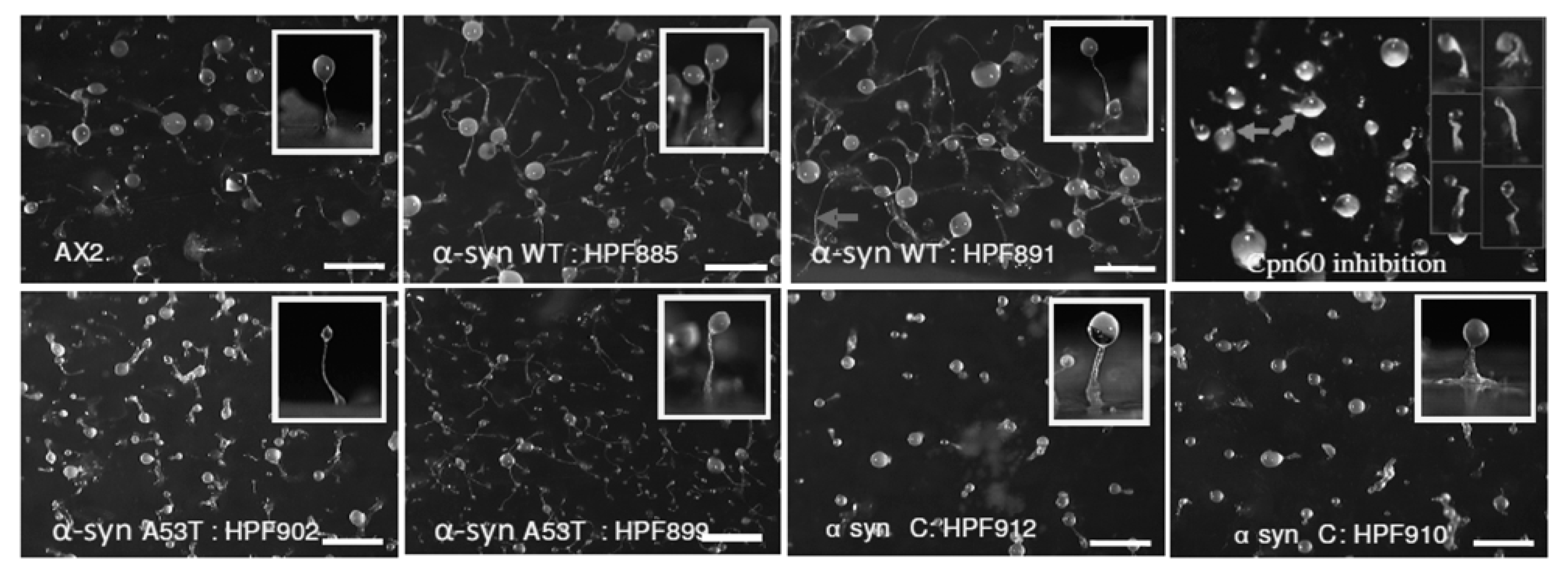
3.4. Strains Expressing C-Terminally Truncated α-Synuclein Display Aberrant Fruiting Bodies
3.5. α-Synuclein Impairs Growth on Plates but Not in Liquid Medium
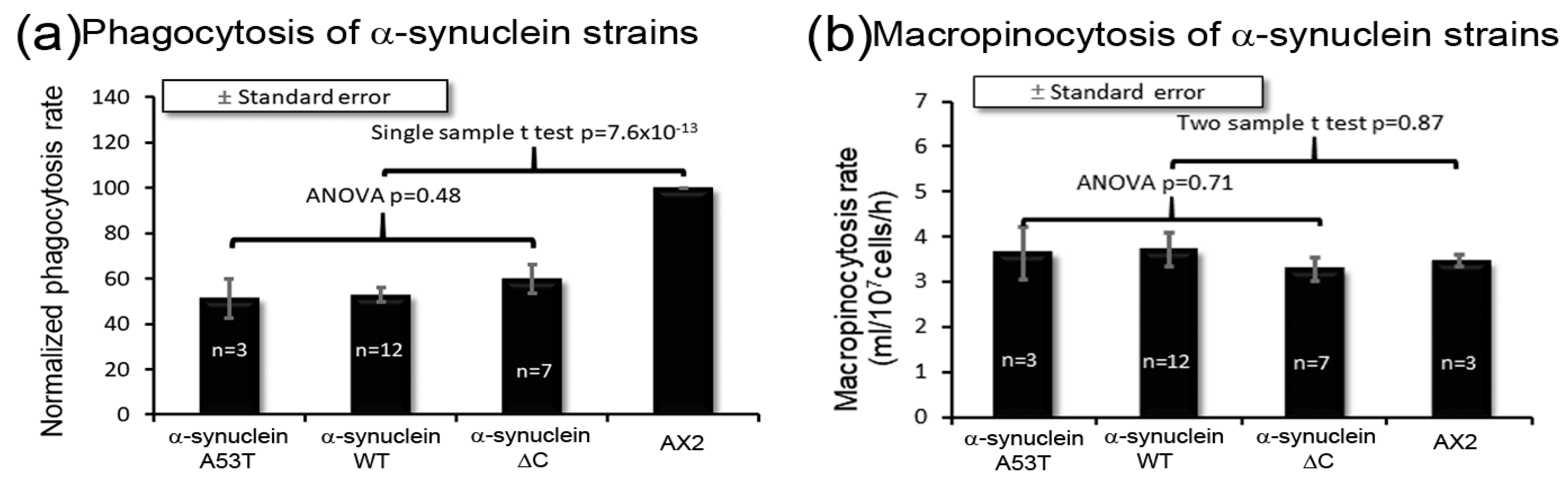
3.6. α-Synuclein Causes an Impairment of Phagocytosis, Which Results in Reduced Growth Rates on Bacterial Lawns
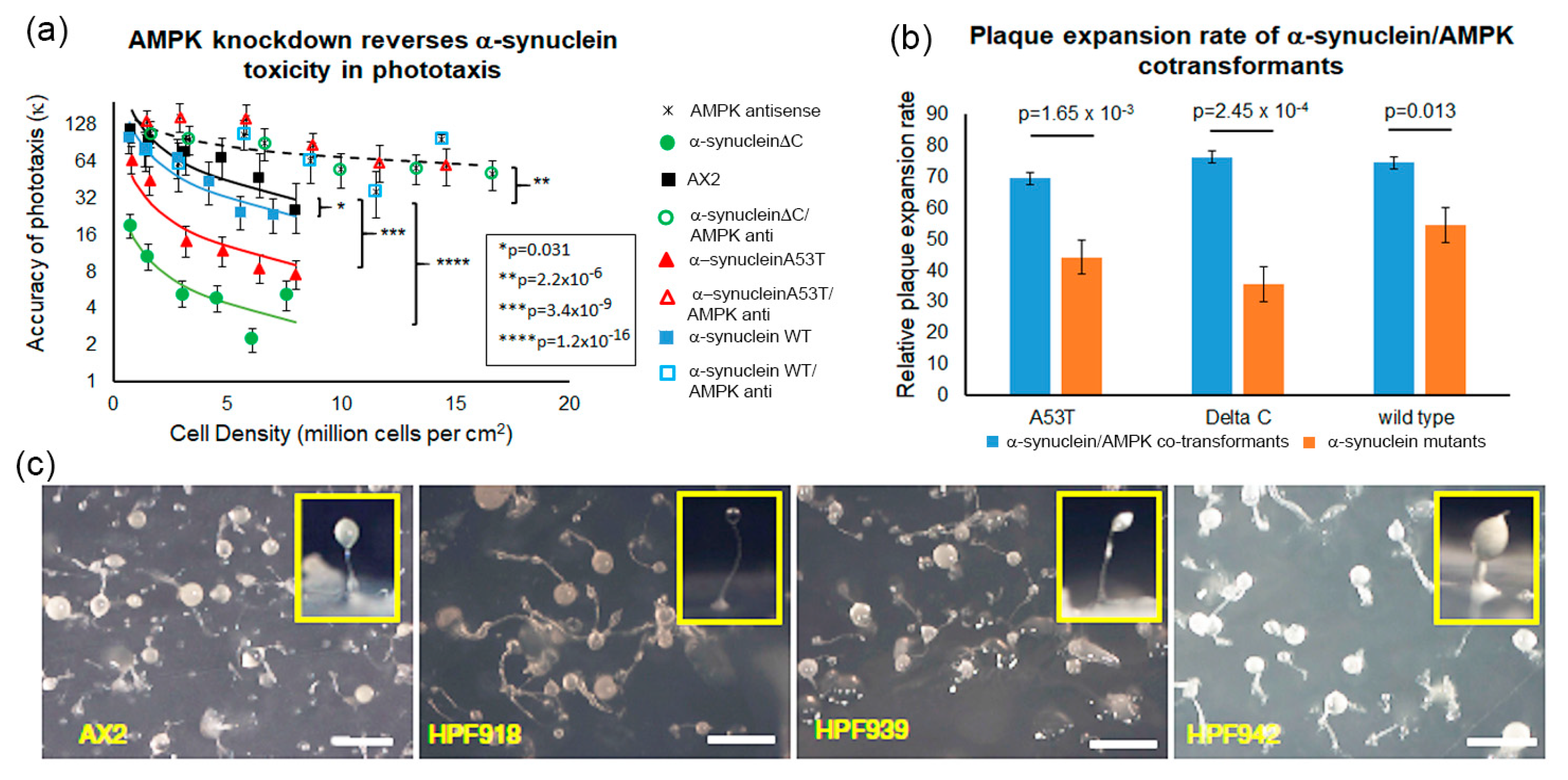
3.7. AMPK-Dependent and AMPK-Independent Pathways
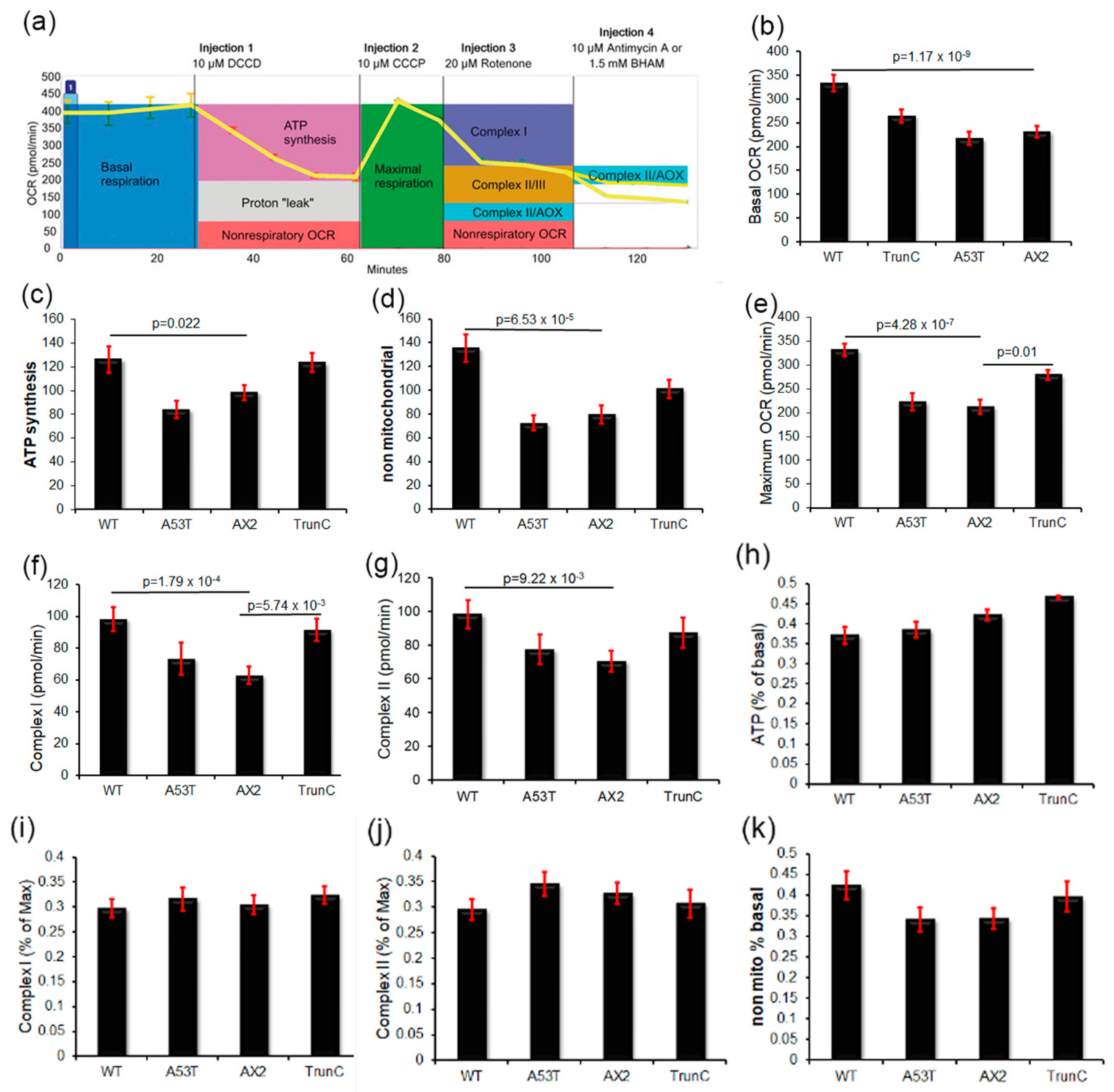
3.8. Seahorse Respirometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
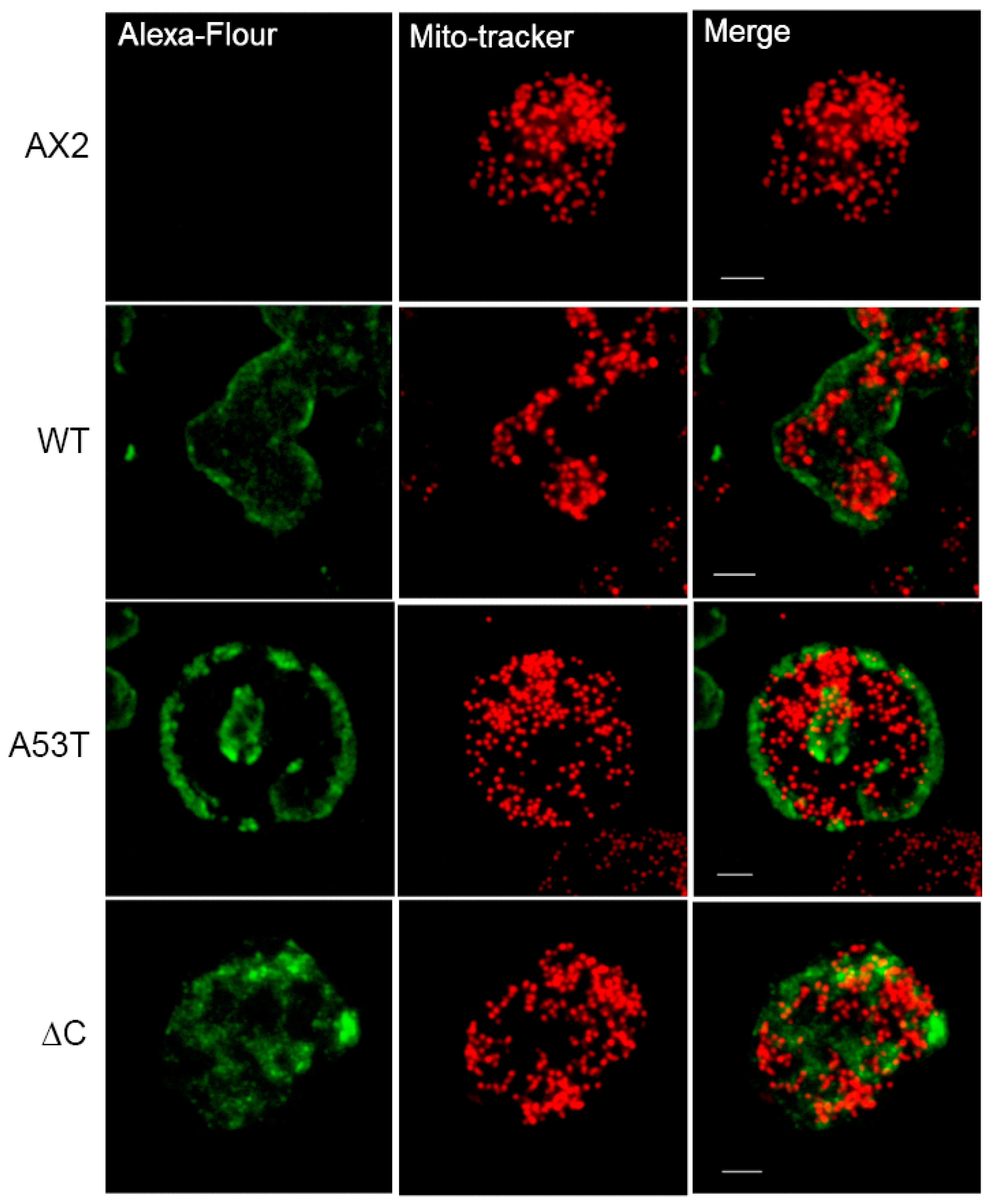
Appendix A. α-Synuclein Does Not Colocalise with Mitochondria
References
- Stichel, C.C.; Zhu, X.R.; Bader, V.; Linnartz, B.; Schmidt, S.; Lubbert, H. Mono- and double-mutant mouse models of Parkinson’s disease display severe mitochondrial damage. Hum. Mol. Genet. 2007, 16, 2377–2393. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 2013, 47, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Leverenz, J.B.; Umar, I.; Wang, Q.; Montine, T.J.; McMillan, P.J.; Tsuang, D.W.; Jin, J.; Pan, C.; Shin, J.; Zhu, D.; et al. Proteomic identification of novel proteins in cortical lewy bodies. Brain Pathol. 2007, 17, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Flagmeier, P.; Meisl, G.; Vendruscolo, M.; Knowles, T.P.; Dobson, C.M.; Buell, A.K.; Galvagnion, C. Mutations associated with familial Parkinson’s disease alter the initiation and amplification steps of alpha-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2016, 113, 10328–10333. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. Alpha-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Paik, S.R.; Yang, C.-H. Structural and Functional Implications of C-Terminal Regions of R-Synuclein†. Biochemistry 2002, 41, 13782–13790. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; West, N.; Colla, E.; Pletnikova, O.; Troncoso, J.C.; Marsh, L.; Dawson, T.M.; Jäkälä, P.; Hartmann, T.; Price, D.L.; et al. Aggregation promoting C-terminal truncation of α-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc. Natl. Acad. Sci. USA 2005, 102, 2162–2167. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nguyen, L.T.; Burlak, C.; Chegini, F.; Guo, F.; Chataway, T.; Ju, S.; Fisher, O.S.; Miller, D.W.; Datta, D.; et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein alpha-synuclein. Proc. Natl. Acad. Sci. USA 2016, 113, 9587–9592. [Google Scholar] [CrossRef] [PubMed]
