Angiogenic Activity of Cytochalasin B-Induced Membrane Vesicles of Human Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. MSCs Isolation and Characterization
2.2. CIMVs Production
2.3. Characterization of the CIMVs
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Proteome Analysis
2.3.3. Multiplex Analysis
2.3.4. Flow Cytometry Analysis
2.3.5. Cytoplasmic Membrane Staining
2.4. Animals
2.5. Angiogenic Activity Test In Vivo
2.6. Statistical Analysis
3. Results
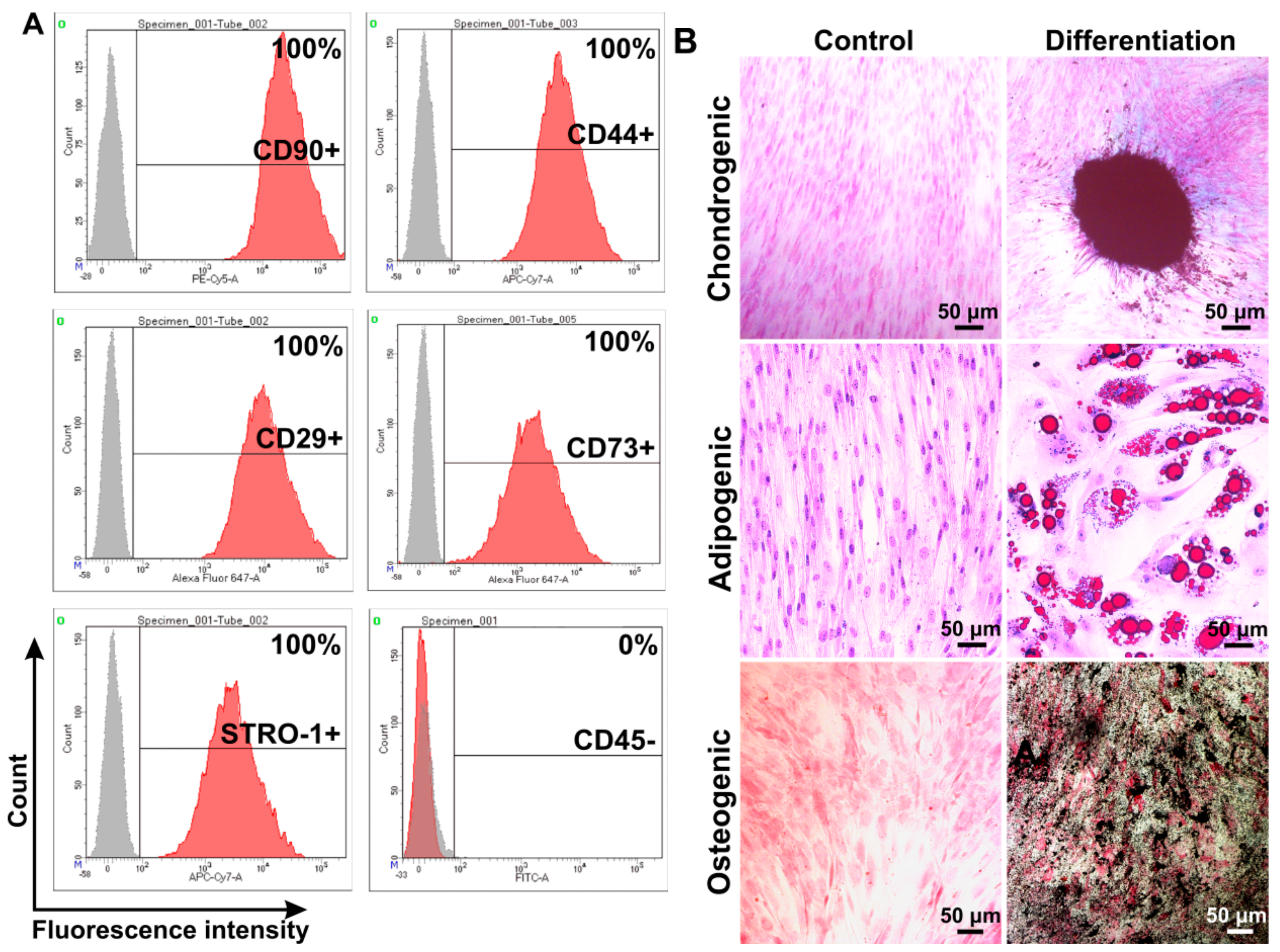
3.1. Isolation and Characterization of Human Adipose-Derived MSCs
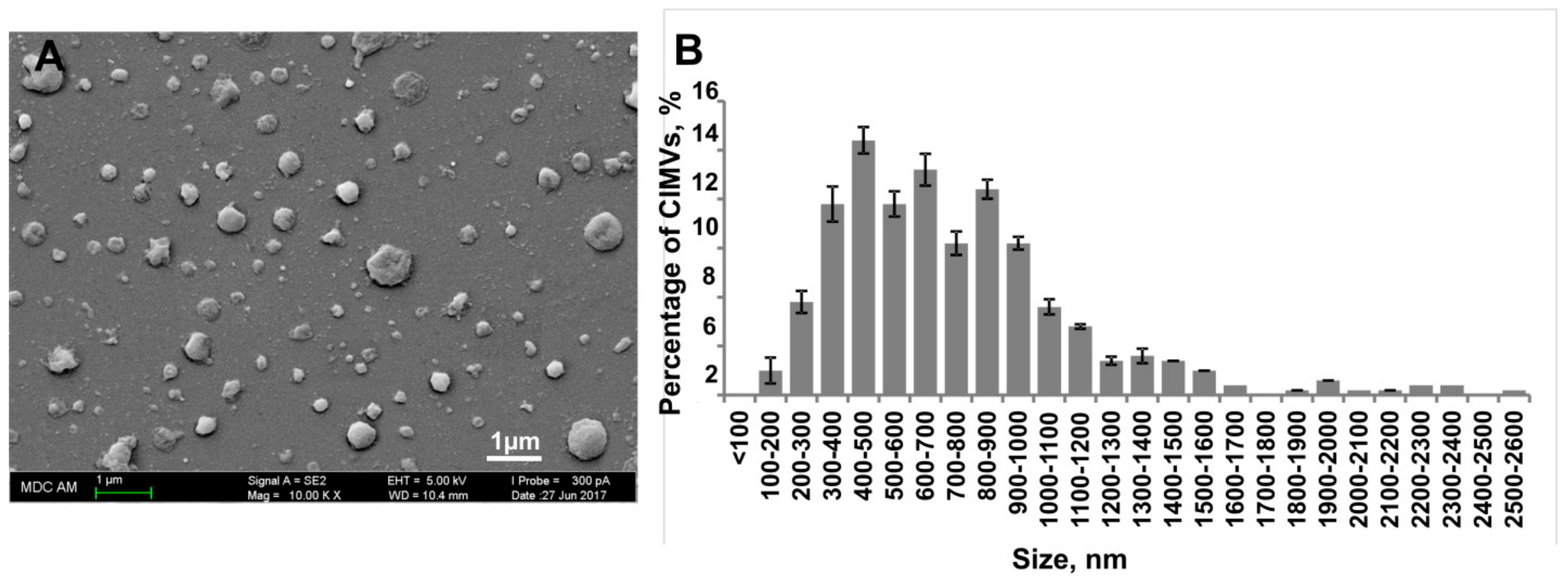
3.2. Characterization of Human CIMVs-MSCs
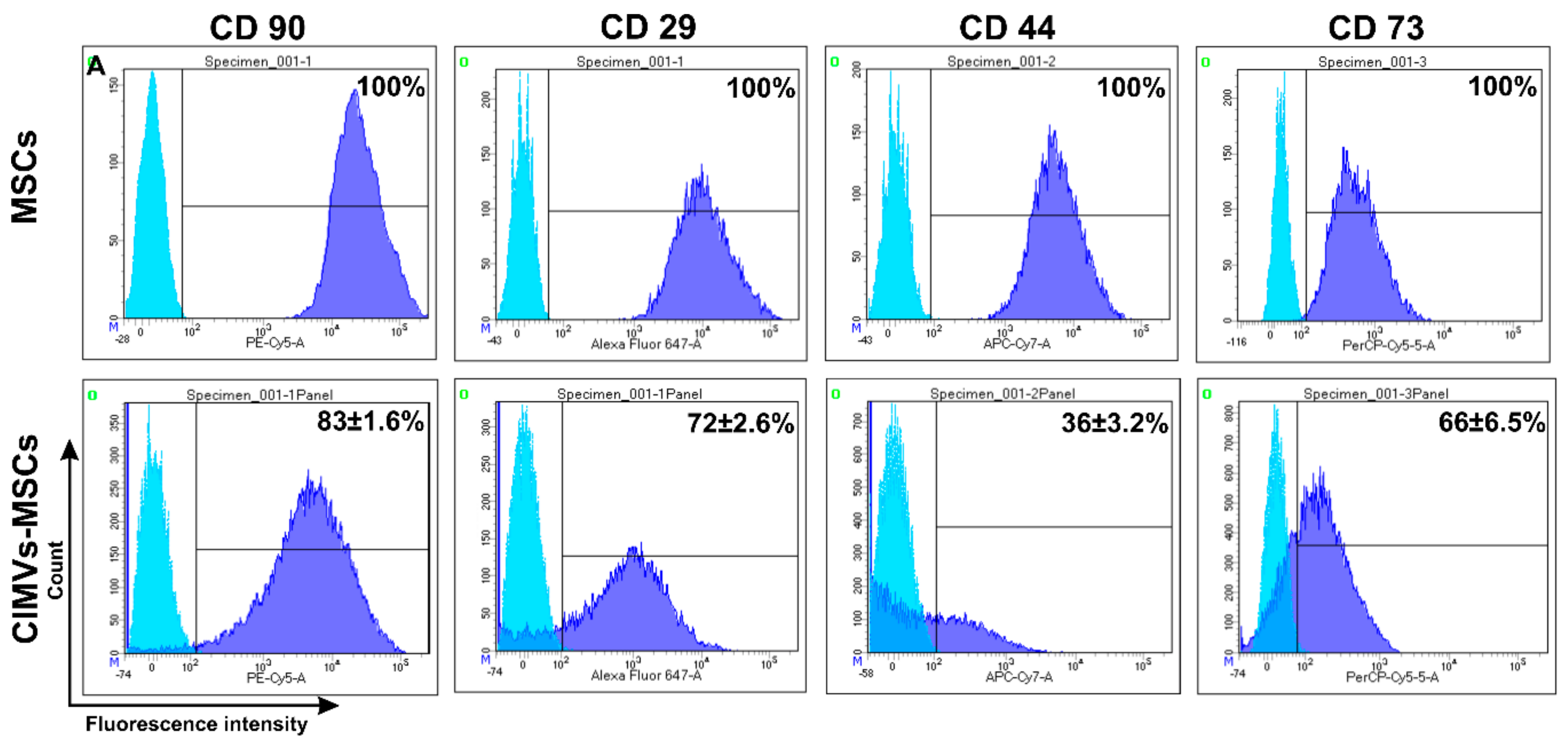
3.3. Immunophenotype of Human CIMVs-MSCs
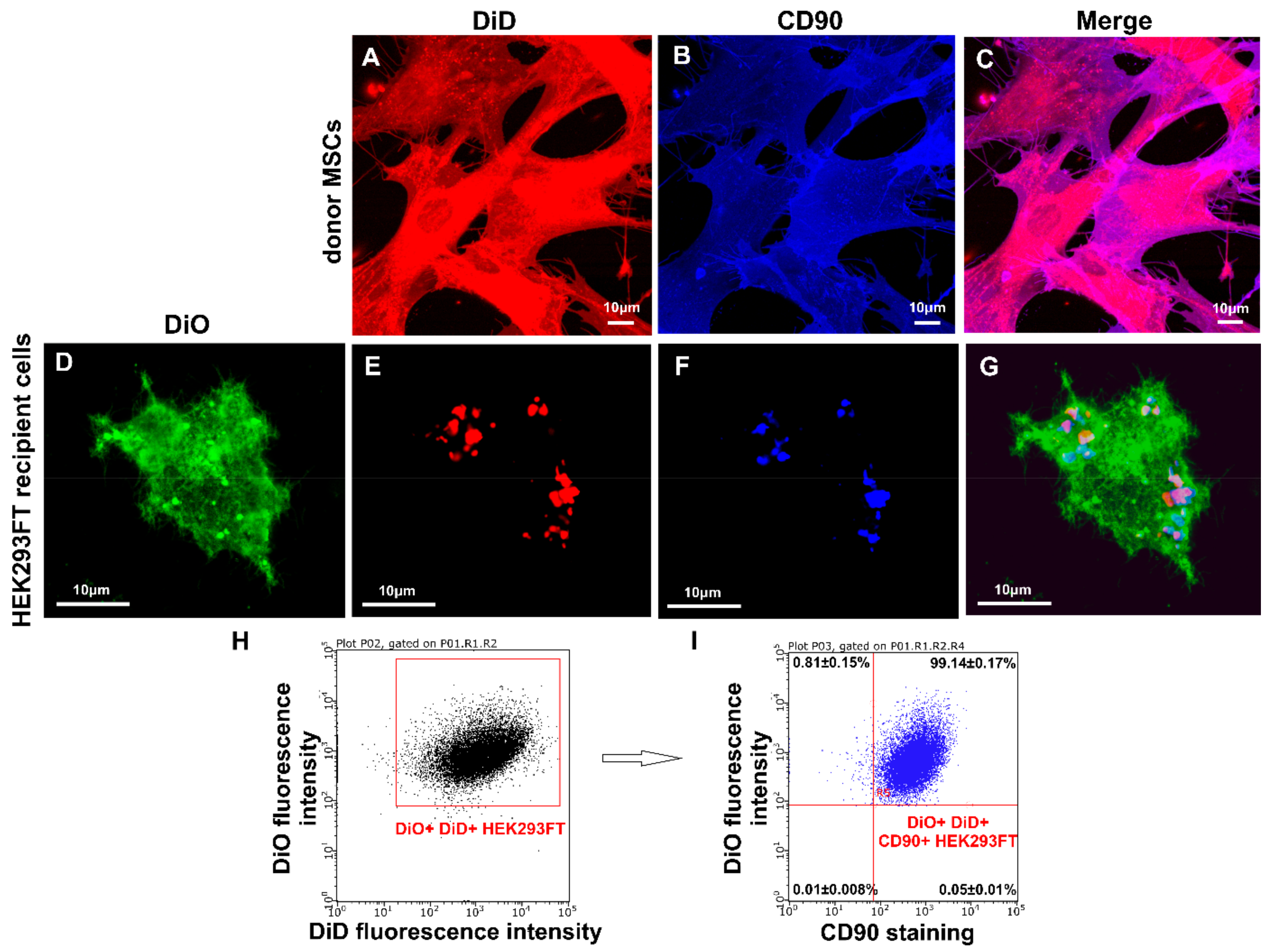
3.4. Transfer of Cell Surface Receptors to the Recipient Cell Membrane by CIMVs-MSCs
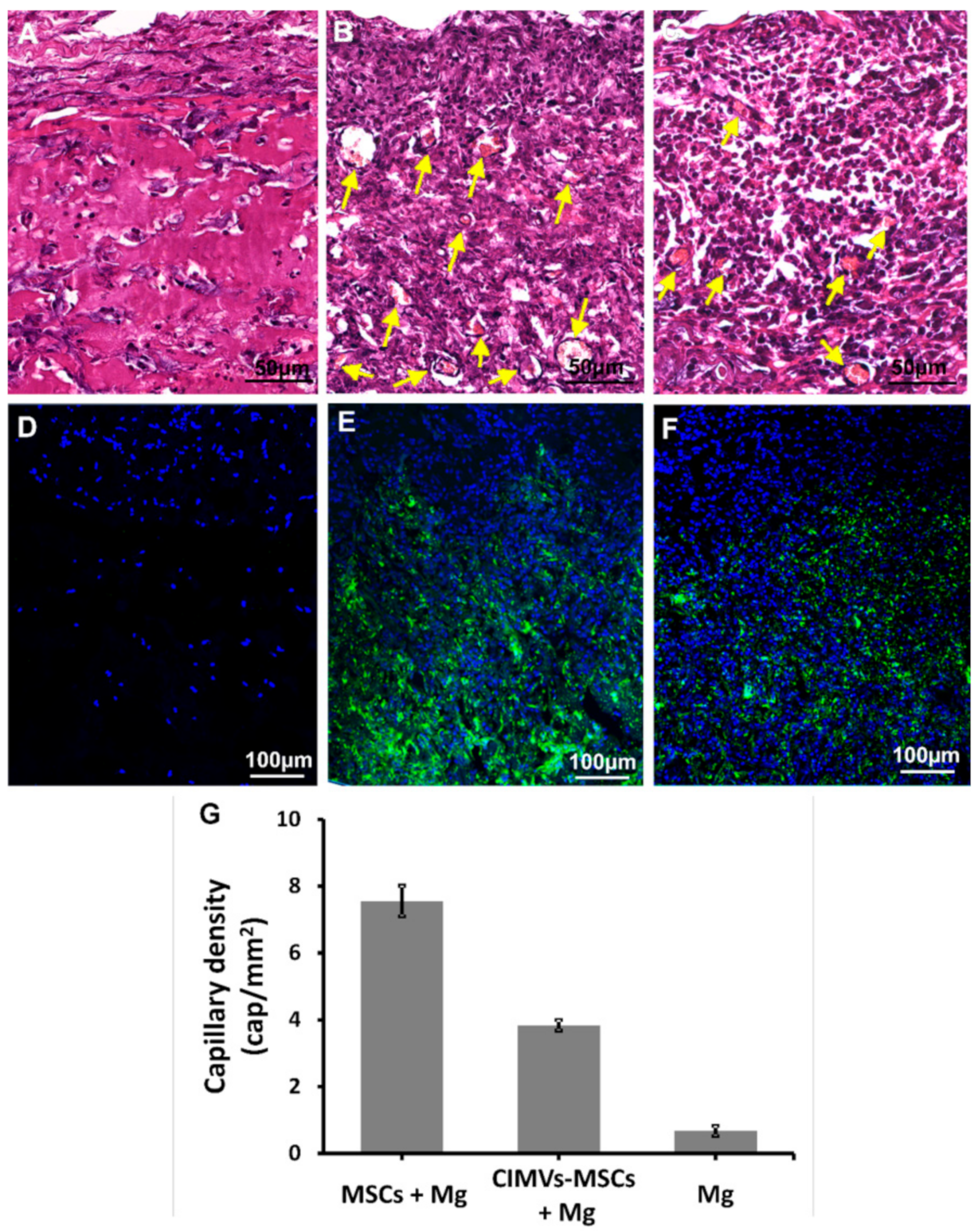
3.5. CIMVs-MSCs Stimulated Angiogenesis In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rohban, R.; Pieber, T.R. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017, 2017, 5173732. [Google Scholar] [CrossRef] [PubMed]
- Rosland, G.V.; Svendsen, A.; Torsvik, A.; Sobala, E.; McCormack, E.; Immervoll, H.; Mysliwietz, J.; Tonn, J.C.; Goldbrunner, R.; Lonning, P.E.; et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009, 69, 5331–5339. [Google Scholar] [CrossRef] [PubMed]
