Abstract
Ca2+ is essential for virus entry, viral gene replication, virion maturation, and release. The alteration of host cells Ca2+ homeostasis is one of the strategies that viruses use to modulate host cells signal transduction mechanisms in their favor. Host calcium-permeable channels and pumps (including voltage-gated calcium channels, store-operated channels, receptor-operated channels, transient receptor potential ion channels, and Ca2+-ATPase) mediate Ca2+ across the plasma membrane or subcellular organelles, modulating intracellular free Ca2+. Therefore, these Ca2+ channels or pumps present important aspects of viral pathogenesis and virus–host interaction. It has been reported that viruses hijack host calcium channels or pumps, disturbing the cellular homeostatic balance of Ca2+. Such a disturbance benefits virus lifecycles while inducing host cells’ morbidity. Evidence has emerged that pharmacologically targeting the calcium channel or calcium release from the endoplasmic reticulum (ER) can obstruct virus lifecycles. Impeding virus-induced abnormal intracellular Ca2+ homeostasis is becoming a useful strategy in the development of potent antiviral drugs. In this present review, the recent identified cellular calcium channels and pumps as targets for virus attack are emphasized.
1. Introduction
Viruses exploit the environment of host cells to replicate, thereby inducing host cells’ dysfunction. Virus–host interaction is the foundation of pathogenesis and closely associated with disease severity and incidence. The prevention and therapy of virus infections are often confounded by the high mutation rates that facilitate the viral evasion of antiviral strategies that target virally encoded proteins. Modulations of the intracellular environment have become an important strategy in antiviral drug discovery and development. In mammalian cells, Ca2+, as an important second messenger, mediates the sensor input and responses output for almost all known cellular progress, such as stress responses, synaptic plasticity, immunodefenses, protein transport, and endosome formation [1,2]. It has been demonstrated that the host cell dysfunction following infection with a virus is accompanied by abnormal intracellular Ca2+ concentration [3]. A virus can hijack the host intracellular Ca2+ system to achieve successful replication via multiple routes; for instance, viral proteins directly bind to Ca2+ or disturb the membrane permeability for Ca2+ by manipulating Ca2+ apparatus.
The host cell plasma membrane is the first barrier against the invasion of viruses. Various Ca2+ channels and pumps are distributed on the cell plasma membrane. Therefore, these membrane proteins become the direct target of virus infection. Interaction between viruses and these membrane proteins is the foremost approach of viruses perturbing the host cell calcium signal system. This interaction may inhibit or stimulate calcium influx and modulate free cytosolic Ca2+ concentrations. After entry into the host cell, viruses stimulate or inhibit the calcium release from internal stores via an effect on calcium-permeable channels, transporters, and exchangers on organellar membranes. Then, the change in cytosolic calcium concentration may trigger further distortion of the host cell system, which benefits virus survival and replication.
This review concentrates on host cell membranes’ calcium channels and pumps in viral infection. Blockers for these membrane proteins or preventing viruses from grabbing these host calcium-signaling components may lower the probability of virus stability, replication, and release, as well as infection-related host–cell apoptosis and reactive oxygen species production, neurotoxicity, and enterotoxin, making these membrane proteins potential targets for antiviral drugs.
2. Calcium Channels and Pumps in Host Ca2+ Homeostasis
Cellular Ca2+ is from two major sources: the internal Ca2+ store (mainly endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR)) and the extracellular medium. Calcium channels on cell plasma membrane mediate the entry of Ca2+ from the extracellular medium. These channels are activated by specific stimuli, such as voltage-gated calcium channels (VGCCs), which are stimulated by membrane depolarization, specific receptor-operated channels (ROC), which are stimulated by external agonists, or intracellular messengers and store-operated calcium channel (SOC), which are stimulated by the depletion of internal Ca2+ stores. The IP3 receptor (IP3R) and the ryanodine receptors (RyR) are the main players in mediating the release of Ca2+ from the internal stores. Inositol-1,4,5-triphosphate (IP3) activates IP3R, triggers Ca2+ release from stores, and further increases IP3R’s sensitivity to Ca2+. Calcium pumps (the plasma membrane Ca2+-ATPase (PMCA), sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA)) and the Na+/Ca2+ exchanger (NCX) are responsible for transporting Ca2+ from the cytosol to external medium or into cellular calcium stores (Figure 1). The normal function of these calcium channels and pump is important for cells to maintain intracellular Ca2+ homeostasis.
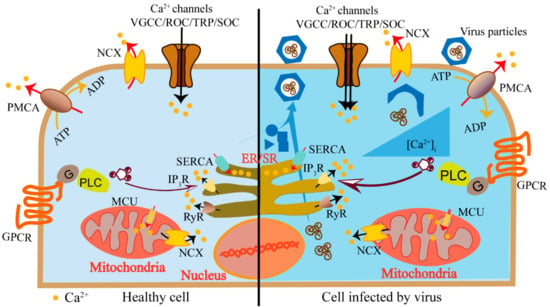
Figure 1.
Schematics of host cell elevated cytosolic calcium concentration induced by a virus. Calcium channels (voltage-gated calcium channels (VGCCs), receptor-operated channels (ROC), store-operated Ca2+ (SOC), channels and transient receptor potential (TRP) channels) mediate the entry of Ca2+ from extracellular medium (black arrows). The IP3 receptor (IP3R) and the ryanodine receptors (RyR) on the endoplasmic reticulum (ER) mediate the release of Ca2+ from internal stores (black arrows). Calcium pumps (the plasma membrane Ca2+-ATPase (PMCA), sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA)) and the Na+/Ca2+ exchanger (NCX) are responsible for transporting Ca2+ from the cytosol to external medium or into cellular calcium stores (red arrows). Viruses utilize these calcium components to elevate cytosolic calcium concentration to activate Ca2+-dependent/sensitive enzymes and transcriptional factors to promote virus replication (right panel).
