Abstract
Meniscal injuries have posed a challenging problem for many years, especially considering that historically the meniscus was considered to be a structure with no important role in the knee joint. This led to earlier treatments aiming at the removal of the entire structure in a procedure known as a meniscectomy. However, with the current understanding of the function and roles of the meniscus, meniscectomy has been identified to accelerate joint degradation significantly and is no longer a preferred treatment option in meniscal tears. Current therapies are now focused to regenerate, repair, or replace the injured meniscus to restore its native function. Repairs have improved in technique and materials over time, with various implant devices being utilized and developed. More recently, strategies have applied stem cells, tissue engineering, and their combination to potentiate healing to achieve superior quality repair tissue and retard the joint degeneration associated with an injured or inadequately functioning meniscus. Accordingly, the purpose of this current review is to summarize the current available pre-clinical and clinical literature using stem cells and tissue engineering for meniscal repair and regeneration.
1. Introduction
The meniscus is an essential member of the knee joint and without its proper functioning, pathologic force distribution and instability occur in the knee, negatively affecting overall joint biomechanics [1,2]. Due to the avascular and hypocellular nature of meniscal tissue, it possesses a capacity for healing once damaged [3,4]. Though the guarded prognosis of meniscectomy was noticed as early as 1923 [5], many surgeons still perform total or partial removal of the meniscus to address meniscal tears. This management option remains popular as modern-day medicine is yet to find an effective evidence-based solution. It is now generally accepted that every effort should be made to repair and retain as much native meniscal tissue as possible [6]. This change in approach has led to the introduction of several novel reparative techniques and strategies to restore meniscal function in individuals with meniscal injuries. Owing to the complex phenotype of meniscal tissue, tissue regeneration using stem cell therapy may hold the key to tackling meniscal tears. The use of both meniscal cells and mesenchymal stem cells (MSCs) have proven effective in regenerating meniscal tissue, however meniscal cell harvest poses an unacceptable donor site morbidity and tear site cells have little to no chondrogenic potential [7]. Therefore, the majority of research has been focused on stem cells where there is a reasonable amount of pre-clinical data but limited clinical data.
Meniscal replacement strategies in the form of a collagen meniscal implant and a polyurethane polymer scaffold have been employed over recent years with promising clinical results. However, these implants have been unsuccessful in emulating normal meniscal biomechanics and radiological follow-up does not demonstrate images resembling that of normal meniscus [8,9,10,11]. Meniscal allograft transplantation has also been an option in selected healthcare systems but has several limitations ranging from graft availability, congruence, biocompatibility, fixation problems, and potential infection [8]. With the current preferred management option being meniscal repair, the purpose of this article is to comprehensively review the current status of stem cell treatments in both pre-clinical and clinical studies, dividing them into injection-based and tissue-engineered cell therapies.
2. Anatomy of the Meniscus
The menisci are composed of two semilunar shaped structures divided into a medial and lateral component of which both are biphasic and fibrocartilaginous. They are composed of a dense extracellular matrix (ECM) with low cellularity and vascular supply exclusively to the outer 10–15% of tissue [12,13,14,15]. The menisci are attached to the tibial plateau at anterior and posterior roots and are part of a meniscal ligament complex consisting of the medial collateral ligament (MCL), the transverse ligament, the meniscotibial and meniscofemoral ligaments [12,16,17] (Figure 1).
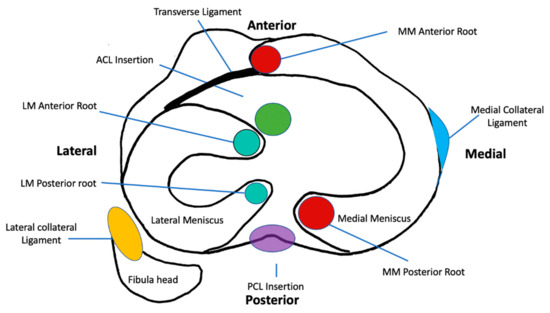
Figure 1.