- Kunter, U.; Rong, S.; Boor, P.; Eitner, F.; Muller-Newen, G.; Djuric, Z.; van Roeyen, C.R.; Konieczny, A.; Ostendorf, T.; Villa, L.; et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J. Am. Soc. Nephrol. 2007, 18, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, M.; Bostani, T.; Roell, W.; Xia, Y.; Dewald, O.; Nygren, J.M.; Fries, J.W.; Tiemann, K.; Bohlen, H.; Hescheler, J.; et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 2007, 110, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Kim, O.Y.; Gho, Y.S. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014, 47, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Choi, H.; Kim, Y.; Lee, H.C.; Choi, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapeutics and as a Drug Delivery Platform. Stem Cell Transl. Med. 2019, 8, 880–886. [Google Scholar] [CrossRef]
- Bian, S.Y.; Zhang, L.P.; Duan, L.F.; Wang, X.; Min, Y.; Yu, H.P. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control. Release 2017, 264, 112–126. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Borrelli, D.A.; Matsuda, A.; Parasramka, M.; Shukla, N.; Lee, D.D.; Patel, T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl. 2017, 23, 791–803. [Google Scholar] [CrossRef]
- Deng, M.Y.; Xiao, H.; Zhang, H.N.; Peng, H.L.; Yuan, H.; Xu, Y.X.; Zhang, G.S.; Hu, Z.P. Mesenchymal Stem Cell-Derived Extracellular Vesicles Ameliorates Hippocampal Synaptic Impairment after Transient Global Ischemia. Front. Cell Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Gomzikova, M.O.; Rizvanov, A.A. Current Trends in Regenerative Medicine: From Cell to Cell-Free Therapy. Bionanoscience 2017, 7, 240–245. [Google Scholar] [CrossRef]
- Hong, P.; Yang, H.; Wu, Y.; Li, K.; Tang, Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: A comprehensive review. Stem Cell Res. Ther. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Simons, M. Exosomes: Vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013, 352, 33–47. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chen, X.; Gueydan, C.; Han, J.H. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Del Piccolo, N.; Placone, J.; He, L.; Agudelo, S.C.; Hristova, K. Production of plasma membrane vesicles with chloride salts and their utility as a cell membrane mimetic for biophysical characterization of membrane protein interactions. Anal. Chem. 2012, 84, 8650–8655. [Google Scholar] [CrossRef]
- Wu, H.W.; Oliver, A.E.; Ngassam, V.N.; Yee, C.K.; Parikh, A.N.; Yeh, Y. Preparation, characterization, and surface immobilization of native vesicles obtained by mechanical extrusion of mammalian cells. Integr. Biol. UK 2012, 4, 685–692. [Google Scholar] [CrossRef]