- Periquet, M.; Fulga, T.; Myllykangas, L.; Schlossmacher, M.G.; Feany, M.B. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J. Neurosci. 2007, 27, 3338–3346. [Google Scholar] [CrossRef] [PubMed]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Iwai, A.; Masliah, E.; Yoshimoto, M.; Ge, N.; Fianagan, L.; de Silva, H.A.R.; Kittei, A.; Saitoh, T. The precursor protein of non-ap component of Alzheimer’s Disease Amyloid is a presynaptic protein of the central nervous system. Neuron 1995, 14, 467–475. [Google Scholar] [CrossRef]
- Masliah, E.; Iwai, A.; Mallory, M.; Uéda, K.; Saitoh, T. Altered presynaptic protein NACP is associated with plaque formation and neurodegeneration in Alzheimer’s disease. Am. J. Patbol. 1996, 148, 201–210. [Google Scholar]
- Hurtig, H.I.; Trojanowski, J.Q.; Galvin, J.; Ewbank, D.; Schmidt, M.L.; Lee, V.M.-Y.; Clark, C.M.; Glosser, G.; Stern, M.B.; Gollomp, S.M.; et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology 2000, 54, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.B.; Murphy, D.D.; Grider, T.; Rueter, S.; Brasaemle, D.; Nussbaum, R.L. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein alpha-synuclein. J. Biol. Chem. 2002, 277, 6344–6352. [Google Scholar] [CrossRef] [PubMed]
- McLean, P.J.; Kawamata, H.; Ribich, S.; Hyman, B.T. Membrane Association and Protein Conformation of α-Synuclein in Intact Neurons. J. Biol. Chem. 2000, 275, 8812–8816. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.B.; Dieuliis, D.; Leo, P.; Mitchell, D.C.; Nussbaum, R.L. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp. Cell. Res. 2008, 314, 2076–2089. [Google Scholar] [CrossRef]
- Devi, L.; Raghavendran, V.; Prabhu, B.M.; Avadhani, N.G.; Anandatheerthavarada, H.K. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 2008, 283, 9089–9100. [Google Scholar] [CrossRef]
- Parihar, M.S.; Parihar, A.; Fujita, M.; Hashimoto, M.; Ghafourifar, P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol. Life Sci. 2008, 65, 1272–1284. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Zhu, Y.; Cai, Q.; Chan, P.; Ueda, K.; Yu, S.; Yang, H. Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: An immunogold electron microscopic study using a C-terminal specific monoclonal antibody. Brain Res. 2008, 1244, 40–52. [Google Scholar] [CrossRef]