These channels and pumps are activated in a flexible and precise manner to generate specific Ca2+ signaling, satisfying various spatiotemporal requirements. During the viral infections, host cells modulate these calcium-signaling components in response to the infection. On the other hand, viruses utilize these components to create a cellular environment that benefits their own lifecycles [4]. Viruses induce elevated cytosolic calcium concentration to activate Ca2+ dependent/sensitive enzymes and transcriptional factors to promote virus replication. Mitochondria could take the Ca2+ to generate more energy to support continuous virus replication. Moreover, regulating the calcium concentration in ER or Golgi may inhibit host proteins trafficking and promote virus protein maturation. The inhibition of host proteins frustrates host antiviral immune responses, while the promotion of virus protein maturation benefits virus propagation (Figure 1). The journey begins when a virus encounters the host cell and attaches to the cell surface. The virus particle penetrates the cytoplasm via direct membrane fusion or receptor-mediated endocytosis followed by exposing its genome to cellular machinery for replication. When the viral proteins and viral genomes are accumulated, they are assembled to form a progeny virion particle and then released [5]. During the viral lifecycles, they utilize various calcium channels and pumps to obviate the cell membrane barriers, enter the host cell, complete replication, acquire infection ability, and release.
3. Viruses Control Host Voltage-Gated Calcium Channels (VGCCs) and Two-Pore Channels (TPCs)
VGCCs are widely found in the membrane of excitable cells [6] and one of the most well studied viral targets because of the availability of specific channel blockers. There are several different types of VGCCs: L-type (Long-conducting) channels are mostly found in skeletal and smooth myocytes, bone (osteoblasts), and ventricular myocytes; N-type (Non-L or Neuronal), P/Q-type (Purkinje and Granular), and R-type (dihydropyridine- Resistant) channels display longer-lasting conductance and are expressed throughout the nervous system; T-type (Transient) channels produce short-term conductance and are found in neurons and cells that have pacemaker activity [6]. The activation of the channels results in a Ca2+ cascade, which is associated with numerous host physiological functions including excitation–contraction–relaxation coupling of muscles, synaptic transmission, immunoprotection, etc.
Since 1984, it has been known that verapamil, the blocker of VGCCs, inhibits influenza A virus (IAV) infection [7]. Moreover, IAV infection induces Ca2+ influx, and the elevated intracellular Ca2+ promotes endocytic uptake of IAV [8]. Until recently, the underlying mechanism was revealed by Fujioka et al. [9]. They reported that several VGCC blockers and siRNA against the CaV1.2 channel (L-type channel) inhibited H1N1 and H3N2 IAV infection in multiple cell lines. IAV attaches to the target cells by the viral hemagglutinin protein (HA), which is a type I transmembrane protein embedded in the viral envelope, binding to sialic acids [10]. Fujioka et al. showed that the virus HA binds to domain IV of CaV1.2, which contains two potential sialylated asparagine residues (N1436 and N1487) [9,11]. When CaV1.2 mutants in one or both these residues were replaced with glutamine (N1436Q, N1487Q, and N1436Q + N1487Q), the interaction between HA and the Cav1.2 was attenuated compared with the wild-type fragment. They demonstrated that a VGCC blocker, diltiazem, significantly prolonged the survival of IAV-infected mice and allowed the recovery of the survivors. Therefore, CaV1.2 may serve as a host cell surface receptor that binds IAV and is critical for IAV entry (Figure 2A). New world hemorrhagic fever arenaviruses (NWA) are another virus that is reportedly sensitive to the VGCC blocker club member [12,13]. An siRNA screen with Junín virus glycoprotein-pseudotyped viruses identified that VGCCs are involved in NWA entry. Treatment with the channel blocker gabapentin, an FDA (U.S. Food and Drug Administration)-approved analgesic that targets the channel α2δ2 subunit, protects against NWA infection [13]. This research work demonstrated that the interaction between a virus and VGCCs promotes virus entry at the virus-cell fusion step.
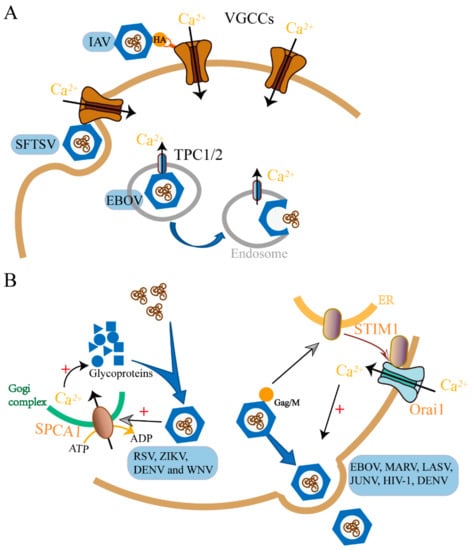
Figure 2.
Examples of viruses interplaying with host calcium channels or pumps to achieve viral entry (A) and release (B). VGCCs are important for influenza A virus (IAV) and severe fever with thrombocytopenia syndrome virus (SFTSV) entry into the host cell as well as TPC1/2 for EBOV (A). RSV, Zika virus (ZIKA), dengue virus (DENV) and West Nile virus (WNV) hijack SPCA1 to facilitate their release as well as Ebola virus (EBOV), MARV, LASV, JUNV, HIV-1 and DENV manipulates STIM1/ORAI1 (B). For a complete list of definitions, see Table 1.