Schematic diagram of the axial section at the level of the tibial plateau depicting the anatomy, attachments, and relations of the menisci. MM, Medial Meniscus; PCL, Posterior Cruciate Ligament; LM, Lateral Meniscus; ACL, Anterior Cruciate Ligament.
Within the meniscus fibrils, fibers, and fascicles are arranged in diverse patterns depending on the region of tissue [18]. The innermost region consists of small unorganized woven radial collagen fibrils with a structure similar to that of cartilage [19,20], with greater proteoglycan content. The outer region consists of intertwined collagen fibrils in a circumferential orientation with radially oriented three-dimensional arrays of fibers known as “tie-fibers” (Figure 2). They lie perpendicular to the circumferential collagen fibers and originate from the joint capsule creating a complex honeycomb network [18,21]. The root attachments of the meniscus to the tibia are more ligamentous-like structures with a fibrocartilaginous enthesis [22].
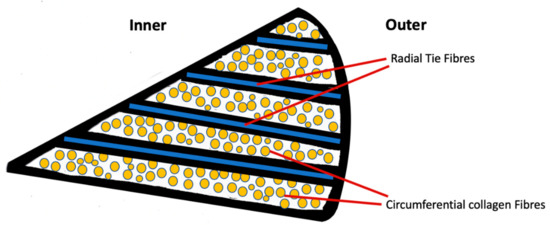
Figure 2.
Schematic cross-sectional diagram of the meniscus displaying the circumferential arrangement of collagen fibers and radial tie fibers.
The biochemical composition of meniscal tissue is 72% water, 22% collagen, 0.8% glycosaminoglycan (GAG) and 0.012% DNA [23]. Within the meniscal ECM, there is a greater amount of collagen I in the outer red–red zone and a greater amount of collagen II in the inner white–white zone [24]. Numerous proteoglycans exist within meniscal tissue of which the most abundant is aggrecan, others include biglycan, decorin, fibromodulin, lubricin, and elastin [25,26,27]. These proteoglycans provide the meniscus with its viscoelastic, low friction, yet strong phenotype.
The cellularity of meniscal tissue is composed of oval fibrochondrocytes and spindle-shaped fibroblast-like meniscus cells near the outer region connected via long cell extensions [12,28,29]. The cells present in the inner region of the meniscus are chondrocyte-like and are more rounded and embedded within the ECM [30]. Within the superficial zone of the meniscus, one more population of cells described has a flattened, fusiform morphology without any cell extensions. These have been postulated to be progenitor cells with regenerative capabilities [31].
3. Functions of the Meniscus
The meniscus plays an important role in normal knee joint mechanics and function by the transmission of joint reaction forces, lubrication, nutrition to the cartilage and shock absorption [32,33]. During standard weight-bearing, the forces applied to the meniscus are known as “hoop stresses”. These are circumferential forces generated as a result of vertical axial forces being converted to horizontal tensile forces owing to the meniscal tissues circumferential collagen fiber arrangement [34]. Shear forces are similarly developed between collagen fibers when the meniscus undergoes radial deformation [35]. The wedge shape of the meniscus allows for better articulation and stability for the rounded femoral condyle on the flat tibial plateau [2,36]. The medial meniscus has also been demonstrated to have a considerable contribution to preventing anterior tibial translation alongside the anterior cruciate ligament (ACL) [37].
It is hypothesized that through a system of micro canals within the meniscal tissue there is the transport of synovial fluid in order to nourish the articular cartilage by compressing synovial fluid into the cartilage reducing friction on the chondral surface [38,39]. Another key feature of the meniscus is the presence of proprioceptive mechanical receptors, in the form of Pacinian corpuscles and Ruffini endings located in the anterior and posterior horns of the menisci contributing to joint position sense and afferent sensory feedback [40,41,42].
It is important to note the several roles of the meniscus and focus on interventions restoring it to full capacity. It can be certainly agreed that the complex phenotype of the meniscus is in accordance with its complex functionality.
