Interplay between Podoplanin, CD44s and CD44v in Squamous Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Skin TPA Treatment and Chemical Carcinogenesis
2.2. Cell Culture
2.3. Plasmids and Cell Transfections
2.4. RT–PCR
2.5. Immunofluorescence and Confocal Microscopy
2.6. Western Blot and Co-Immunoprecipitation Experiments
3. Results
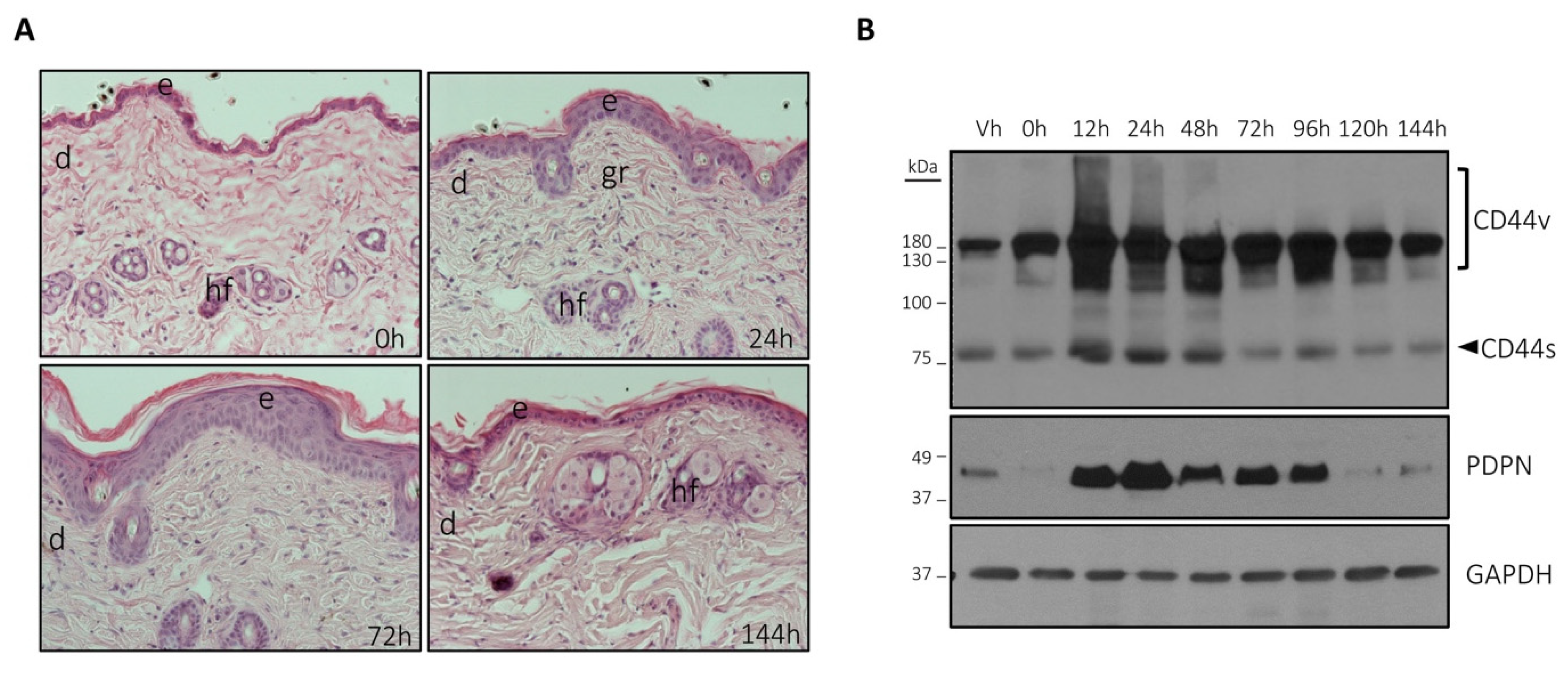
3.1. The Expression of Podoplanin and CD44 Is Coordinately Induced by TPA in Mouse Skin
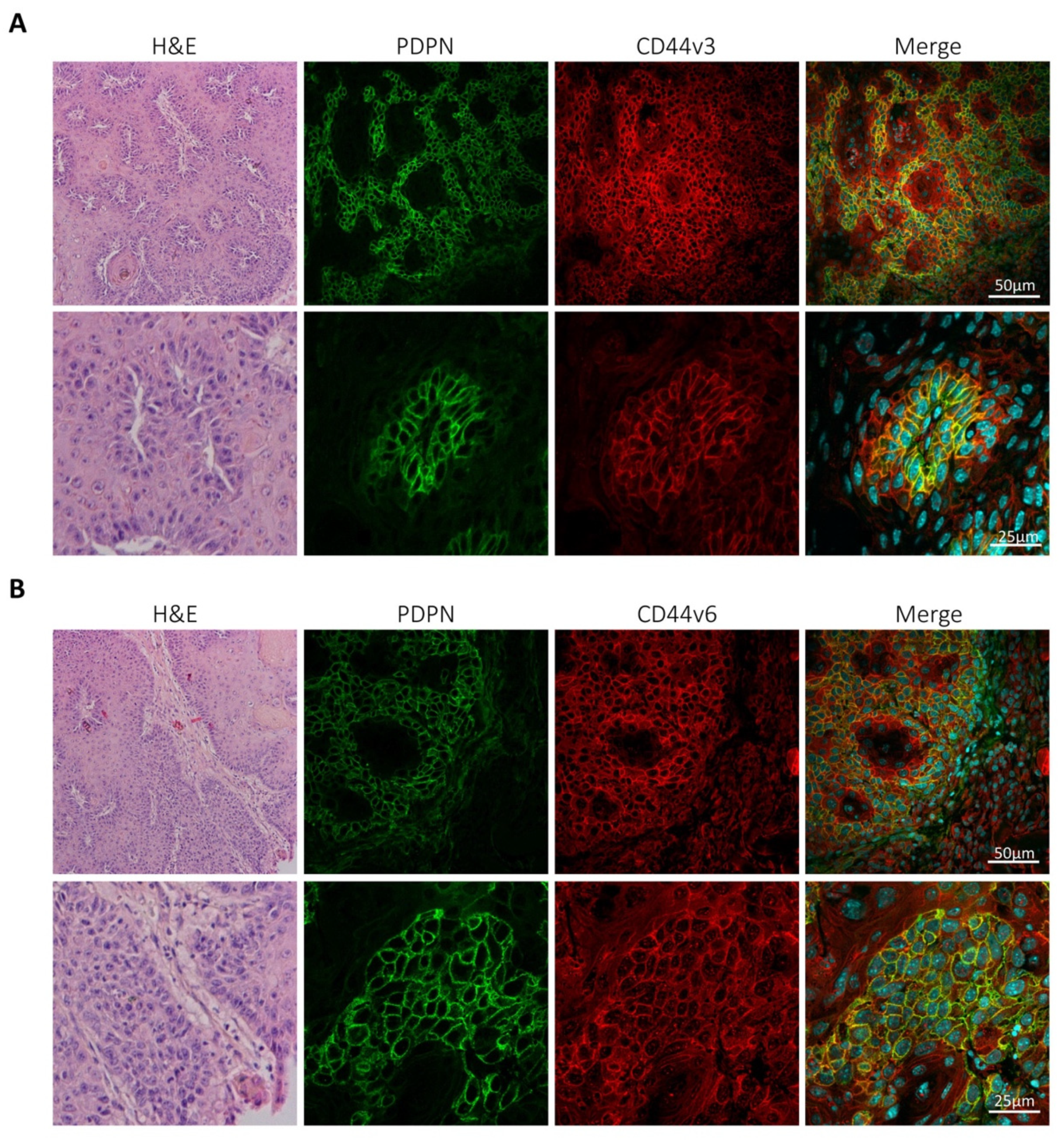
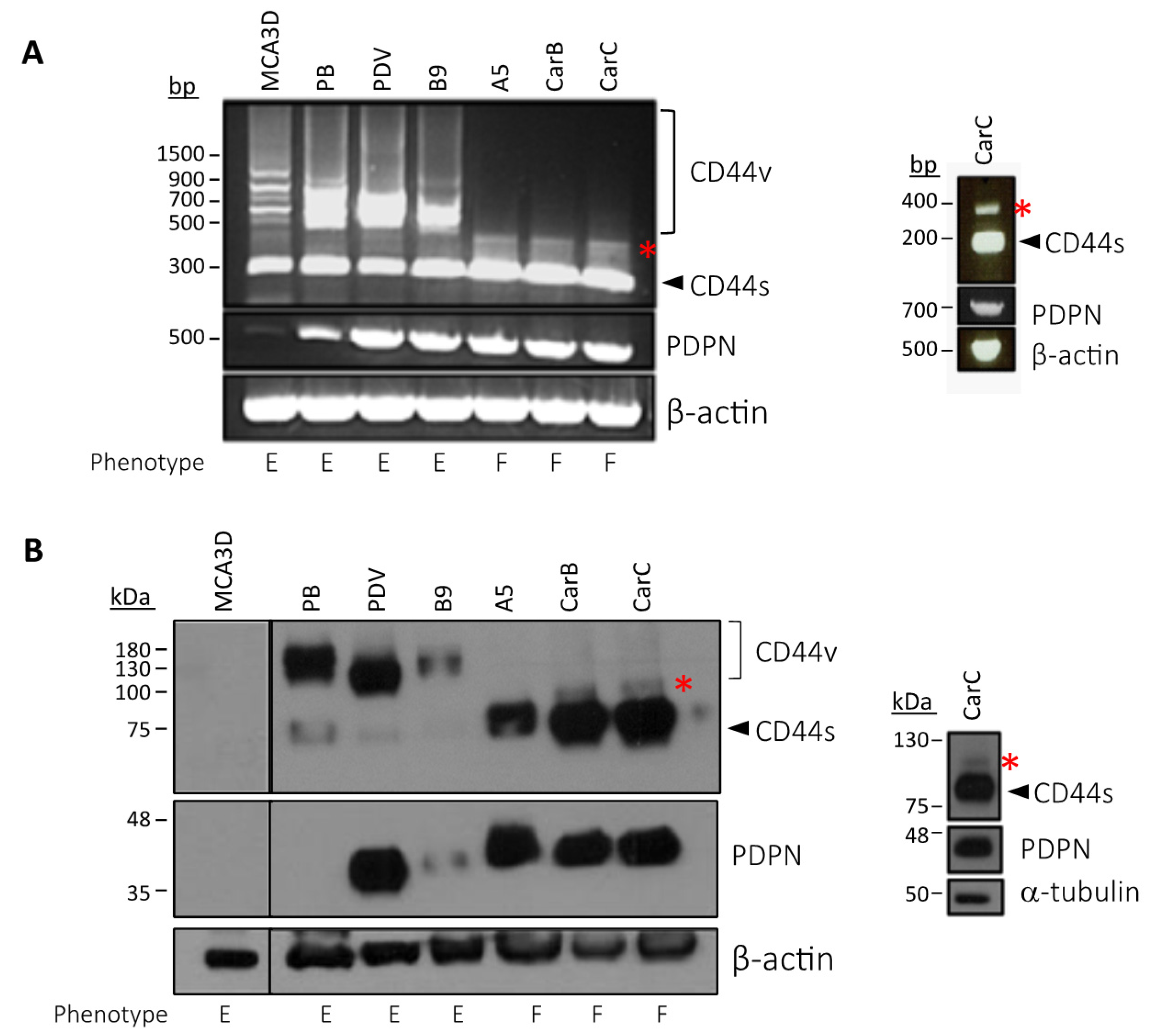
3.2. Podoplanin Co-Localizes with CD44v in Chemically-Induced Skin Tumors In Vivo and Is Co-Expressed with CD44s and CD44v in Transformed Mouse Epidermal Cell Lines
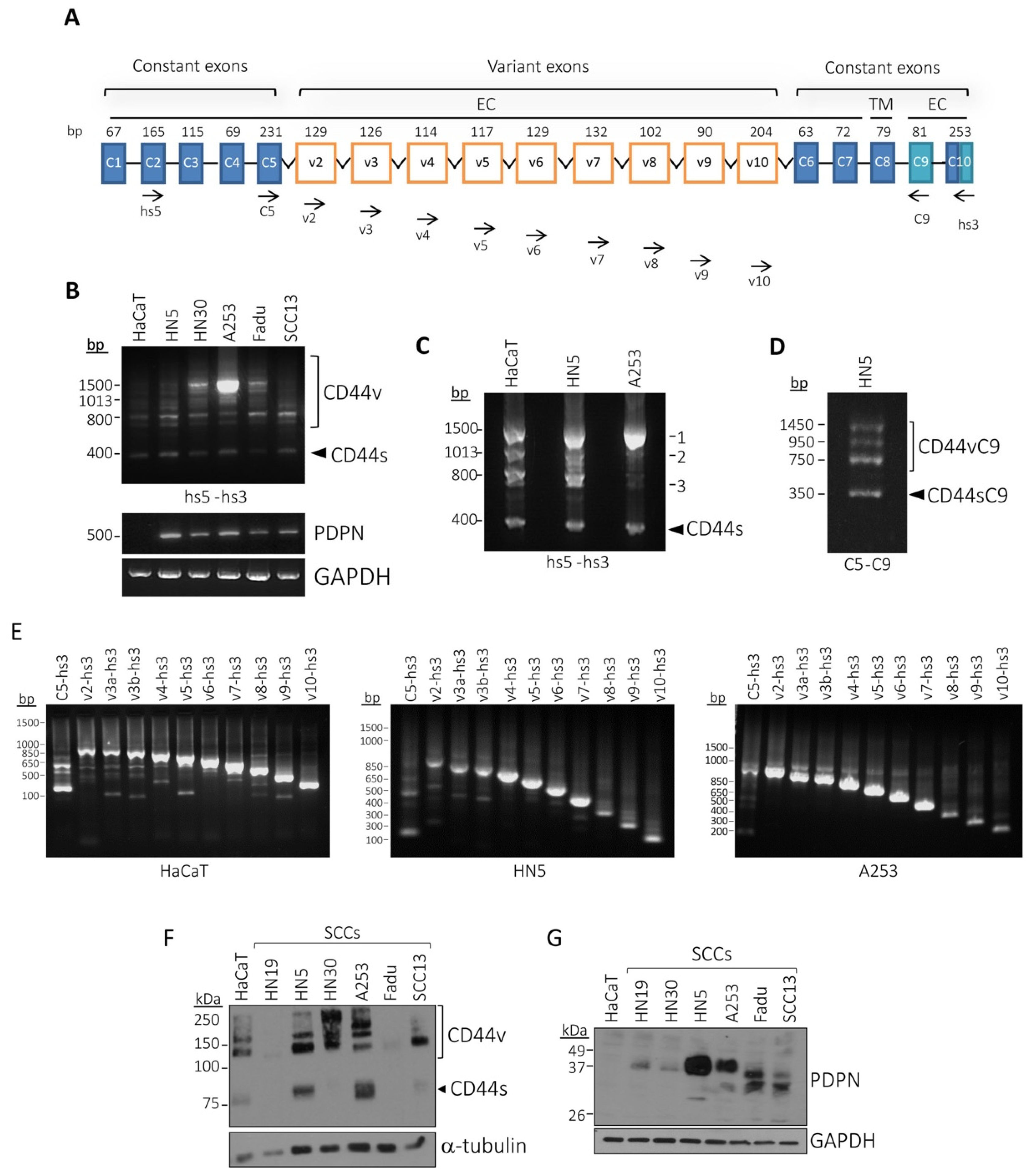
3.3. Characterization of CD44v Isoforms Expressed in Human SCC Cell Lines
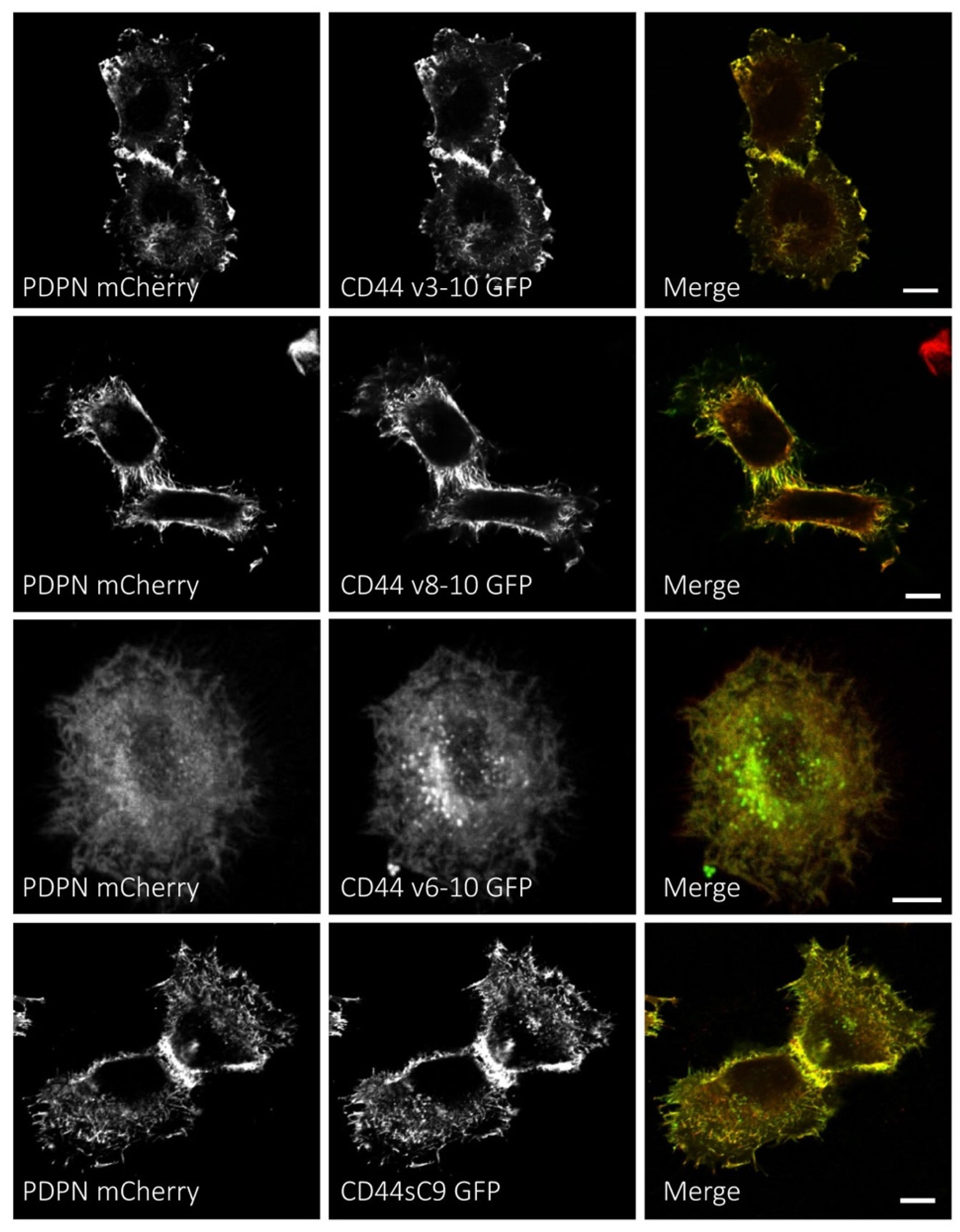
3.4. Podoplanin Colocalizes with CD44s and CD44v Isoforms at the Plasma Membrane of Human SCC Cells
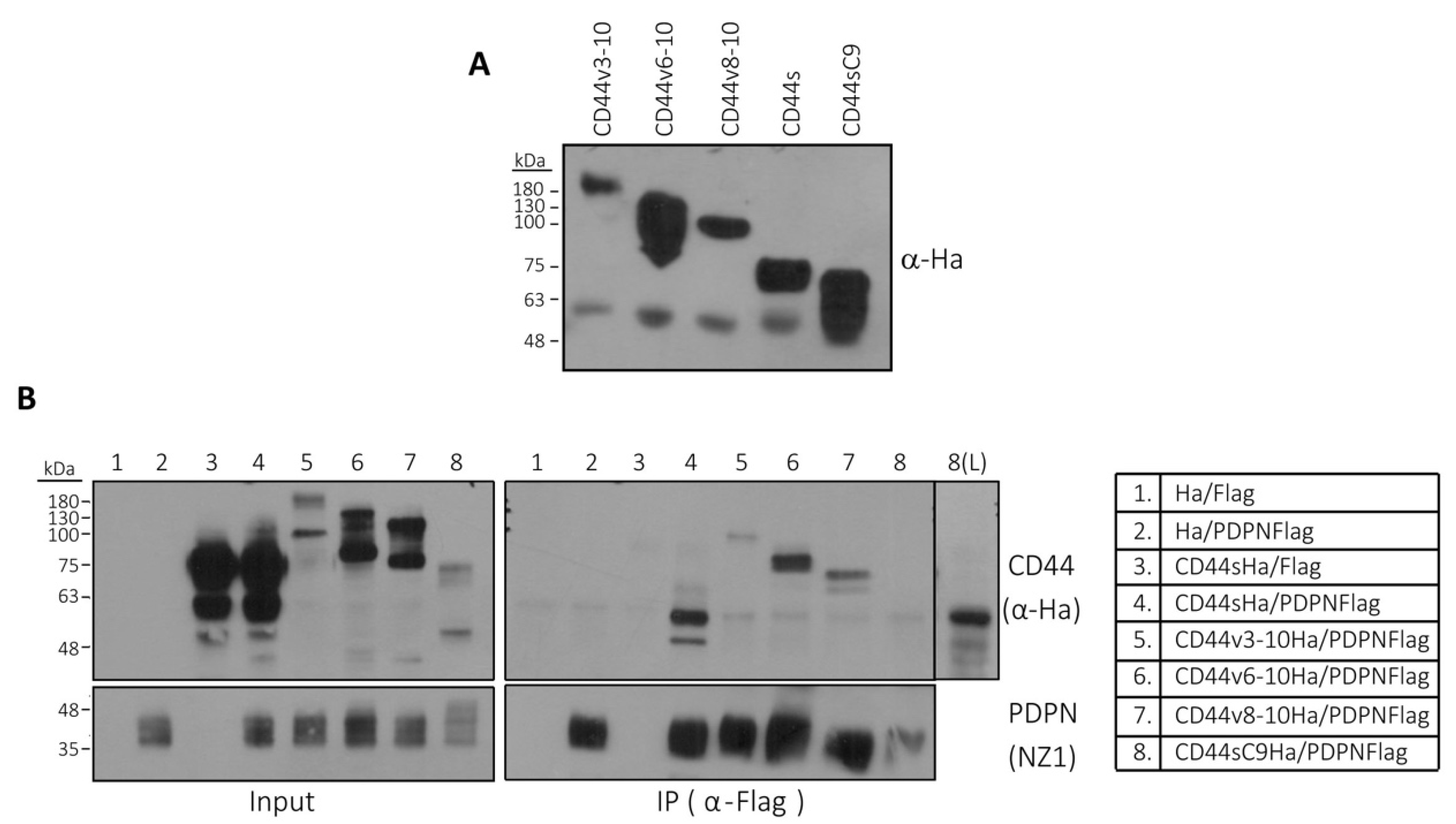
3.5. Podoplanin Interacts with CD44s and CD44v Isoforms
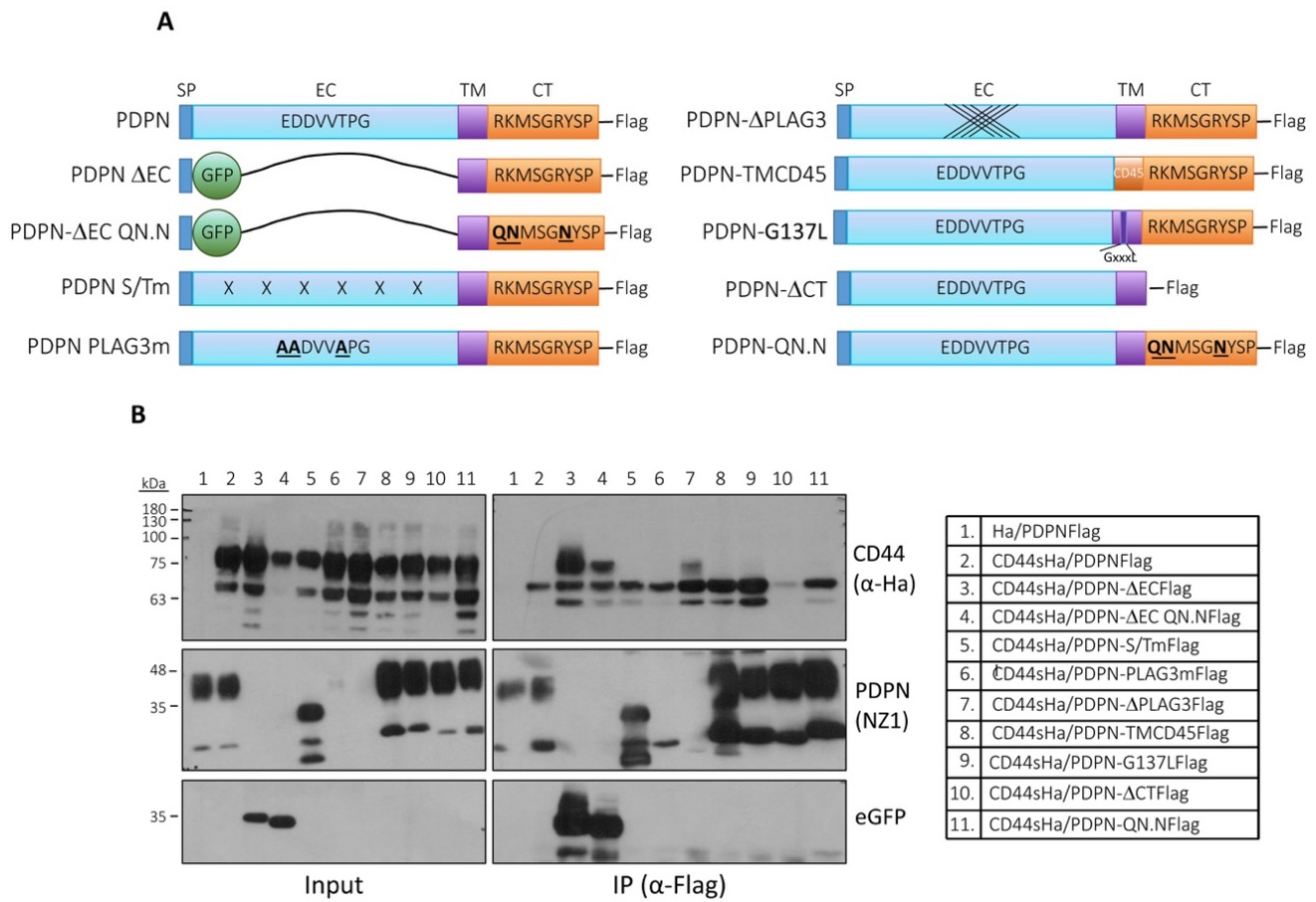
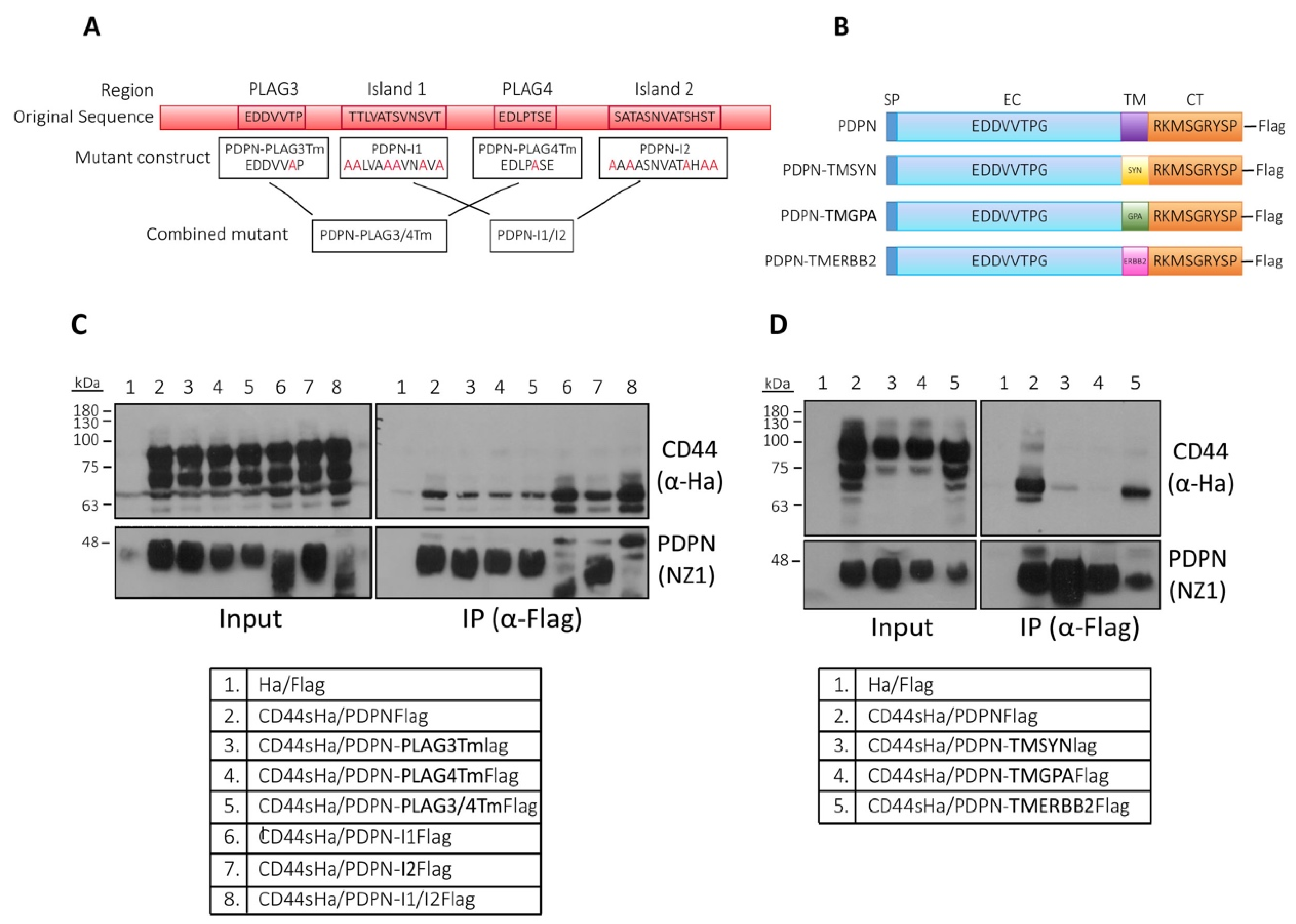
3.6. Analysis of the Structural Domains Involved in Podoplanin–CD44 Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Management of advanced and high-stage tumors. J. Am. Acad. Dermatol. 2018, 78, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martín-Villar, E. Podoplanin in Inflammation and Cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef]
- Krishnan, H.; Rayes, J.; Miyashita, T.; Ishii, G.; Retzbach, E.P.; Sheehan, S.A.; Takemoto, A.; Chang, Y.-W.; Yoneda, K.; Asai, J.; et al. Podoplanin: An emerging cancer biomarker and therapeutic target. Cancer Sci. 2018, 109, 1292–1299. [Google Scholar] [CrossRef]
- Renart, J.; Carrasco-Ramírez, P.; Fernández-Muñoz, B.; Martín-Villar, E.; Montero, L.; Yurrita, M.M.; Quintanilla, M. Chapter Four—New Insights into the Role of Podoplanin in Epithelial–Mesenchymal Transition. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 317, pp. 185–239. [Google Scholar]
- Ugorski, M.; Dziegiel, P.; Suchanski, J. Podoplanin—A small glycoprotein with many faces. Am. J. Cancer Res. 2016, 6, 370–386. [Google Scholar]
- Suzuki-Inoue, K. CLEC-2/podoplanin and thromboinflammation. Blood 2017, 129, 1896–1898. [Google Scholar] [CrossRef][Green Version]