- Zigoneanu, I.G.; Yang, Y.J.; Krois, A.S.; Haque, E.; Pielak, G.J. Interaction of alpha-synuclein with vesicles that mimic mitochondrial membranes. Biochim. Biophys. Acta 2012, 1818, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Yang, R.; Guo, J.-C.; Ren, H.-M.; Zha, X.-L.; Cheng, J.-S.; Cai, D.-F. Localization of a-synuclein to mitochondria within midbrain of mice. Clin. Neurosci. Neuropathol. 2007, 18, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.R. The biochemistry of Parkinson’s disease. Annu. Rev. Biochem. 2005, 74, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Dawson, T.M.; Dawson, V.L. Molecular Pathways of Neurodegeneration in Parkinson’s Disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef] [PubMed]
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef]
- Lu, M.; Su, C.; Qiao, C.; Bian, Y.; Ding, J.; Hu, G. Metformin prevents dopaminergic neuron death in MPTP/P-Induced mouse model of Parkinson’s Disease via Autophagy and Mitochondrial ROS Clearance. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef]
- Bayliss, J.A.; Lemus, M.B.; Santos, V.V.; Deo, M.; Davies, J.S.; Kemp, B.E.; Elsworth, J.D.; Andrews, Z.B. Metformin prevents nigrostriatal dopamine degeneration independent of AMPK activation in dopamine neurons. PLoS ONE 2016, 11, e0159381. [Google Scholar] [CrossRef]
- Curry, D.W.; Stutz, B.; Andrews, Z.B.; Elsworth, J.D. Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s Disease. J. Parkinsons Dis. 2018, 8, 161–181. [Google Scholar] [CrossRef]
- Kang, H.; Khang, R.; Ham SJo, M.; Lee, B.D.; Lee, Y.I.; Jo, A.; Park, C.; Kim, H.; Seo, J.; Paek, S.H.; et al. Activation of the ATF2/CREB-PGC-1α pathway by metformin leads to dopaminergic neuroprotection. Oncotarget 2017, 8, 48603. [Google Scholar] [CrossRef] [PubMed]
- Dulovic, M.; Jovanovic, M.; Xilouri, M.; Stefanis, L.; Harhaji-Trajkovic, L.; Kravic-Stevovic, T.; Paunovic, V.; Ardah, M.T.; El-Agnaf, O.M.; Kostic, V.; et al. The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiol. Dis. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Ogawa, S.; Yoshino, R.; Angata, K.; Iwamoto, M.; Pi, M.; Kuroe, K.; Matsuo, K.; Morio, T.; Urushihara, H.; Yanagisawa, K.; et al. The mitochondrial DNA of Dictyostelium discoideum: Complete sequence, gene content and genome organization. Mol. Gen. Genet. 2000, 263, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, L.; Pachebat, J.A.; Glöckner, G.; Rajandream, M.A.; Sucgang, R.; Berriman, M.; Song, J.; Olsen, R.; Szafranski, K.; Xu, Q.; et al. The genome of the social amoeba Dictyostelium discoideum. Nature 2005, 435, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Francione, L.M.; Fisher, P.R. Heteroplasmic mitochondrial disease in Dictyostelium discoideum. Biochem. Pharmacol. 2011, 82, 1510–1520. [Google Scholar] [CrossRef]