- Pick, H.; Schmid, E.L.; Tairi, A.P.; Ilegems, E.; Hovius, R.; Vogel, H. Investigating cellular signaling reactions in single attoliter vesicles. J. Am. Chem. Soc. 2005, 127, 2908–2912. [Google Scholar] [CrossRef]
- Peng, L.H.; Zhang, Y.H.; Han, L.J.; Zhang, C.Z.; Wu, J.H.; Wang, X.R.; Gao, J.Q.; Mao, Z.W. Cell Membrane Capsules for Encapsulation of Chemotherapeutic and Cancer Cell Targeting in Vivo. ACS Appl. Mater. Inter. 2015, 7, 18628–18637. [Google Scholar] [CrossRef] [PubMed]
- Gomzikova, M.O.; Zhuravleva, M.N.; Miftakhova, R.R.; Arkhipova, S.S.; Evtugin, V.G.; Khaiboullina, S.F.; Kiyasov, A.P.; Persson, J.L.; Mongan, N.P.; Pestell, R.G.; et al. Cytochalasin B-induced membrane vesicles convey angiogenic activity of parental cells. Oncotarget 2017, 8, 70496–70507. [Google Scholar] [CrossRef] [PubMed]
- Katina, M.N.; Gaifullina, R.F.; Hayatova, Z.G.; Emene, C.; Rizvanov, A.A. Isolation, culture and differentiation of rat (Rattus norvegicus) and hamster (Mesocricetus auratus) adipose derived multipotent mesenchymal stromal cells. Cell. Transpl. Tissue Eng. 2012, 7, 82–87. (In Russian) [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.; Boyer, C.; Thinard, R.; Remy, S.; Dugast, A.S.; Dubayle, D.; Dey, N.D.; Boeffard, F.; Delecrin, J.; Heymann, D.; et al. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. J. Cell. Mol. Med. 2009, 13, 2547–2558. [Google Scholar] [CrossRef]