- Mahtab, E.A.F.; Wijffels, M.C.E.F.; Akker, N.M.S.V.D.; Hahurij, N.D.; Lie-Venema, H.; Wisse, L.J.; DeRuiter, M.C.; Uhrin, P.; Zaujec, J.; Binder, B.R.; et al. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: Correlation with abnormal epicardial development. Dev. Dyn. 2008, 237, 847–857. [Google Scholar] [CrossRef]
- Mahtab, E.A.F.; Vicente-Steijn, R.; Hahurij, N.D.; Jongbloed, M.R.M.; Wisse, L.J.; DeRuiter, M.C.; Uhrin, P.; Zaujec, J.; Binder, B.R.; Schalij, M.J.; et al. Podoplanin deficient mice show a rhoa-related hypoplasia of the sinus venosus myocardium including the sinoatrial node. Dev. Dyn. 2009, 238, 183–193. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Millien, G.; Hinds, A.; Cao, Y.; Seldin, D.C.; Williams, M.C. T1α, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev. Biol. 2003, 256, 62–73. [Google Scholar] [CrossRef]
- Schacht, V.; Ramirez, M.I.; Hong, Y.-K.; Hirakawa, S.; Feng, D.; Harvey, N.; Williams, M.; Dvorak, A.M.; Dvorak, H.F.; Oliver, G.; et al. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003, 22, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Uhrin, P.; Zaujec, J.; Breuss, J.M.; Olcaydu, D.; Chrenek, P.; Stockinger, H.; Fuertbauer, E.; Moser, M.; Haiko, P.; Fässler, R.; et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 2010, 115, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Astarita, J.L.; Acton, S.E.; Turley, S.J. Podoplanin: Emerging functions in development, the immune system, and cancer. Front. Immunol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Núñez, L.; Langan, S.A.; Nash, G.B.; Watson, S.P. The physiological and pathophysiological roles of platelet CLEC-2. Thromb. Haemost. 2013, 109, 991–998. [Google Scholar] [CrossRef]
- Scholl, F.G.; Gamallo, C.; Vilaró, S.; Quintanilla, M. Identification of PA2.26 antigen as a novel cell-surface mucin-type glycoprotein that induces plasma membrane extensions and increased motility in keratinocytes. J. Cell Sci. 1999, 112, 4601–4613. [Google Scholar]
- Martín-Villar, E.; Megías, D.; Castel, S.; Yurrita, M.M.; Vilaró, S.; Quintanilla, M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J. Cell Sci. 2006, 119, 4541–4553. [Google Scholar] [CrossRef]
- Martín-Villar, E.; Scholl, F.G.; Gamallo, C.; Yurrita, M.M.; Muñoz-Guerra, M.; Cruces, J.; Quintanilla, M. Characterization of human PA2.26 antigen (T1α–2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int. J. Cancer 2005, 113, 899–910. [Google Scholar] [CrossRef]
- Scholl, F.G.; Gamallo, C.; Quintanilla, M. Ectopic Expression of PA2.26 Antigen in Epidermal Keratinocytes Leads to Destabilization of Adherens Junctions and Malignant Progression. Lab. Investig. 2000, 80, 1749–1759. [Google Scholar] [CrossRef]