- Bokko, P.B.; Francione, L.; Bandala-Sanchez, E.; Ahmed, A.U.; Annesley, S.J.; Huang, X.; Khurana, T.; Kimmel, A.R.; Fisher, P.R. Diverse cytopathologies in mitochondrial disease are caused by AMP-activated protein kinase signaling. Mol. Biol. Cell. 2007, 18, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.; Compton, K.; Cox, E.C. Green fluorescent protein production in the cellular slime molds Polysphondylium pallidurn and Dictyostelium discoideum. Genes (Basel) 1995, 165, 127–130. [Google Scholar]
- Nellen, W.; Silan, C.; Firtel, R.A. DNA-Mediated Transformation in Dictyostelium discoideum: Regulated Expression of an Actin Gene Fusion. Mol. Cell. Biol. 1984, 4, 2890–2898. [Google Scholar] [CrossRef]
- Wilczynska, Z.; Fisher, P.R. Analysis of a complex plasmid insertion in a phototaxis-deficient transformant of Dictyostelium discoideum selected on a Micrococcus luteus lawn. Plasmid 1994, 32, 182–194. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vietnam, Austria, 2020. [Google Scholar]
- Maselli, A.; Laevsky, G.; Knecht, D.A. Kinetics of binding, uptake and degradation of live fluorescent (DsRed) bacteria by Dictyostelium discoideum. Microbiology 2002, 148, 413–420. [Google Scholar] [CrossRef]
- Klein, G.; Satre, M. Kinetics of fluid-phase pinocytosis in dictyostelium discoideum amoebae. Biochem. Biophys. Res. Commun. 1986, 138, 1146–1152. [Google Scholar] [CrossRef]
- Annesley, S.J.; Fisher, P.R. Dictyostelium slug phototaxis. Methods Mol. Biol. 2009, 571, 67–76. [Google Scholar] [PubMed]
- Fisher, P.R.; Smith, E.; Williams, K.L. An extracellular chemical signal controlling phototactic behavior by D. discoideum slugs. Cell 1981, 23, 799–807. [Google Scholar] [CrossRef]
- Lay, S.; Sanislav, O.; Annesley, S.J.; Fisher, P.R. Mitochondrial Stress Tests Using Seahorse Respirometry on Intact Dictyostelium discoideum Cells. Methods Mol. Biol. 2016, 1407, 41–61. [Google Scholar]
- Barth, C.; Fraser, D.J.; Fisher, P.R. Co-insertional Replication Is Responsible for Tandem Multimer Formation during Plasmid Integration into the Dictyostelium Genome. Plasmid 1998, 39, 141–153. [Google Scholar] [CrossRef]
- Kotsifas, M.; Barth, C.; de Lozanne, A.; Lay, S.T.; Fisher, P.R. Chaperonin 60 and mitochondrial disease in Dictyostelium. J. Muscle Res. Cell Motil. 2002, 23, 839–852. [Google Scholar] [CrossRef]
- Wilczynska, Z.; Barth, C.; Fisher, P.R. Mitochondrial Mutations Impair Signal Transduction in Dictyostelium discoideum Slugs. Biochem. Biophys. Res. Commun. 1997, 234, 39–43. [Google Scholar] [CrossRef]