- Isakova, I.A.; Lanclos, C.; Bruhn, J.; Kuroda, M.J.; Baker, K.C.; Krishnappa, V.; Phinney, D.G. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS ONE 2014, 9, e87238. [Google Scholar] [CrossRef]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem Cells Int. 2012, 2012, 461718. [Google Scholar] [CrossRef]
- Docheva, D.; Haasters, F.; Schieker, M. Mesenchymal Stem Cells and Their Cell Surface Receptors. Curr. Rheumatol. Rev. 2008, 4, 6. [Google Scholar] [CrossRef]
- Mack, M.; Kleinschmidt, A.; Bruhl, H.; Klier, C.; Nelson, P.J.; Cihak, J.; Plachy, J.; Stangassinger, M.; Erfle, V.; Schlondorff, D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 2000, 6, 769–775. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Mairuhu, A.T.; Italiano, J.E. Platelet- and megakaryocyte-derived microparticles. Semin. Thromb. Hemost. 2010, 36, 881–887. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, D.Y.; Yun, S.J.; Choi, S.M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Corsalini, M.; Schierano, G.; Pettini, F.; Di Venere, D.; Carossa, S. Cytokine, Chemokine, and Growth Factor Profile Characterization of Undifferentiated and Osteoinduced Human Adipose-Derived Stem Cells. Stem Cells Int. 2017, 2017, 6202783. [Google Scholar] [CrossRef] [PubMed]