- Martín-Villar, E.; Borda-d’Agua, B.; Carrasco-Ramirez, P.; Renart, J.; Parsons, M.; Quintanilla, M.; Jones, G.E. Podoplanin mediates ECM degradation by squamous carcinoma cells through control of invadopodia stability. Oncogene 2015, 34, 4531–4544. [Google Scholar] [CrossRef]
- Acton, S.E.; Farrugia, A.J.; Astarita, J.L.; Mourão-Sá, D.; Jenkins, R.P.; Nye, E.; Hooper, S.; van Blijswijk, J.; Rogers, N.C.; Snelgrove, K.J.; et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature 2014, 514, 498–502. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Kato, Y.; Kitano, T.; Osawa, M. Conservation of a platelet activating domain of Aggrus/podoplanin as a platelet aggregation-inducing factor. Gene 2006, 378, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Morita-Matsumoto, K.; Kato, M.; Kaneko, M.K.; Kato, Y.; Yamaguchi, Y. A Platform of C-type Lectin-like Receptor CLEC-2 for Binding O-Glycosylated Podoplanin and Nonglycosylated Rhodocytin. Structure 2014, 22, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, T.; Takemoto, A.; Takagi, S.; Takatori, K.; Sato, S.; Takami, M.; Fujita, N. Targeting a novel domain in podoplanin for inhibiting platelet-mediated tumor metastasis. Oncotarget 2015, 7, 3934–3946. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, A.Y.; Poulter, N.S.; Gitz, E.; Navarro-Nuñez, L.; Wang, Y.-J.; Hughes, C.E.; Thomas, S.G.; Nieswandt, B.; Douglas, M.R.; Owen, D.M.; et al. Syk and Src Family Kinases Regulate C-type Lectin Receptor 2 (CLEC-2)-mediated Clustering of Podoplanin and Platelet Adhesion to Lymphatic Endothelial Cells. J. Biol. Chem. 2014, 289, 35695–35710. [Google Scholar] [CrossRef] [PubMed]
- Séverin, S.; Pollitt, A.Y.; Navarro-Nuñez, L.; Nash, C.A.; Mourão-Sá, D.; Eble, J.A.; Senis, Y.A.; Watson, S.P. Syk-dependent Phosphorylation of CLEC-2 a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J. Biol. Chem. 2011, 286, 4107–4116. [Google Scholar] [CrossRef]
- Tamura, S.; Suzuki-Inoue, K.; Tsukiji, N.; Shirai, T.; Sasaki, T.; Osada, M.; Satoh, K.; Ozaki, Y. Podoplanin-positive periarteriolar stromal cells promote megakaryocyte growth and proplatelet formation in mice by CLEC-2. Blood 2016, 127, 1701–1710. [Google Scholar] [CrossRef]
- Kunita, A.; Kashima, T.G.; Morishita, Y.; Fukayama, M.; Kato, Y.; Tsuruo, T.; Fujita, N. The platelet aggregation-inducing factor aggrus/podoplanin promotes pulmonary metastasis. Am. J. Pathol. 2007, 170, 1337–1347. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Sato, S.; Naito, M.; Kato, Y.; Mishima, K.; Arai, H.; Tsuruo, T.; Fujita, N. Tetraspanin family member CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and suppresses pulmonary metastasis. Blood 2008, 112, 1730–1739. [Google Scholar] [CrossRef]