The effect on VGCCs is not only restricted to virus entry. A high-throughput screening of an FDA-approved drug library for inhibitors of Japanese encephalitis virus (JEV) identified five hit drugs, three of which are VGCCs blockers (manidipine, cilnidipine, and benidipine hydrochloride) [14]. Recombinant viral particles (RVPs) with the luciferase-reporting replicon were used to quantify the efficiency of JEV replication, which confirmed that these drugs inhibited JEV infection at the stage of replication. These drugs were subsequently validated for their antiviral activities against other flavivirus, such as Zika virus (ZIKV), dengue virus (DENV), and West Nile virus (WNV). Similarly, another research group screened the FDA-approved drug library and found that nifedipine and benidipine hydrochloride inhibited severe fever with thrombocytopenia syndrome virus (SFTSV) replication in vitro [12]. Moreover, the retrospective clinical investigation on SFTS patients showed that nifedipine can significantly inhibit SFTSV infection. These studies indicate that the VGCCs blockers are excellent candidates for broad-spectrum anti-virus treatment. Most of these FDA-approved VGCC blockers are clinically used to treat cardiovascular diseases. Similar to a double-edged sword, the cardiovascular effect of VGCC blockers may limit their antiviral application. Therefore, repurposing these drugs requires more analysis before clinical trials.
Further examples of virus-regulating VGCCs to service their replication can be found in HIV-1 and herpes simplex virus (HSV)-1. Two HIV-1 proteins, the membrane glycoprotein gp120 [15,16] and the transcriptical transactivator Tat [17,18], have been identified to induce the elevation of host intracellular Ca2+ in various cell types, including neuronal, immune, and epithelial cells, via targeting the activity of VGCCs. The detailed information about the dysregulation of the L-type calcium channel by Tat can be found in the review [16,17].
Taking together, the infections of viruses could increase the host’s intracellular calcium to facilitate viral entry and replication by manipulating host VGCCs. In addition, herpes simplex virus (HSV)-1 could downregulate the VGCCs on infected neuronal cells to escape detection by host cells. T-type Ca2+ channels were reported as the targets of HSV-1 in sensory-like ND7-23 cells [19,20]. HSV-1 infection of differentiated ND7-23 cells causes a significant loss of T-type Ca2+ channels from the membrane, which depends on viral replication and protein synthesis. This downregulation of T-type Ca2+ channel expression may alter the ability of sensory neurons to transmit pain information. Thus, the lower expression of VGCCs may diminish the detection for viral infection by the host, which benefits virus survival and further infection.
Ebola virus (EBOV) used to be considered a VGCC blocker-sensitive virus, and several research groups independently reported that compounds blocking L-type channels (such as verapamil, nimodipine, and diltiazem) inhibited EBOV infection in vivo [21,22,23]. However, gabapentin, representing a fifth distinct class of L-type channel inhibitor, had no effect even at high concentrations. It has been shown that verapamil, nimodipine, and diltiazem also inhibit the activity of two-pore channels (TPCs) [24]. TPCs are intracellular voltage-gated and receptor-operated calcium permeable channels, playing an integral role in membrane trafficking pathways [25,26]. Mouse embryonic fibroblasts (MEFs) lacking TPC1 or TPC2 expression (Tpcn1−/−, Tpcn2−/−) resisted EBOV infection [27]. It turns out that the target of EBOV may not be classical L-type calcium channels but rather endosomal calcium channels termed TPCs (Figure 2A). The calcium channels inhibitors prevented virus–endosome membrane fusion and virus capsid releasing into the cell cytoplasm, which is a late entry step. EBOV acts on TPCs, which control the movement of endosomes containing virus particles, and thereby facilitate its intracellular trafficking [27,28]. The channel inhibitor, tetrandrine, significantly enhanced the survival of mice challenged with mouse-adapted EBOV without any detectable side effects. This indicates that tetrandrine is highly effective against EBOV disease in mice.
4. Store-Operated Calcium (SOC) Channel in Viral Assembly and Egress
SOC channels are the major Ca2+ entry pathways in non-excitable cells. The protein Orai1 on the plasma membrane and STIM1 (stromal interaction molecule) on ER are the molecular identities that are responsible for SOC channels activation. The depletion of ER Ca2+ stores promotes STIM1 proteins aggregation and interaction with Orai1 to open the channel, mediating Ca2+ entry [29,30].
Most enveloped viruses are released extracellularly via exocytosis, also called budding, as an analogy of buds in plants [31,32]. The budding process of the enveloped viruses is triggered by a peptide motif (termed late (L) domains), which was discovered in the Gag polyproteins of retroviruses and M (matrix) proteins of rhabdoviruses [33]. These L domains interact with cellular proteins to promote the formation of virus vesicles that bud away from the cytoplasm [32,34]. Research works established that this essential final step of related viruses depends on the host Ca2+ signal mediated by SOC channels (STIM1/Orai1).
The research work done on four distinct hemorrhagic fever viruses (Ebolavirus (EBOV), Marburgvirus (MARV), Lassa Virus (LASV), and Junín Virus (JUNV)) demonstrated that EBOV, MARV VP40, and JUNV Z proteins trigger host cell Ca2+ signals by activating the ER Ca2+ sensing protein STIM1 and the plasma membrane ORAI1 channel. The STIM1/Orai1-mediated Ca2+ signal is critical for EBOV and related viruses budding from host cells [35]. The suppression of STIM1 expression and genetic inactivation or the pharmacological blockade of ORAI1 inhibits infections of EBOV, MARV, and JUNV in cultured cells (Figure 2B). Obviously, the matrix proteins or live virus activates STIM1 and the ORAI channel. Similarly to hemorrhagic fever viruses, the HIV-1 matrix protein Gag, directing HIV-1 budding, and mediating VLP (Virus-like Particle) formation also exhibit dependence on Ca2+ regulation [36,37]. Therefore, further study is needed to validate the role for STIM1 and the ORAI channel in HIV- 1 budding.