- Darcy, P.K.; Wilczynska, Z.; Fisher, P.R. Genetic analysis of dictyostelium slug phototaxis mutants. Genetics 1994, 137, 977–985. [Google Scholar]
- Mishizen-Eberz, A.J.; Guttmann, R.P.; Giasson, B.I.; Day, G.A., 3rd; Hodara, R.; Ischiropoulos, H.; Lee, V.M.; Trojanowski, J.Q.; Lynch, D.R. Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J. Neurochem. 2003, 86, 836–847. [Google Scholar] [CrossRef]
- Dufty, B.M.; Warner, L.R.; Hou, S.T.; Jiang, S.X.; Gomez-Isla, T.; Leenhouts, K.M.; Oxford, J.T.; Feany, M.B.; Masliah, E.; Rohn, T.T. Calpain-cleavage of alpha-synuclein: Connecting proteolytic processing to disease-linked aggregation. Am. J. Pathol. 2007, 170, 1725–1738. [Google Scholar] [CrossRef]
- Michell, A.W.; Tofaris, G.K.; Gossage, H.; Tyers, P.; Spillantini, M.G.; Barker, R.A. The effect of truncated human α-synuclein (1–120) on dopaminergic cells in a transgenic mouse model of Parkinson’s Disease. Cell Transplant. 2007, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.; Yang, S.; Sauchanka, O.; Spillantini, M.G.; Anichtchik, O. Behavioural deficits in transgenic mice expressing human truncated (1–120 amino acid) alpha-synuclein. Exp. Neurol. 2015, 264, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Tofaris, G.K.; Reitbock, P.G.; Humby, T.; Lambourne, S.L.; O’Connell, M.; Ghetti, B.; Gossage, H.; Emson, P.C.; Wilkinson, L.S.; Goedert, M.; et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): Implications for Lewy body disorders. J. Neurosci. 2006, 26, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Annesley, S.J.; Jasim, R.A.F.; Musco, V.J.; Sanislav, O.; Fisher, P.R. The Parkinson’s disease-associated protein DJ-1 plays a positive nonmitochondrial role in endocytosis in Dictyostelium cells. Dis. Model Mech. 2017, 10, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, C.L.; Annesley, S.J.; Gordon, S.E.; Mroczek, K.; Perugini, M.A.; Lawson, V.A.; Fisher, P.R.; Finkelstein, D.I.; Hill, A.F. Misfolded alpha-synuclein causes hyperactive respiration without functional deficit in live neuroblastoma cells. Dis. Model Mech. 2020, 13. [Google Scholar] [CrossRef]
- Annesley, S.J.; Lay, S.T.; de Piazza, S.W.; Sanislav, O.; Hammersley, E.; Allan, C.Y.; Francione, L.M.; Bui, M.Q.; Chen, Z.P.; Ngoei, K.R.; et al. Immortalized Parkinson’s disease lymphocytes have enhanced mitochondrial respiratory activity. Dis. Model Mech. 2016, 9, 1295–1305. [Google Scholar] [CrossRef]