4. Meniscus Pathology
Meniscal injuries may be acute or degenerative and be as a result of macro-trauma or chronic repetitive attrition commonly encountered in middle-aged and older patients. Acute tears are usually in association with a traumatic event where a combination of compressive, shear, and rotational forces are applied across the meniscus from the femoral condyles onto the tibial plateau. Acute tears are classified into different patterns: Longitudinal, radial, and horizontal, these can progress to more complex tears. In certain situations, tears may displace the tissue and it may get lodged between the femoral condyles, thereby locking the knee joint in flexion. Degenerative meniscal lesions occur more gradually over time and are usually associated with osteoarthritis (OA) [43,44]. Data suggests that the incidence of degenerative tears is higher than earlier believed, as many tears remain asymptomatic [45,46,47]. Degenerative tears are more frequently located in the posterior horn of the medial meniscus and are of horizontal-cleavage or flap tears with some element of tissue destruction [48]. Besides tear morphology, the overall position of the meniscus is important to evaluate. Extrusion of the meniscus can occur concomitantly with certain tear types, particularly root and radial tears, and usually occurs in degenerative lesions in the setting of OA [49,50,51].
The healing potential of a meniscal tear is largely dictated by the tear location. The meniscus has been described to have an inner white–white avascular zone and an outer red-red vascular zone. Between these is a red–white zone of less, but still some degree of, vascularity (Figure 3). Tears involving the inner zone have the least healing potential due to a lack of blood supply [3,52].
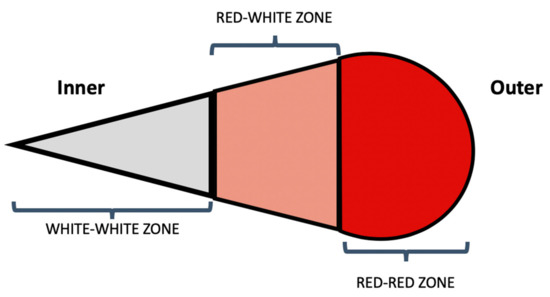
Figure 3.
Schematic cross-sectional diagram of the body of the meniscus representing the vascular zones of the meniscus.
When the meniscal function is compromised in the event of an injury, the biomechanics of the knee is deranged. There is increased stress on the cartilage in the joint which can lead to cartilage loss, bony changes, and OA progression [53,54,55,56]. Studies have even shown trabecular bone variations as a result of the loss of meniscal function. In settings of meniscal extrusion, the meniscus no longer absorbs hoops stresses, joint space is reduced and there is an increased possibility in the occurrence of bone marrow lesions [57,58].
5. Types of Mesenchymal Stem Cells
Stem cell therapies in musculoskeletal medicine have employed numerous sources of stem cells, and more recently the breakthrough of the induced pluripotent cell has meant cells can now be reprogrammed to perform as stem cells [59]. Treatment strategy focus has been primarily on cartilage, meniscus, and bone to treat chondral defects, meniscal injuries, and fractures. MSCs have been of keen interest in stem cell treatments due to their ease of availability and differentiation capabilities [60]. Cell source is an important consideration for successful outcomes in stem cell therapies [61] and common sources include bone marrow [62], adipose [63], synovium [64], and blood [65]. There is no absolute best cell source as each source has its advantages, disadvantages, and differentiation capacities. Table 1 has been constructed based on studies by Sakaguchi et al. on human MSCs [64] and Yoshimura et al. on rat MSCs [66]. Both studies concluded that synovial tissue was the superior choice of tissue when comparing osteogenic, chondrogenic and adipogenic capacities of the three cell sources. Additional literature has also found synovium to be a superior and effective cell source of MSCs [61,67,68]. Bone marrow has been a popular cell source in the majority of studies. The principal difficulties associated with bone marrow MSCs (BMMSC) is the harvesting process being painful, and their limited differentiation potential with in vitro expansion [69]. Adipose-derived stem cells (ADSCs) have gained popularity for their high yield [70] and ease to procure through liposuction. Literature does, however, report ADSCs to be inferior to synovial MSCs in terms of their chondrogenic and osteogenic differentiation capacities [64]. The clinical advantages and disadvantages of the discussed cell sources have also been outlined in Table 1 [64,69,70,71]. Concerning meniscal tissue, the ideal cell source remains to determined and despite showing varying differentiation capacities between sources in different models the literature still lacks evidence to state one cell source superior to another in meniscal regeneration.