Infections of other enveloped RNA viruses that buds in similar mechanisms may also be inhibited by STIM1 and ORAI1 inhibitors. Indeed, SOC channel antagonists significantly reduced DENV yield [38]. When the human hepatic HepG2 and Huh-7 cells are infected by DENV, STIM1 and ORAI1 were shown to be co-localized in infected cells, indicating activation of the SOC channel [38,39]. Therefore, DENV infection alters cell Ca2+ homeostasis possibly via promoting the interaction between STIM1 and ORAI1.
It is possible that the viral proteins trigger the Ca2+ depletion in ER or prevent ER refilled with Ca2+ to maintain resting ER Ca2+ levels [40,41,42]. Alternately, these viral proteins may directly modify STIM1 and uncouple the activation of STIM1 from Ca2+ levels [35,43]. Thus, STIM1/ORAI might represent a conserved target to regulating the budding of enveloped RNA viruses and possibly DNA viruses that rely on similar host cellular proteins for budding. The example is that the hepatitis B virus X (HBx) protein upregulates the activity of the STIM1–ORAI1 channel complex [44]. The mechanisms by which these viruses activate STIM1 and ORAI1 represent novel therapeutic targets for controlling budding.
Calcium influx through the SOC channel also contributes to the elevated cytosolic calcium concentration induced by rotavirus (RV) infection. It has been well established that the dramatically increased cellular Ca2+ is the hallmark of rotavirus (RV) infection [45,46]. Rotavirus nonstructural protein 4 (NSP4) is an ER-localized viroporin that functionally depletes ER calcium. Thus, in rotavirus-infected cells, STIM1 is constitutively active and colocalizes with the ORAI1 channel. The knockdown of STIM1 or the pharmacological inhibition of SOC channels significantly reduced rotavirus yield, indicating that the SOC channel plays a critical role in the RV lifecycle [47,48].
5. Host Transient Receptor Potential (TRP) Channels and Receptor-Operated Calcium (ROC) Channels
5.1. TRP Channels
The TRP channel is a non-selective cation channel predominately permeable for Ca2+ [49,50]. It is divided into six sub-families according to their amino acid sequence: TRP canonical or classical (TRPC), TRP vanilloid (TRPV), TRP melastatin (TRPM), TRP ankyrin (TRPA), TRP polycystin (TRPP), and TRP mucolipin (TRPML) [50]. They are ubiquitously expressed in different tissues and cell types and are a key player in the regulation of intracellular calcium. Nevertheless, reports about virus control TRP channels are not numerous.
TRPV4 mediates intracellular Ca2+ signals in response to several stimuli, including hypotonic cell swelling, mechanical forces, moderating heat, and UVB radiation [51]. When cells are exposed to the Zika virus or the purified viral envelope protein, TRPV4 mediates Ca2+ influx and drives the nuclear translocation of DEAD-box RNA helicase (DDX3X) [52]. DDX3X is an ATP-dependent RNA helicase from the DEAD-box helicase family and is involved in multiple stages of the RNA metabolism, from transcription to translation [53,54]. Moreover, diverse RNA viruses hijack DDX3X to multiply efficiently. Targeting TRPV4 reduces the infectivity of dengue, hepatitis C, and Zika viruses [52]. Overall, the research work demonstrated the role of TRPV4 in the regulation of DDX3X-dependent control of the RNA metabolism and viral infectivity [52].
The TRPV1, TRPA1, and TRPM8 channels are directly activated by chemical, thermal, and mechanical stimuli. They are potentially associated with respiratory virus-induced airway hypersensitivity [55]. The infection of respiratory viruses, such as respiratory syncytial virus (RSV), measles virus (MV), and human rhinovirus (HRV), was reported to increase the expression of these channels in sensory neurons and human bronchial epithelium cells [56,57]. The increase in TRPA1 and TRPV1 levels can be mediated by soluble factors induced by infection, whereas TRPM8 requires virus replication [56,57]. These reports may explain the possible mechanism by which respiratory viruses induce cough. Alternatively, the up-regulated TRP channels may be utilized by the respiratory viruses to create a favorable calcium environment. Further investigation is required to determine the possible pathway by which this happens.
5.2. N-Methyl-D-Aspartate (NMDA) Receptors
NMDArs and IP3Rs are the two receptor-operated calcium (ROC) channels reported to be involved in virus–host interactions. NMDAr, which is activated by the endogenously synthesized excitant amino acid, glutamate, is widely expressed throughout the mammalian central nervous system and is particularly enriched in the cerebral cortex and hippocampus.
The Zika virus infection is a “neurodegenerative” disease. Costa and colleagues showed that blockage of the NMDAr channel activity with FDA-approved memantine or other antagonists prevents neuronal cell death and microgliosis induced by Zika in vitro and in vivo, without affecting the ability of Zika to replicate in the host [58,59]. It seems that NMDAr mainly contributes to Zika, triggering the neuronal cell death progress. Anyway, the blockade of NMDAr may be a viable treatment for patients at risk for Zika infection-induced neurodegeneration [59]. NMDAr blockade also significantly abrogated neuronal cell death and inflammatory response triggered by JEV infection [60]. Therefore, NMDAr are probably common attack targets of flavivirus, inducing host neuron cell death.