- Bartels, T.; Ahlstrom, L.S.; Leftin, A.; Kamp, F.; Haass, C.; Brown, M.F.; Beyer, A.K. The N-Terminus of the Intrinsically Disordered Protein a-Synuclein Triggers Membrane Binding and Helix Folding. Biophys. J. 2010, 99, 2116–2124. [Google Scholar] [CrossRef]
- Dixon, C.; Mathias, N.; Zweig, R.M.; Davis, D.A.; Gross, D.S. Alpha-synuclein targets the plasma membrane via the secretory pathway and induces toxicity in yeast. Genetics 2005, 170, 47–59. [Google Scholar] [CrossRef]
- Outeiro, T.F. Yeast Cells Provide Insight into Alpha-Synuclein Biology and Pathobiology. Science 2003, 302, 1772–1775. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Hao, S.; Hu, H.; Wang, C.C. Translocation of alpha-synuclein expressed in Escherichia coli. J. Bacteriol. 2007, 189, 2777–2786. [Google Scholar] [CrossRef]
- McFarland, M.A.; Ellis, C.E.; Markey, S.P.; Nussbaum, R.L. Proteomics analysis identifies phosphorylation-dependent alpha-synuclein protein interactions. Mol. Cell. Proteom. 2008, 7, 2123–2137. [Google Scholar] [CrossRef]
- Lautenschlager, J.; Stephens, A.D.; Fusco, G.; Strohl, F.; Curry, N.; Zacharopoulou, M.; Michel, C.H.; Laine, R.; Nespovitaya, N.; Fantham, M.; et al. C-terminal calcium binding of alpha-synuclein modulates synaptic vesicle interaction. Nat. Commun. 2018, 9, 712. [Google Scholar] [CrossRef]
- Hejjaoui, M.; Butterfield, S.; Fauvet, B.; Vercruysse, F.; Cui, J.; Dikiy, I.; Prudent, M.; Olschewski, D.; Zhang, Y.; Eliezer, D.; et al. Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: Alpha-synuclein phosphorylation at tyrosine 125. J. Am. Chem. Soc. 2012, 134, 5196–5210. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, W.; Cherny, D.; Subramaniam, V.; Jovin, T.M. Impact of the Acidic C-Terminal Region Comprising Amino Acids 109-140 on R-Synuclein Aggregation in Vitro†. Biochemistry 2004, 43, 16233–16242. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.H.; Hager, H.; Nielsen, M.S.; Højrup, P.; Gliemann, J.; Jakes, R. alpha-Synuclein Binds to Tau and Stimulates the Protein Kinase A-catalyzed Tau Phosphorylation of Serine Residues 262 and 356*. J. Biol. Chem. 1999, 274, 25481–25489. [Google Scholar] [CrossRef]
- Paik, S.R.; Shin, H.-J.; Lee, J.-H.; Chang, C.-S.; Kim, J. Copper(II)-induced self-oligomerization of α-synuclein. Biochem. J. 1999, 340, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.; Stephens, S.; Titus, M.A.; Chisholm, R.L. SadA, a novel adhesion receptor in Dictyostelium. J. Cell Biol. 2002, 159, 1109–1119. [Google Scholar] [CrossRef]
- Cornillon, S.; Froquet, R.; Cosson, P. Involvement of Sib proteins in the regulation of cellular adhesion in Dictyostelium discoideum. Eukaryot. Cell 2008, 7, 1600–1605. [Google Scholar] [CrossRef]