- Schinkothe, T.; Bloch, W.; Schmidt, A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008, 17, 199–206. [Google Scholar] [CrossRef]
- Niehage, C.; Steenblock, C.; Pursche, T.; Bornhauser, M.; Corbeil, D.; Hoflack, B. The cell surface proteome of human mesenchymal stromal cells. PLoS ONE 2011, 6, e20399. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Lopatina, T.; Mazzeo, A.; Bruno, S.; Tetta, C.; Kalinina, N.; Romagnoli, R.; Salizzoni, M.; Porta, M.; Camussi, G. The Angiogenic Potential of Adipose Mesenchymal Stem Cell-derived Extracellular Vesicles is modulated by Basic Fibroblast Growth Factor. Stem Cell Res. Ther. 2014, 4, 1–7. [Google Scholar] [CrossRef]

| Cytokine | MSCs (pg/mL) | CIMVs-MSCs (pg/mL) | p Value |
|---|---|---|---|
| EGF | 141.3 ± 20.6 | 160.7 ± 67.7 | <0.37 |
| FGF-2 | 12114 ± 1433.9 | 10278.5 ± 1020.3 | <0.14 |
| Eotaxin/CCL11 | 48.6 ± 6.2 | 28.9 ± 22.4 | <0.18 |
| * TGF-α | 3 ± 0.1 | 1.6 ± 0.3 | <0.02 |
| G-CSF | 1327.4 ± 457.1 | 1144.3 ± 615 | <0.38 |
| Flt-3L | 19.6 ± 21.4 | 19.3 ± 7.8 | <0.49 |
| GM-CSF | 25.4 ± 6.5 | 15.6 ± 6 | <0.13 |
| Fractalkine/CX3CL | 376.9 ± 28.4 | 548.7 ± 155.8 | <0.07 |
| IFNα2 | 162 ± 43.6 | 83.5 ± 61.7 | <0.14 |
| IFN-γ | 7.5 ± 1.1 | 7.5 ± 3.8 | <0.49 |