5.3. IP3 Receptors
The engagement of receptors or agonist binding to the cell surface receptors activate phospholipase C (PLC), which hydrolyses phosphatidylinositol-4,5-biphosphate (PIP2) to produce IP3. The activation of IP3R triggers Ca2+ release from stores and further increases IP3R’s sensitivity to Ca2+. Targeting IP3Rs is more universal among different virus types. In turn, this process generates a more profound effect on host cell physiology, including metabolic stress, neurotoxicity, and enterotoxicity. Modifying IP3 and directly interfering with IP3R are the main ways in which viruses affect calcium release through IP3R. For example, human cytomegalovirus (HCMV) UL37x1 protein interacts with the host P2Y2 purinergic receptor to increase intracellular Ca2+ levels via the PLC–IP3 pathway, and this activity is important to viral replication [61]. HIV-1 Gp120 and Tat upregulate intracellular IP3 [37,62], while HIV Nef [63,64] and p12(I) human T-cell lymphotropic virus type 1 (HTLV-1) [65] activate IP3R directly as an agonist. Glycoprotein E of HSV redistributes the density of IP3Rs within infected cells [66]. Some virus proteins activate IP3R through both ways. Enterotoxin NSP4 of RV and HCMV UL37x1-encoded protein increases the basal permeability of the ER as IP3 diffuses and binds to IP3Rs to stimulate Ca2+ release. These virus proteins deplete ER Ca2+ storage during early stages of viral infection to increase the replication ability of viruses. On the other hand, the depletion of ER calcium storage triggers the calcium influx through SOCs (STIM1/ORAI1).
6. Calcium Pumps
The calcium pumps (Ca2+ ATPase) and the Na+–Ca2+ exchanger are the main regulators of intracellular Ca2+ concentrations [67]. Three Ca2+ ATPase types (pumps) have been described in animal cells located in the membranes of the sarcoplasmic reticulum (SERCA pump), in those of the Golgi network (Secretory Pathway Ca2+ ATPase, SPCA pump), and in the plasma membrane (PMCA pump) [68]. At the end of a stimulus, these pumps participate in returning the cell to its resting state by the decrease in the cytosolic Ca2+ concentration. For example, SERCA transports Ca2+ from the cytoplasm into the lumen of the ER. PMCA pumps function to remove Ca2+ from the cell. They serve as the main regulators of intracellular and extracellular Ca2+ concentrations. All three calcium pumps are reported to be involved in different viral lifecycles.
Maturation is the last assembly step of a virus particle. In this step, the virus acquires the infectivity, which is essential for its lifecycle. The results obtained from a genome-wide knockout screen indicated that SPCA1 in the Golgi network is critical for human respiratory syncytial virus (RSV) infection [69,70,71]. Further study demonstrated that similar to RSV, other viruses from the Paramyxoviridae, Flaviviridae, and Togaviridae families also failed to spread in SPCA1-deficient cells. Studies on this mechanism revealed that Ca2+ pumped into Golgi by SPCA1 is the trigger to produce normal functional viral glycoproteins that are essential for virus spread. Therefore, SPCA1 may be a promising target for therapeutic intervention against a diverse set of viruses (Figure 2B). However, in this study, herpes simplex 1 (HSV-1), vesicular stomatitis (VSV), bunyamwera (BUNV), and LCMV were unaffected by the loss of SPCA1. That is probably because the maturation of these viral glycoproteins requires different triggers.
Cui et al. [72] screened the natural product library and found that cyclopiazonic acid, an inhibitor of SERCA, was shown to have activity in the low micromolar range against RSV strains at the step of virus genome replication. Further study found that another SERCA inhibitor BHQ had a similar effect [72]. It is probable that SERCA inhibitors prevent cytosolic calcium returning to the ER from the cytosol via SERCA, resulting in an increase in intracellular calcium concentration. A continuous higher concentration of intracellular calcium may impair viral genome replication and/or transcription, thereby reducing virus yield.
7. Conclusions
The journey of viral infections is also a procedure during which a virus grabs the host intracellular calcium signaling system and homeostasis to benefit its own lifecycle. The disturbance of the host Ca2+ system by virus may suppress T-cell responsiveness, antiapoptotic, and other protentional functions. In this review, we summarized the calcium permeable channels and calcium pumps located on host cell membranes that may be potential therapeutic targets for viral infections (Table 1). However, the calcium channel family is continuously being refreshed with the discovery of new members, and the effect of viruses on the host intracellular calcium environment is not often achieved through a single pathway; it is more complicated. Both upstream messengers and downstream Ca2+ binding proteins are targets for pharmacological intervention. Thus, explicating the entire picture of all aspects of the calcium–virus interplay at the molecular level will be a challenge in the future.

Table 1.
Calcium channels/pumps utilized by a virus.
The prevention of and therapeutic efforts against virus infections are often challenged by the high mutation rates of viral proteins. However, those host calcium apparatus proteins required by viral replication and transmission are highly conserved, essentially immutable, and thus are potentially an Achilles’ heel of virus infection. As described above, almost all the calcium permeable channels or calcium pumps are possible attack targets of viruses and customized host intracellular calcium signaling via effects on these channel proteins. Targeting those host cellular proteins required by virus infection may be one treatment strategy with therapeutic potential, which may avoid or delay the development of resistance. The identified calcium-permeable proteins discussed above offer suitable target proteins for inhibiting virus infection. VGCCs and NMDA receptors are two of most well studied targets because of the availability of specific channel antagonists. Moreover, some of those compounds are FDA-approved drugs with clinical application. For example, verapamil, amlodipine, verapamil, and diltiazem, which are widely used to treat cardiovascular disease, have shown inhibition for various virus infection. Memantine, which is an FDA-approved Alzheimer’s drug, reduces neurological complications associated with Zika virus infection. Obviously, this direction depends on the identification of selective and potent small molecule modulators of calcium channels. Currently, aside from some blockers for VGCCs and the NMDA receptor, almost no other channel modulators can be used in clinics, although important progress has been made in recent years toward high quality and widely available channel modulators. Furthermore, modulators should be generated with properties that make them suitable for in vivo use. This review outlines promising progress made with the potentiation for a host cell calcium channels modulator to become an antiviral drug. We believe that modulators for host calcium channels are the pots of gold for antiviral drug development.