- Gebbie, L.; Benghezal, M.; Cornillon, S.; Froquet, R.; Cherix, N.; Malbouyres, M.; Lefkir, Y.; Grangeasse, C.; Fache, S.; Dalous, J.; et al. Phg2, a kinase involved in adhesion and focal site modeling in Dictyostelium. Mol. Biol. Cell 2004, 15, 3915–3925. [Google Scholar] [CrossRef]
- Seastone, D.J.; Lee, E.; Bush, J.; Knecht, D.; Cardelli, J. Overexpression of a Novel Rho Family GTPase, RacC, Induces Unusual Actin-based Structures and Positively Affects Phagocytosis in Dictyostelium discoideum. Mol. Biol. Cell 1998, 9, 2891–2904. [Google Scholar] [CrossRef]
- Seastone, D.J.; Zhang, L.; Buczynski, G.; Rebstein, P.; Weeks, G.; Spiegelman, G.; Cardelli, J. The Small Mr Ras-like GTPase Rap1 and the Phospholipase C Pathway Act to Regulate Phagocytosis in Dictyostelium discoideum. Mol. Biol. Cell 1999, 10, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Temesvari, L.; Zhang, L.; Fodera, B.; Janssen, K.-P.; Schleicher, M.; Cardelli, J.A. Inactivation of lmpA, Encoding a LIMPII-related Endosomal Protein, Suppresses the Internalization and Endosomal Trafficking Defects in Profilin-null Mutants. Mol. Biol. Cell 2000, 11, 2019–2031. [Google Scholar] [CrossRef]
- Titus, M.A. A class VII unconventional myosin is required for phagocytosis. Curr. Biol. 1999, 9, 1297–1303. [Google Scholar] [CrossRef]
- Haenseler, W.; Zambon, F.; Lee, H.; Vowles, J.; Rinaldi, F.; Dugga, G.; Houlden, H.; Gwinn, K.; Wray, S.; Luk, K.C.; et al. Excess α-synuclein compromises phagocytosis in iPSC-derived macrophages. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Gardai, S.J.; Mao, W.; Schüle, B.; Babcock, M.; Schoebel, S.; Lorenzana, C.; Alexander, J.; Kim, S.; Glick, H.; Hilton, K.; et al. Elevated alpha-synuclein impairs innate immune cell function andprovides a potential peripheral biomarker for Parkinson’s disease. PLoS ONE 2013, 8, e71634. [Google Scholar] [CrossRef]
- Rojanathammanee, L.; Murphy, E.J.; Combs, C.K. Expression of mutant alpha-synuclein modulates microglial phenotype in vitro. J. Neuroinflamm. 2011, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.B.; Devos, S.L.; Kfoury, N.; Li, M.; Jacks, R.; Yanamandra, K.; Ouidja, M.O.; Brodsky, F.M.; Marasa, J.; Bagchi, D.P.; et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA 2013, 110, E3138–E3147. [Google Scholar] [CrossRef]
- Fitzner, D.; Schnaars, M.; Van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.-K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 1, 447–458. [Google Scholar] [CrossRef]