| GRO | 661.3 ± 234.1 | 278.6 ± 117.5 | <0.06 |
| IL-10 | 3.3 ± 1.1 | 3.4 ± 0.7 | <0.47 |
| * MCP-3/CCL7 | 60 ± 5 | 37.2 ± 9.3 | <0.05 |
| IL-12p40 | 31.4 ± 12.2 | 27.9 ± 13.4 | <0.40 |
| MDC/CCL22 | 20.7 ± 3.9 | 22.2 ± 6.4 | <0.41 |
| IL-12p70 | 7.4 ± 0.9 | 7 ± 2.9 | <0.42 |
| IL-15 | 18.7 ± 12.5 | 19 ± 5.8 | <0.49 |
| * sCD40L | 9 ± 1.3 | 3.8 ± 2.2 | <0.05 |
| IL-17A | 5.3 ± 0.9 | 2.2 ± 1.5 | <0.06 |
| IL-1RA | 46 ± 35 | 40.6 ± 29 | <0.44 |
| IL-1a | 13.2 ± 1.6 | 10.8 ± 4.8 | <0.29 |
| IL-9 | 136.3 ± 57.1 | 127.6 ± 68.3 | <0.45 |
| * IL-1b | 8.7 ± 0.5 | 4.5 ± 1.1 | <0.02 |
| IL-2 | 9.9 ± 5 | 4 ± 1.9 | <0.13 |
| IL-4 | 18.8 ± 1.6 | 23.7 ± 13.1 | <0.33 |
| IL-5 | 3.8 ± 1.6 | 1.7 ± 0.2 | <0.11 |
| IL-6 | 1833.5 ± 235.4 | 1497.3 ± 513.8 | <0.24 |
| IL-7 | 21 ± 13.3 | 41.3 ± 16.3 | <0.11 |
| IL-8 | 1612.7 ± 487.2 | 721.3 ± 299.2 | <0.08 |
| IP-10 | 108.6 ± 44.9 | 268.1 ± 86.7 | <0.07 |
| MCP-1/CCL2 | 622.4 ± 238.9 | 1164.6 ± 505.1 | <0.09 |
| MIP_1a/CCL3 | 38.7 ± 2.5 | 28.5 ± 5.6 | <0.07 |
| MIP-1b/CCL4 | 82.4 ± 3.7 | 35.4 ± 29.8 | <0.08 |
| TNF-α | 5.1 ± 1.3 | 2 ± 1.1 | <0.06 |
| * TNF-β | 4.8 ± 0.3 | 2.8 ± 0.3 | <0.01 |