Author Contributions
X.C. and W.Z. conceived this article; X.C. and R.C. wrote the original draft and prepared figures; R.C. and W.Z. edited and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China, grant numbers 81773631 and 81900402), National Science and Technology Major Projects, grant number 2018ZX09711003 and Fundamental Research Funds for the Central Universities grant number G2019KY0501.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martinez de Victoria, E. Calcium, essential for health. Nutr. Hosp. 2016, 33 (Suppl. S4), 341. [Google Scholar]
- Berridge, M.J.; Bootman, M.D.; Lipp, P. Calcium—A life and death signal. Nature 1998, 395, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M. Modulation of host cell intracellular Ca2+. Parasitol. Today 1996, 12, 145–150. [Google Scholar] [CrossRef]
- Clark, K.B.; Eisenstein, E.M. Targeting host store-operated Ca2+ release to attenuate viral infections. Curr. Top. Med. Chem. 2013, 13, 1916–1932. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-van Horn, S.R.; Sarnow, P. Making the Mark: The Role of Adenosine Modifications in the Life Cycle of RNA Viruses. Cell Host Microbe 2017, 21, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Atlas, D. Voltage-gated calcium channels function as Ca2+-activated signaling receptors. Trends Biochem. Sci. 2014, 39, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nugent, K.M.; Shanley, J.D. Verapamil inhibits influenza A virus replication. Arch. Virol. 1984, 81, 163–170. [Google Scholar] [CrossRef]
- Fujioka, Y.; Tsuda, M.; Nanbo, A.; Hattori, T.; Sasaki, J.; Sasaki, T.; Miyazaki, T.; Ohba, Y. A Ca2+-dependent signalling circuit regulates influenza a virus internalization and infection. Nat. Commun. 2013, 4, 2763. [Google Scholar] [CrossRef]
- Fujioka, Y.; Nishide, S.; Ose, T.; Suzuki, T.; Kato, I.; Fukuhara, H.; Fujioka, M.; Horiuchi, K.; Satoh, A.O.; Nepal, P. A Sialylated Voltage-Dependent Ca2+ Channel Binds Hemagglutinin and Mediates Influenza A Virus Entry into Mammalian Cells. Cell Host Microbe 2018, 23, 809–818. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Srinivasan, A.; Raman, R.; Viswanathan, K.; Raguram, S.; Tumpey, T.M.; Sasisekharan, V.; Sasisekharan, R. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat. Biotechnol. 2008, 26, 107–113. [Google Scholar] [CrossRef]
- Lazniewska, J.; Weiss, N. Glycosylation of voltage-gated calcium channels in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, L.K.; Li, S.F.; Zhang, S.F.; Wan, W.W.; Zhang, Y.L.; Xin, Q.L.; Dai, K.; Hu, Y.Y.; Wang, Z.B. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res. 2019, 29, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, M.; Cuevas, C.D.; Thomas, M.; Cherry, S.; Ross, S.R. siRNA screen for genes that affect Junin virus entry uncovers voltage-gated calcium channels as a therapeutic target. Sci. Transl. Med. 2013, 5, 204ra131. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Guo, J.; Wang, P.; Zhang, L.; Xiao, G.; Wang, W. Screening of FDA-Approved Drugs for Inhibitors of Japanese Encephalitis Virus Infection. J. Virol. 2017, 91, e01055-17. [Google Scholar] [CrossRef]
- Dreyer, E.B.; Kaiser, P.K.; Offermann, J.T.; Lipton, S.A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science 1990, 248, 364–367. [Google Scholar] [CrossRef]
- Haughey, N.J.; Mattson, M.P. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J. Acquir. Immune. Defic. Syndr. 2002, 31 (Suppl. S2), S55–S61. [Google Scholar] [CrossRef]
- Hu, X.T. HIV-1 Tat-Mediated Calcium Dysregulation and Neuronal Dysfunction in Vulnerable Brain Regions. Curr. Drug Targets 2016, 17, 4–14. [Google Scholar] [CrossRef]
- Kruman, I.I.; Nath, A.; Mattson, M.P. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp. Neurol. 1998, 154, 276–288. [Google Scholar] [CrossRef]
- Zhang, Q.; Hsia, S.C.; Martin-Caraballo, M. Regulation of T-type Ca2+ channel expression by herpes simplex virus-1 infection in sensory-like ND7 cells. J. Neurovirol. 2017, 23, 657–670. [Google Scholar] [CrossRef]
- Zhang, Q.; Hsia, S.C.; Martin-Caraballo, M. Regulation of T-type Ca2+ channel expression by interleukin-6 in sensory-like ND7/23 cells post-herpes simplex virus (HSV-1) infection. J. Neurochem. 2019, 151, 238–254. [Google Scholar] [CrossRef]
- Kolokoltsov, A.A.; Saeed, M.F.; Freiberg, A.N.; Holbrook, M.R.; Davey, R.A. Identification of novel cellular targets for therapeutic intervention against Ebola virus infection by siRNA screening. Drug Dev. Res. 2009, 70, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Gehring, G.; Rohrmann, K.; Atenchong, N.; Mittler, E.; Becker, S.; Dahlmann, F.; Pohlmann, S.; Vondran, F.W.; David, S.; Manns, M.P. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J. Antimicrob. Chemother. 2014, 69, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Madrid, P.B.; Chopra, S.; Manger, I.D.; Gilfillan, L.; Keepers, T.R.; Shurtleff, A.C.; Green, C.E.; Iyer, L.V.; Dilks, H.H.; Davey, R.A. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS ONE 2013, 8, e60579. [Google Scholar] [CrossRef] [PubMed]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.T. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Ma, J.; Parrington, J.; Calcraft, P.J.; Galione, A.; Evans, A.M. Calcium signaling via two-pore channels: Local or global, that is the question. Am. J. Physiol. Cell Physiol. 2010, 298, C430–C441. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J. Ca2+ dialogue between acidic vesicles and ER. Biochem. Soc. Trans. 2016, 44, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef]