Table 1.
Summary table showing differentiation capacities as well as advantages and disadvantages of bone marrow, adipose and synovium mesenchymal stem cells (MSCs) [64,66,67,68,69,70,71].
6. Mechanism of Meniscal Repair
The precise mechanism by which a meniscal regeneration occurs remains unknown, it is, however, thought to occur via both extrinsic and intrinsic pathways [72,73,74]. The extrinsic pathway is dependent on the tear site vascularity, where undifferentiated MSCs and growth factors can encourage the repair. The more direct intrinsic pathway occurs via the self-healing capability of the meniscal tissue and is not always a strong contributor to repair [75]. It is known that after meniscal injury the number of MSCs in the synovial fluid increases providing endogenous cells required for repair [76].
As with all healing, angiogenesis is a vital factor in meniscal tear repair too, promoting repair by supplying growth factors and inflammatory processes. The significance of angiogenesis has been demonstrated in a rabbit meniscal defect model where angiogenin treated defects had significantly better healing rates than the control group [77]. This is following other studies that have shown good healing rates in the vascular rich red-red zone of the meniscus [78,79]. Some literature has also shown synovium to contribute some element of vascularity to injured meniscal sites [80,81]. The role of growth factors remains dependent on the injury site vascularity and their anabolic effects have shown to improve MSC differentiation and phenotype [52]. Such growth factors are secreted as a result of the paracrine functions of MSCs into exosomes, allowing them to modulate angiogenesis, cell migration, differentiation, and numerous additional processes [82]. For this reason, research has focused on the application of various growth factors within scaffolds, to meniscal injury models with the hope of an enhanced healing response. Of note, recently transforming growth factor (TGF-β3) and connective tissue growth factor (CTGF) have shown positive results in ovine model meniscal repairs, with the ability to induce cell differentiation towards native zone-specific matrix phenotypes [83,84]. This highlights the key roles of MSCs and growth factors in successful meniscal healing to generate cellular phenotypes resembling that of normal meniscal tissue.
Mechanical factors also affect meniscal healing considerably and can have undesirable effects on healing when the meniscus is loaded pathologically. This is the rationale behind stabilizing tears with sutures to immobilize tear sites. Though tear site stability seems to be more important than complete immobilization of the joint [85,86]. Normal physiologic loading of the meniscus has been shown to have anti-inflammatory and overall anabolic effects while pathological loading has the exact opposite effect increasing catabolism, inflammation, and cell death [87]. Overall it can be summarized that meniscal repair is very much dependent on vascularity and stability of the tear site. Good vascularity facilitates pluripotent stem cells and endogenous growth factors to interact and mediate the production of repair meniscal tissue.