- Francione, L.; Smith, P.K.; Accari, S.L.; Taylor, P.E.; Bokko, P.B.; Bozzaro, S.; Beech, P.L.; Fisher, P.R. Legionella pneumophila multiplication is enhanced by chronic AMPK signalling in mitochondrially diseased Dictyostelium cells. Dis. Model Mech. 2009, 2, 479–489. [Google Scholar] [CrossRef]
- Salt, I.P.; Johnson, G.; Ashcroft, S.J.H.; Hardie, D.G. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β cells, and may regulate insulin release. Biochem. J. 1998, 335, 533–539. [Google Scholar] [CrossRef]
- Zhang, C.S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.W.; Zhu, M.; et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 2017, 548, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Rafaeloff-Phail, R.; Ding, L.; Conner, L.; Yeh, W.-K.; McClure, D.; Guo, H.; Emerson, K.; Brooks, H. Biochemical Regulation of Mammalian AMP-activated Protein Kinase Activity by NAD and NADH. J. Biol. Chem. 2004, 279, 52934–52939. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Hayashi, T.; Miyamoto, L.; Yonemitsu, S.; Nakano, M.; Tanaka, S.; Ebihara, K.; Masuzaki, H.; Hosoda, K.; Inoue, G.; et al. Possible involvement of the α1 isoform of 5’AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E166–E173. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Vidydhara, D.J.; Lee, J.E.; Chandra, S.S. Role of the endolysosomal system in Parkinson’s disease. J. Neurochem. 2019, 150, 487–506. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, S.; Allan, C.Y.; Mroczek, K.; Pearce, X.; Sanislav, O.; Fisher, P.R.; Annesley, S.J. Cytotoxicity and Mitochondrial Dysregulation Caused by α-Synuclein in Dictyostelium discoideum. Cells 2020, 9, 2289. https://doi.org/10.3390/cells9102289
Fernando S, Allan CY, Mroczek K, Pearce X, Sanislav O, Fisher PR, Annesley SJ. Cytotoxicity and Mitochondrial Dysregulation Caused by α-Synuclein in Dictyostelium discoideum. Cells. 2020; 9(10):2289. https://doi.org/10.3390/cells9102289
Chicago/Turabian StyleFernando, Sanjanie, Claire Y. Allan, Katelyn Mroczek, Xavier Pearce, Oana Sanislav, Paul R. Fisher, and Sarah J. Annesley. 2020. "Cytotoxicity and Mitochondrial Dysregulation Caused by α-Synuclein in Dictyostelium discoideum" Cells 9, no. 10: 2289. https://doi.org/10.3390/cells9102289
APA StyleFernando, S., Allan, C. Y., Mroczek, K., Pearce, X., Sanislav, O., Fisher, P. R., & Annesley, S. J. (2020). Cytotoxicity and Mitochondrial Dysregulation Caused by α-Synuclein in Dictyostelium discoideum. Cells, 9(10), 2289. https://doi.org/10.3390/cells9102289