- Shirai, T.; Inoue, O.; Tamura, S.; Tsukiji, N.; Sasaki, T.; Endo, H.; Satoh, K.; Osada, M.; Sato-Uchida, H.; Fujii, H.; et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J. Thromb. Haemost. JTH 2017, 15, 513–525. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol. Pathol. MP 1999, 52, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Screaton, G.R.; Bell, M.V.; Bell, J.I.; Jackson, D.G. The identification of a new alternative exon with highly restricted tissue expression in transcripts encoding the mouse Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons between mouse, human, and rat. J. Biol. Chem. 1993, 268, 12235–12238. [Google Scholar] [PubMed]
- Prochazka, L.; Tesarik, R.; Turanek, J. Regulation of alternative splicing of CD44 in cancer. Cell. Signal. 2014, 26, 2234–2239. [Google Scholar] [CrossRef]
- Marhaba, R.; Zöller, M. CD44 in cancer progression: Adhesion, migration and growth regulation. J. Mol. Histol. 2004, 35, 211–231. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V. CD44 Acts as a Signaling Platform Controlling Tumor Progression and Metastasis. Front. Immunol. 2015, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Bourguignon, L.Y.W. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am. J. Pathol. 2011, 178, 956–963. [Google Scholar] [CrossRef]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef]
- Morath, I.; Hartmann, T.N.; Orian-Rousseau, V. CD44: More than a mere stem cell marker. Int. J. Biochem. Cell Biol. 2016, 81, 166–173. [Google Scholar] [CrossRef]
- Martín-Villar, E.; Fernández-Muñoz, B.; Parsons, M.; Yurrita, M.M.; Megías, D.; Pérez-Gómez, E.; Jones, G.E.; Quintanilla, M. Podoplanin associates with CD44 to promote directional cell migration. Mol. Biol. Cell 2010, 21, 4387–4399. [Google Scholar] [CrossRef]
- Quintanilla, M.; Ramirez, J.R.; Pérez-Gómez, E.; Romero, D.; Velasco, B.; Letarte, M.; López-Novoa, J.M.; Bernabéu, C. Expression of the TGF- β coreceptor endoglin in epidermal keratinocytes and its dual role in multistage mouse skin carcinogenesis. Oncogene 2003, 22, 5976–5985. [Google Scholar] [CrossRef][Green Version]