7. Pre-Clinical Studies
7.1. Stem Cell Injection
Simple MSC injections from different sources have been employed in various animal models to evaluate their effects on tissue regeneration and healing. Recently, synovium has been identified as a good source of MSCs as these cells have a high potential for proliferation and chondrogenic differentiation [64,88,89]. Nakagawa et al. [90] combined allogeneic synovial MSCs and a suture repair to a meniscal defect model in a porcine model. In their study, the time to outcome assessment was only 12 weeks, though they reported superior results than in an isolated suture repair group. The MSC group demonstrated higher histology scores, collagen deposition and greater tensile strength in the repair site. They noted no immunologic reactions despite not using any immunosuppressive drugs in the subjects. A similar study employing allogeneic synovial MSCs was performed by Hatsushika et al. [91] using multiple doses of intraarticular synovial MSC injections in a porcine model. The defect model was somewhat large where the entire anterior half of the medial meniscus was removed. Subjects injected with MSCs showed defect filling with synovial tissue at 2 weeks. At 16 weeks when compared to the control group, the MSC group had superior quality tissue with improved safranin-o and collagen I and II staining. They concluded that synovial derived MSCs promoted meniscal regeneration and were more effective with repeated intraarticular injection use, though the optimal number of injections was yet to be determined. Both studies [91,92] did also mention that this was an acute meniscal tear model and that regenerative results may be different in a chronic scenario as demonstrated by Ruiz-Iban et al. [92]. In this study, rabbit meniscal lesions were created, and some subjects underwent an acute treatment protocol while others were treated after 3 weeks to simulate tear chronicity. Meniscal healing was significantly better in the acutely treated groups, thereby confirming tear chronicity having a role to play in tissue healing.
A study by Ferris et al. [93] administered an intraarticular injection of autologous BMMSCs to horse stifle joints after the diagnosis of a meniscal tear by arthroscopy. This model is more accurate in that the time from injury to injection simulated that of a normal clinical scenario as opposed to the creation of a defect and immediate subsequent treatment. The subjects received only debridement followed by the intraarticular BMMSC injection, no suture repair was performed alongside the treatment. Eighteen out of twenty-four horses with documented meniscal lesions returned to work with 9 horses reaching their previous levels of activity. The outcomes of this study were compared to previous reports and they reported significant positive outcomes in BMMSC injections for the treatment of meniscal lesions in horses. Another study employing only autologous BMMSCs in a canine meniscus tear model found better healing responses in injected subjects compared to the controls [94]. Injected subjects exhibited significantly better histology with marked angiogenesis, fibroblast proliferation, chondrogenesis, and collagen deposition. They concluded that BMMSCs were effective in regenerating meniscus tissue and could function by either BMMSC differentiation or mediator release signaling a healing mechanism. From these models, it is evident that MSCs have a role to play in meniscal regeneration and that subjects who received some form of stem cell injection, whether combined with a repair or not, did display superior healing responses and histology.
As mentioned earlier, it has still not been determined which cell source is superior for MSC treatments, but each has shown promising results with their own advantages and disadvantages. In addition, allogeneic cells have also shown promising results, which would have its own benefits for manufacturing, cost, and development of single-stage treatment strategies. Table 2 summarizes pre-clinical stem cell injection data studies included in our review.

Table 2.
Summary of pre-clinical studies using stem cell injections.
7.2. Tissue Engineering
Tissue engineering techniques concerning meniscus regeneration have employed MSCs in combination with engineered scaffolds and growth factors to achieve more efficient and better-quality repair/regenerate tissue. Several scaffolds have been developed ranging from synthetic polymers to more natural tissue-derived sources. In vitro studies have demonstrated the outcomes of using different cell types [7] and the benefits of adding growth factors to cultures that can promote GAG production and improve cell differentiation enhancing the bioactivity of the overall scaffold for integration [95]. Such study models have also shed light on the effects of inhibitory effects on the meniscal repair by interleukin-1 and tumor necrosis factor-α commonly upregulated in injured joints [96,97,98]. The mechanical variations of the inner and outer zone cells of the meniscus have also been thought to bring about variation in gene expression and protein regulation [99]. The challenge in scaffold optimization is in finding a delicate balance between mechanical strength and bioactivity.
Zhang et al. [100] in a goat model demonstrated the healing capacity of BMMSCs transfected with human insulin-like growth factor 1 (hIGF-1) using a calcium alginate gel for delivery into a full-thickness meniscal defect. Their study included three control groups: one group with cells and no hIGF-1 transfection, one with the alginate gel alone, and one without any form of treatment. The group repaired with hIGF-1 transfected cells developed the best reparative tissue with margins difficult to delineate from the neighboring native tissue. This group also demonstrated a greater number of cells, more cartilaginous tissue, and higher GAG content than the control groups. This is a clear example of an in vivo application of a growth factor (hIGF-1) in combination with MSCs promoting and enhancing the meniscal regenerate.