- Kintzer, A.F.; Stroud, R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 258–262. [Google Scholar] [CrossRef]
- Hogan, P.G.; Lewis, R.S.; Rao, A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010, 28, 491–533. [Google Scholar] [CrossRef]
- Nwokonko, R.M.; Cai, X.; Loktionova, N.A.; Wang, Y.; Zhou, Y.; Gill, D.L. The STIM-Orai Pathway: Conformational Coupling between STIM and Orai in the Activation of Store-Operated Ca2+ Entry. Adv. Exp. Med. Biol. 2017, 993, 83–98. [Google Scholar]
- Sepulveda, C.S.; Garcia, C.C.; Damonte, E.B. Determining the Virus Life-Cycle Stage Blocked by an Antiviral. Methods Mol. Biol. 2018, 1604, 371–392. [Google Scholar] [PubMed]
- Hartlieb, B.; Weissenhorn, W. Filovirus assembly and budding. Virology 2006, 344, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Lamb, R.A. Mechanisms for enveloped virus budding: Can some viruses do without an ESCRT? Virology 2008, 372, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Licata, J.M.; Simpson-Holley, M.; Wright, N.T.; Han, Z.; Paragas, J.; Harty, R.N. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: Involvement of host proteins TSG101 and VPS-4. J. Virol. 2003, 77, 1812–1819. [Google Scholar] [CrossRef]
- Han, Z.; Madara, J.J.; Herbert, A.; Prugar, L.I.; Ruthel, G.; Lu, J.; Liu, Y.; Liu, W.; Liu, X.; Wrobel, J.E. Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention. PLoS Pathog. 2015, 11, e1005220. [Google Scholar] [CrossRef]
- Ehrlich, L.S.; Carter, C.A. HIV Assembly and Budding: Ca2+ Signaling and Non-ESCRT Proteins Set the Stage. Mol. Biol. Int. 2012, 2012, 851670. [Google Scholar] [CrossRef]
- Ehrlich, L.S.; Medina, G.N.; Carter, C.A. ESCRT machinery potentiates HIV-1 utilization of the PI (4,5) P (2)-PLC-IP3R-Ca2+ signaling cascade. J. Mol. Biol. 2011, 413, 347–358. [Google Scholar] [CrossRef]
- Dionicio, C.L.; Pena, F.; Constantino-Jonapa, L.A.; Vazquez, C.; Yocupicio-Monroy, M.; Rosales, R.; Zambrano, J.L.; Ruiz, M.C.; Del Angel, R.M.; Ludert, J.E. Dengue virus induced changes in Ca2+ homeostasis in human hepatic cells that favor the viral replicative cycle. Virus Res. 2018, 245, 17–28. [Google Scholar] [CrossRef]
- Cheshenko, N.; Liu, W.; Satlin, L.M.; Herold, B.C. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol. Biol. Cell 2007, 18, 3119–3130. [Google Scholar] [CrossRef]
- Robinson, L.C.; Marchant, J.S. Enhanced Ca2+ leak from ER Ca2+ stores induced by hepatitis C NS5A protein. Biochem. Biophys. Res. Commun. 2008, 368, 593–599. [Google Scholar] [CrossRef]
- Zhadina, M.; Bieniasz, P.D. Functional interchangeability of late domains, late domain cofactors and ubiquitin in viral budding. PLoS Pathog. 2010, 6, e1001153. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.C.; Aristimuno, O.C.; Diaz, Y.; Pena, F.; Chemello, M.E.; Rojas, H.; Ludert, J.E.; Michelangeli, F. Intracellular disassembly of infectious rotavirus particles by depletion of Ca2+ sequestered in the endoplasmic reticulum at the end of virus cycle. Virus Res. 2007, 130, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.D.; Harty, R.N. Calcium and filoviruses: A budding relationship. Future Microbiol. 2016, 11, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.H.; Liu, Z.J.; Yi, J.H.; Wang, J.; Liu, Y.N. Hepatitis B Virus X Protein Upregulates Intracellular Calcium Signaling by Binding C-terminal of Orail Protein. Curr. Med. Sci. 2018, 38, 26–34. [Google Scholar] [CrossRef]
- Michelangeli, F.; Ruiz, M.C.; del Castillo, J.R.; Ludert, J.E.; Liprandi, F. Effect of rotavirus infection on intracellular calcium homeostasis in cultured cells. Virology 1991, 181, 520–527. [Google Scholar] [CrossRef]
- Pham, T.; Perry, J.L.; Dosey, T.L.; Delcour, A.H.; Hyser, J.M. The Rotavirus NSP4 Viroporin Domain is a Calcium-conducting Ion Channel. Sci. Rep. 2017, 7, 43487. [Google Scholar] [CrossRef]
- Hyser, J.M.; Utama, B.; Crawford, S.E.; Broughman, J.R.; Estes, M.K. Activation of the endoplasmic reticulum calcium sensor STIM1 and store-operated calcium entry by rotavirus requires NSP4 viroporin activity. J. Virol. 2013, 87, 13579–13588. [Google Scholar] [CrossRef]
- Chang-Graham, A.L.; Perry, J.L.; Strtak, A.C.; Ramachandran, N.K.; Criglar, J.M.; Philip, A.A.; Patton, J.T.; Estes, M.K.; Hyser, J.M. Rotavirus Calcium Dysregulation Manifests as Dynamic Calcium Signaling in the Cytoplasm and Endoplasmic Reticulum. Sci. Rep. 2019, 9, 10822. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Song, X.; Liu, R.; Zhang, J.; Li, Z. The structure of TRPC ion channels. Cell Calcium. 2019, 80, 25–28. [Google Scholar] [CrossRef]