| VEGF | 219.8 ± 90 | 215.4 ± 48.1 | <0.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomzikova, M.O.; Zhuravleva, M.N.; Vorobev, V.V.; Salafutdinov, I.I.; Laikov, A.V.; Kletukhina, S.K.; Martynova, E.V.; Tazetdinova, L.G.; Ntekim, A.I.; Khaiboullina, S.F.; et al. Angiogenic Activity of Cytochalasin B-Induced Membrane Vesicles of Human Mesenchymal Stem Cells. Cells 2020, 9, 95. https://doi.org/10.3390/cells9010095
Gomzikova MO, Zhuravleva MN, Vorobev VV, Salafutdinov II, Laikov AV, Kletukhina SK, Martynova EV, Tazetdinova LG, Ntekim AI, Khaiboullina SF, et al. Angiogenic Activity of Cytochalasin B-Induced Membrane Vesicles of Human Mesenchymal Stem Cells. Cells. 2020; 9(1):95. https://doi.org/10.3390/cells9010095
Chicago/Turabian StyleGomzikova, Marina O., Margarita N. Zhuravleva, Vyacheslav V. Vorobev, Ilnur I. Salafutdinov, Alexander V. Laikov, Sevindzh K. Kletukhina, Ekaterina V. Martynova, Leysan G. Tazetdinova, Atara I. Ntekim, Svetlana F. Khaiboullina, and et al. 2020. "Angiogenic Activity of Cytochalasin B-Induced Membrane Vesicles of Human Mesenchymal Stem Cells" Cells 9, no. 1: 95. https://doi.org/10.3390/cells9010095
APA StyleGomzikova, M. O., Zhuravleva, M. N., Vorobev, V. V., Salafutdinov, I. I., Laikov, A. V., Kletukhina, S. K., Martynova, E. V., Tazetdinova, L. G., Ntekim, A. I., Khaiboullina, S. F., & Rizvanov, A. A. (2020). Angiogenic Activity of Cytochalasin B-Induced Membrane Vesicles of Human Mesenchymal Stem Cells. Cells, 9(1), 95. https://doi.org/10.3390/cells9010095