Moriguchi et al. developed a natural tissue-engineered construct (TEC) consisting of a high- density monolayer culture of allogeneic synovial MSCs in the presence of ascorbic acid [101]. Four-millimeter cylindrical defects were created in a porcine meniscus model and repaired using TEC or left untreated for control. All TEC implanted defects were filled with well-integrated repair tissue, while the controls remained empty or partially filled. Histology of the TEC repair displayed cartilage-like cells with lacunae indicative of fibro-cartilaginous tissue. The incidence of chondral injury 6 months after defect creation was significantly less in the TEC group in comparison to the control. Interestingly, TEC is scaffold-free and yet provides good bioactivity and mechanical support to the repair site. The study concluded that a fibrocartilaginous repair tissue is a desirable result for meniscal defects as meniscal tissue displays mixed characteristics of hyaline cartilage and fibrous tissue. This animal study validated that TEC could be an effective solution to achieve the desirable hybrid tissue qualities required to fill a meniscal defect and retard OA progression that typically follows a meniscal injury. Kondo et al. [102] used autologous synovial MSC aggregates to repair meniscal defects in a primate model. They found the medial meniscus in the repair group to have larger regenerate at both time points of 8 and 16 weeks. The regenerated meniscus also stained better with safranin-o and had T1rho magnetic resonance imaging (MRI) that resembled native meniscal tissue. Both the control and the study group did show OA changes, though the MSC treated group had better scores. This study again demonstrated both the regenerative potential of synovial MSCs and also the use of aggregates alone without the need for a fixation method to treat meniscal injury.
Desando et al. [103] used a hyaluronic acid (HA) scaffold seeded with autologous BMMSCs in an ovine meniscal defect model. No fixation technique was employed as the mesh had intrinsic adhesive properties [104]. The BMMSC seeded HA scaffold group revealed a superior repair with a smooth restored surface and good proteoglycan content compared to the control group. There was greater expression of collagen type I and II and lower expression of matrix metalloproteinase-13 and interleukin 1 beta indicating less collagen degradation [105,106,107]. The bone marrow seeded HA group was therefore determined to be more chondroprotective when compared to the control group. This study confirmed that BMMSCs can enhance a more effective meniscal repair, as well as reduce the biochemical changes associated with the progression of OA. Table 3 summarizes the pre-clinical tissue engineered stem cell studies included in our review.

Table 3.
Summary of pre-clinical studies using tissue engineering.
8. Clinical Studies
8.1. Stem Cell Injection
Clinical studies evaluating the effects of MSC injections in the knee joint are limited, but early clinical data suggests encouraging results. Currently, there have not been any reported safety concerns or side-effects in the clinical use of MSC injections.
There is only one randomized double-blind controlled study to date studying the effects of MSC injections into the knee post medial meniscectomy [108]. The study contained 55 subjects in 3 groups who underwent a percutaneous injection of allogeneic MSCs with one group receiving 50 × 106 cells another 150 × 106 cells and control receiving only HA. At 12 months follow up, MRI scan findings reported a significant increase in meniscal volume in 24% of patients receiving 50 × 106 cells and 6% receiving 150 × 106 cells. None of the control group patients demonstrated an increase in meniscal volume. The study is limited to MRI scan being the only objective outcome measure, but the study methodology is rigorous in that it has the advantage of being blinded and randomized.