- Zhao, M.; Sano, D.; Pickering, C.R.; Jasser, S.A.; Henderson, Y.C.; Clayman, G.L.; Sturgis, E.M.; Ow, T.J.; Lotan, R.; Carey, T.E.; et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 7248–7264. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Baumann, U.; Reymond, J.-L. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 2004, 32, e115. [Google Scholar] [CrossRef] [PubMed]
- Konig, H.; Moll, J.; Ponta, H.; Herrlich, P. Trans-acting factors regulate the expression of CD44 splice variants. EMBO J. 1996, 15, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Gandarillas, A.; Scholl, F.G.; Benito, N.; Gamallo, C.; Quintanilla, M. Induction of PA2.26, a cell-surface antigen expressed by active fibroblasts, in mouse epidermal keratinocytes during carcinogenesis. Mol. Carcinog. 1997, 20, 10–18. [Google Scholar] [CrossRef]
- Marks, A.; Sutherland, D.R.; Bailey, D.; Iglesias, J.; Law, J.; Lei, M.; Yeger, H.; Banerjee, D.; Baumal, R. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br. J. Cancer 1999, 80, 569–578. [Google Scholar] [CrossRef]
- Screaton, G.R.; Bell, M.V.; Jackson, D.G.; Cornelis, F.B.; Gerth, U.; Bell, J.I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc. Natl. Acad. Sci. USA 1992, 89, 12160–12164. [Google Scholar] [CrossRef]
- van Weering, D.H.; Baas, P.D.; Bos, J.L. A PCR-based method for the analysis of human CD44 splice products. PCR Methods Appl. 1993, 3, 100–106. [Google Scholar] [CrossRef]
- Fernández-Muñoz, B.; Yurrita, M.M.; Martín-Villar, E.; Carrasco-Ramírez, P.; Megías, D.; Renart, J.; Quintanilla, M. The transmembrane domain of podoplanin is required for its association with lipid rafts and the induction of epithelial-mesenchymal transition. Int. J. Biochem. Cell Biol. 2011, 43, 886–896. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Kato, Y.; Kameyama, A.; Ito, H.; Kuno, A.; Hirabayashi, J.; Kubota, T.; Amano, K.; Chiba, Y.; Hasegawa, Y.; et al. Functional glycosylation of human podoplanin: Glycan structure of platelet aggregation-inducing factor. FEBS Lett. 2007, 581, 331–336. [Google Scholar] [CrossRef]
- Takemoto, A.; Miyata, K.; Fujita, N. Platelet-activating factor podoplanin: From discovery to drug development. Cancer Metastasis Rev. 2017, 36, 225–234. [Google Scholar] [CrossRef]
- Kato, Y.; Kaneko, M.K. A Cancer-specific Monoclonal Antibody Recognizes the Aberrantly Glycosylated Podoplanin. Sci. Rep. 2014, 4, 5924. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Minami-Hori, M.; Takahashi, H.; Iizuka, H. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: Regulation by TGF-β and STAT-3 activating cytokines, IFN-γ, IL-6, and IL-22. J. Dermatol. Sci. 2012, 65, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Fujii, M.; Iinuma, S.; Minami-Hori, M.; Takahashi, H.; Ishida-Yamamoto, A.; Iizuka, H. Podoplanin expression is inversely correlated with granular layer/filaggrin formation in psoriatic epidermis. J. Dermatol. 2013, 40, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Asai, J.; Hirakawa, S.; Sakabe, J.; Kishida, T.; Wada, M.; Nakamura, N.; Takenaka, H.; Mazda, O.; Urano, T.; Suzuki-Inoue, K.; et al. Platelets Regulate the Migration of Keratinocytes via Podoplanin/CLEC-2 Signaling during Cutaneous Wound Healing in Mice. Am. J. Pathol. 2016, 186, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Shatirishvili, M.; Burk, A.S.; Franz, C.M.; Pace, G.; Kastilan, T.; Breuhahn, K.; Hinterseer, E.; Dierich, A.; Bakiri, L.; Wagner, E.F.; et al. Epidermal-specific deletion of CD44 reveals a function in keratinocytes in response to mechanical stress. Cell Death Dis. 2016, 7, e2461. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Reinke, L.M.; Damerow, M.S.; Perez, D.; Chodosh, L.A.; Yang, J.; Cheng, C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J. Clin. Investig. 2011, 121, 1064–1074. [Google Scholar] [CrossRef]
- Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.G.; Wu, K. The role of CD44 in epithelial-mesenchymal transition and cancer development. OncoTargets Ther. 2015, 8, 3783–3792. [Google Scholar] [CrossRef]
- Ma, X.; Dighe, A.; Erkenbrack, E.; Maziarz, J.; Neumann, E.; Hei, Y.-Y.; Liu, Y.; Suhail, Y.; Kshitiz, K.; Levchenko, A.; et al. Human vulnerability to cancer malignancy is enhanced by evolution of higher mesenchymal CD44 expression compared to other mammals. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, S.J.; Wong, G.; de Heer, A.-M.; Xia, W.; Bourguignon, L.Y.W. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope 2009, 119, 1518–1530. [Google Scholar] [CrossRef]
- Akisik, E.; Bavbek, S.; Dalay, N. CD44 variant exons in leukemia and lymphoma. Pathol. Oncol. Res. POR 2002, 8, 36–40. [Google Scholar] [CrossRef]
- Beham-Schmid, C.; Heider, K.H.; Hoefler, G.; Zatloukal, K. Expression of CD44 splice variant v10 in Hodgkin’s disease is associated with aggressive behaviour and high risk of relapse. J. Pathol. 1998, 186, 383–389. [Google Scholar] [CrossRef]