- Hicks, G.A. TRP channels as therapeutic targets: Hot property, or time to cool down? Neurogastroenterol. Motil. 2006, 18, 590–594. [Google Scholar] [CrossRef]
- Garcia-Elias, A.; Mrkonjic, S.; Jung, C.; Pardo-Pastor, C.; Vicente, R.; Valverde, M.A. The TRPV4 channel. Handb. Exp. Pharm. 2014, 222, 293–319. [Google Scholar]
- Donate-Macian, P.; Jungfleisch, J.; Perez-Vilaro, G.; Rubio-Moscardo, F.; Peralvarez-Marin, A.; Diez, J.; Valverde, M.A. The TRPV4 channel links calcium influx to DDX3X activity and viral infectivity. Nat. Commun. 2018, 9, 2307. [Google Scholar] [CrossRef] [PubMed]
- Ariumi, Y. Multiple functions of DDX3 RNA helicase in gene regulation, tumorigenesis, and viral infection. Front. Genet. 2014, 5, 423. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Echeverria, F.; Hermoso, M.A.; Soto-Rifo, R. RNA helicase DDX3: At the crossroad of viral replication and antiviral immunity. Rev. Med. Virol. 2015, 25, 286–299. [Google Scholar] [CrossRef]
- Fujita, T.; Liu, Y.; Higashitsuji, H.; Itoh, K.; Shibasaki, K.; Fujita, J.; Nishiyama, H. Involvement of TRPV3 and TRPM8 ion channel proteins in induction of mammalian cold-inducible proteins. Biochem. Biophys. Res. Commun. 2018, 495, 935–940. [Google Scholar] [CrossRef]
- Omar, S.; Clarke, R.; Abdullah, H.; Brady, C.; Corry, J.; Winter, H.; Touzelet, O.; Power, U.F.; Lundy, F.; McGarvey, L.P. Respiratory virus infection up-regulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PLoS ONE 2017, 12, e0171681. [Google Scholar] [CrossRef]
- Abdullah, H.; Heaney, L.G.; Cosby, S.L.; McGarvey, L.P. Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: Implications for respiratory virus-induced cough reflex sensitivity. Thorax 2014, 69, 46–54. [Google Scholar] [CrossRef]
- Costa, V.V.; Del Sarto, J.L.; Rocha, R.F.; Silva, F.R.; Doria, J.G.; Olmo, I.G.; Marques, R.E.; Queiroz-Junior, C.M.; Foureaux, G.; Araujo, J.M.S. N-Methyl-d-Aspartate (NMDA) Receptor Blockade Prevents Neuronal Death Induced by Zika Virus Infection. MBio 2017, 8, e00543-17. [Google Scholar] [CrossRef]
- Sirohi, D.; Kuhn, R.J. Can an FDA-Approved Alzheimer’s Drug Be Repurposed for Alleviating Neuronal Symptoms of Zika Virus? MBio 2017, 8, e00916-17. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Ashraf, U.; Zheng, B.; Ye, J.; Zhou, D.; Zhang, H.; Song, Y.; Chen, H.; Zhao, S. Activation of neuronal N-methyl-D-aspartate receptor plays a pivotal role in Japanese encephalitis virus-induced neuronal cell damage. J. Neuroinflammation 2018, 15, 238. [Google Scholar] [CrossRef]
- Chen, S.; Shenk, T.; Nogalski, M.T. P2Y2 purinergic receptor modulates virus yield, calcium homeostasis, and cell motility in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 2019, 116, 18971–18982. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, L.S.; Medina, G.N.; Photiadis, S.; Whittredge, P.B.; Watanabe, S.; Taraska, J.W.; Carter, C.A. Tsg101 regulates PI (4,5) P2/Ca2+ signaling for HIV-1 Gag assembly. Front. Microbiol. 2014, 5, 234. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, L.S.; Medina, G.N.; Khan, M.B.; Powell, M.D.; Mikoshiba, K.; Carter, C.A. Activation of the inositol (1,4,5)-triphosphate calcium gate receptor is required for HIV-1 Gag release. J. Virol. 2010, 84, 6438–6451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manninen, A.; Saksela, K. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J. Exp. Med. 2002, 195, 1023–1032. [Google Scholar] [CrossRef]
- Ding, W.; Albrecht, B.; Kelley, R.E.; Muthusamy, N.; Kim, S.J.; Altschuld, R.A.; Lairmore, M.D. Human T-cell lymphotropic virus type 1 p12 (I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J. Virol. 2002, 76, 10374–10382. [Google Scholar] [CrossRef]
- Cheshenko, N.; Del Rosario, B.; Woda, C.; Marcellino, D.; Satlin, L.M.; Herold, B.C. Herpes simplex virus triggers activation of calcium-signaling pathways. J. Cell Biol. 2003, 163, 283–293. [Google Scholar] [CrossRef]
- Strehler, E.E.; Zacharias, D.A. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev. 2001, 81, 21–50. [Google Scholar] [CrossRef]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Calcium pumps: Why so many? Compr. Physiol. 2012, 2, 1045–1060. [Google Scholar]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Schneider, W.M.; Blomen, V.A.; Scull, M.A.; Hovnanian, A.; Brummelkamp, T.R.; Rice, C.M. Diverse Viruses Require the Calcium Transporter SPCA1 for Maturation and Spread. Cell Host Microbe 2017, 22, 460–470. [Google Scholar] [CrossRef]
- Cervantes-Ortiz, S.L.; Zamorano Cuervo, N.; Grandvaux, N. Respiratory Syncytial Virus and Cellular Stress Responses: Impact on Replication and Physiopathology. Viruses 2016, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Wang, Y.; Wang, L.; Li, G.; Lan, K.; Altmeyer, R.; Zou, G. Cyclopiazonic acid, an inhibitor of calcium-dependent ATPases with antiviral activity against human respiratory syncytial virus. Antivir. Res. 2016, 132, 38–45. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).