- Kagami, T.; Yamade, M.; Suzuki, T.; Uotani, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; Sugimoto, K.; Baba, S.; et al. High expression level of CD44v8-10 in cancer stem-like cells is associated with poor prognosis in esophageal squamous cell carcinoma patients treated with chemoradiotherapy. Oncotarget 2018, 9, 34876–34888. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Earle, C.; Wong, G.; Spevak, C.C.; Krueger, K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 2012, 31, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Todoroki, K.; Ogasawara, S.; Akiba, J.; Nakayama, M.; Naito, Y.; Seki, N.; Kusukawa, J.; Yano, H. CD44v3+/CD24− cells possess cancer stem cell-like properties in human oral squamous cell carcinoma. Int. J. Oncol. 2015, 48, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, N.; Ishii, G.; Kojima, M.; Sanada, M.; Fujii, S.; Ochiai, A. Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma A431. Biochem. Biophys. Res. Commun. 2008, 373, 36–41. [Google Scholar] [CrossRef]
- Islam, F.; Gopalan, V.; Wahab, R.; Smith, R.A.; Lam, A.K.-Y. Cancer stem cells in oesophageal squamous cell carcinoma: Identification, prognostic and treatment perspectives. Crit. Rev. Oncol. Hematol. 2015, 96, 9–19. [Google Scholar] [CrossRef]
- Miyashita, T.; Higuchi, Y.; Kojima, M.; Ochiai, A.; Ishii, G. Single cell time-lapse analysis reveals that podoplanin enhances cell survival and colony formation capacity of squamous cell carcinoma cells. Sci. Rep. 2017, 7, 39971. [Google Scholar] [CrossRef]
- Rahadiani, N.; Ikeda, J.; Makino, T.; Tian, T.; Qiu, Y.; Mamat, S.; Wang, Y.; Doki, Y.; Aozasa, K.; Morii, E. Tumorigenic Role of Podoplanin in Esophageal Squamous-Cell Carcinoma. Ann. Surg. Oncol. 2010, 17, 1311–1323. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Zhu, H.; Chu, A.; Iida, N.; Zhang, L.; Hung, M.C. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J. Biol. Chem. 1997, 272, 27913–27918. [Google Scholar] [CrossRef]
- Bao, W.; Fu, H.-J.; Xie, Q.-S.; Wang, L.; Zhang, R.; Guo, Z.-Y.; Zhao, J.; Meng, Y.-L.; Ren, X.-L.; Wang, T.; et al. HER2 interacts with CD44 to up-regulate CXCR4 via epigenetic silencing of microRNA-139 in gastric cancer cells. Gastroenterology 2011, 141, 2076–2087. [Google Scholar] [CrossRef]
- Perschl, A.; Lesley, J.; English, N.; Hyman, R.; Trowbridge, I.S. Transmembrane domain of CD44 is required for its detergent insolubility in fibroblasts. J. Cell Sci. 1995, 108, 1033–1041. [Google Scholar] [PubMed]
- Tsukita, S.; Oishi, K.; Sato, N.; Sagara, J.; Kawai, A.; Tsukita, S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol. 1994, 126, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Astarita, J.L.; Cremasco, V.; Fu, J.; Darnell, M.C.; Peck, J.R.; Nieves-Bonilla, J.M.; Song, K.; Woodruff, M.C.; Gogineni, A.; Onder, L.; et al. The CLEC-2–podoplanin axis controls fibroblastic reticular cell contractility and lymph node microarchitecture. Nat. Immunol. 2015, 16, 75–84. [Google Scholar] [CrossRef] [PubMed]
- de Winde, C.M.; Makris, S.; Millward, L.; Benjamin, A.C.; Cazzagon, G.; Martinez, V.G.; Acton, S.E. Podoplanin function is switched by partner proteins on fibroblastic reticular cells. BioRxiv 2019, 793141. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Xianglan, Z.; Park, K.-K.; Chung, W.-Y. Functional invadopodia formation through stabilization of the PDPN transcript by IMP-3 and cancer-stromal crosstalk for PDPN expression. Carcinogenesis 2012, 33, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.D.; Dai, L.; Qin, Z.; Parsons, C.; Toole, B.P. CD147: Regulator of hyaluronan signaling in invasiveness and chemoresistance. Adv. Cancer Res. 2014, 123, 351–373. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Wei, Y.; Qiu, Q.; Chew, T.-L.; Kang, Y.; Cheng, C. The CD44s splice isoform is a central mediator for invadopodia activity. J. Cell Sci. 2016, 129, 1355–1365. [Google Scholar] [CrossRef]
- Petropoulos, C.; Guichet, P.-O.; Masliantsev, K.; Wager, M.; Karayan-Tapon, L. Functional invadopodia formed in glioblastoma stem cells are important regulators of tumor angiogenesis. Oncotarget 2018, 9, 20640–20657. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero-Montero, L.; Renart, J.; Ramírez, A.; Ramos, C.; Shamhood, M.; Jarcovsky, R.; Quintanilla, M.; Martín-Villar, E. Interplay between Podoplanin, CD44s and CD44v in Squamous Carcinoma Cells. Cells 2020, 9, 2200. https://doi.org/10.3390/cells9102200
Montero-Montero L, Renart J, Ramírez A, Ramos C, Shamhood M, Jarcovsky R, Quintanilla M, Martín-Villar E. Interplay between Podoplanin, CD44s and CD44v in Squamous Carcinoma Cells. Cells. 2020; 9(10):2200. https://doi.org/10.3390/cells9102200
Chicago/Turabian StyleMontero-Montero, Lucía, Jaime Renart, Andrés Ramírez, Carmen Ramos, Mariam Shamhood, Rocío Jarcovsky, Miguel Quintanilla, and Ester Martín-Villar. 2020. "Interplay between Podoplanin, CD44s and CD44v in Squamous Carcinoma Cells" Cells 9, no. 10: 2200. https://doi.org/10.3390/cells9102200
APA StyleMontero-Montero, L., Renart, J., Ramírez, A., Ramos, C., Shamhood, M., Jarcovsky, R., Quintanilla, M., & Martín-Villar, E. (2020). Interplay between Podoplanin, CD44s and CD44v in Squamous Carcinoma Cells. Cells, 9(10), 2200. https://doi.org/10.3390/cells9102200