Pak et al. [109] reported the results of a single patient who received an ultrasound guided autologous adipose stem cell (ASC) intraarticular injection to the knee joint for treatment of an isolated meniscus tear. The final injection mixture contained the ASCs, platelet-rich plasma (PRP), HA and calcium chloride. This patient also received follow up injections of PRP, HA, and dexamethasone. The patient was followed up for a period of 18 months and reported continued improvement in knee pain scores and superior knee function. A 3-month MRI scan reported almost complete resolution of the meniscal tear. Radiologic evaluation beyond this time point was not available. This study lacked control therefore, it remains difficult to determine the efficacy of this treatment. However, it is worth noting the same patient did undergo PRP and HA injections prior to the stem cell injection and had reported unsatisfactory outcomes with these treatments. Centeno et al. [110] reported a patient who received an intraarticular injection of BMMSCs which were expanded using platelet lysate extracted from the patient’s own blood. After the radiologic MRI diagnosis of degenerative changes in the medial meniscus and medial femoral condyle, the patient was injected with the BMMSCs after expansion in the growth factors present in the platelet lysate. The expanded MSCs were injected into the knee joint along with fresh whole marrow. The patient did also receive 2 subsequent injections of platelet lysate combined with 1ng/mL dexamethasone. The post-procedure 3-month MRI scan showed evidence of increased meniscus volume, and the patient did report improved pain scores. This is encouraging data for a relatively simple procedure, though this case report is limited to one patient without a tissue biopsy, so the exact nature of the regenerated tissue remains unknown.
A recent paper by Onoi et al. [111] reported second-look knee arthroscopy findings in two patients after ASC injections. However, only one of the treated patients had a second-look arthroscopy. This patient underwent a partial meniscectomy for a degenerative tear in the posterior horn of the medial meniscus. At 6 months after ASC injection, the second-look arthroscopy not only showed improved cartilage status but also repair of the resected part of the meniscus. The meniscus tissue was not biopsied, and the sample size was small, but the second-look arthroscopy findings were encouraging.
Sekiya et al. [112] studied the addition of synovial MSCs to degenerative medial meniscus lesions in 5 patients. The patients underwent an initial arthroscopy where the lesions were confirmed, repaired with sutures and finally, a synovial tissue biopsy was performed. The synovial tissue was cultured and expanded for 14 days and a repeat arthroscopy was performed where a synovial MSC cell suspension was delivered to the site of the repair. Patients reported significant clinical score improvement by 2 years and 3D MRI scan results reported no evidence of tear at the repair site. Table 4 summarizes the clinical stem cell injection studies found in the literature.

Table 4.
Summary of clinical studies using stem cell injections.
8.2. Tissue Engineering
The combination of MSCs and tissue engineering is also an emerging field from a clinical standpoint, and a greater number of studies have focused more on finding a solution for cartilage defects. Whitehouse et al. [113] reported a case series of 5 patients where BMMSCs were injected onto a collagen scaffold and sutured into an avascular meniscal tear using vertical mattress sutures. Three out of 5 patients reported positive outcomes beyond 12 months with significantly improved clinical scores and subsequent MRI scans showing in situ repair along with a reduction in the abnormal signal of the scaffold. Two patients had a failure of treatment, sustaining repeat tears at around 15 months. A very recent study by Olivios-Meza et al. [114] combined a polyurethane meniscal scaffold with MSCs for meniscal repair. They divided patients into two groups, one with an acellular scaffold repair and another enriched with MSCs. Scaffolds were arthroscopically implanted into patients who had a history of receiving a meniscectomy in the past. MSCs were obtained from a blood draw after the subjects received 3 days of consecutive 300 μg subcutaneous G-CSF injections in order to increase the peripheral bloodstream MSC pool. After cell separation, CD90+ expression cells were isolated, cultured and seeded on the scaffold. Scaffolds were sutured to the neighboring meniscal tissue and joint capsule to fill the defect with all inside sutures. Outcomes were determined by assessing cartilage adjacent to the repair site with an MRI cartigram. They noted a significant radiologic and clinical improvement in both groups but concluded the addition of MSC to the polyurethane scaffold repair made no difference. This study did, however, have a small sample size with no randomization, and the post-operative MRI scan findings of the meniscal repair site were not reported. Table 5. summarizes the clinical studies available on tissue-engineered meniscal tear treatments.

Table 5.
Summary of clinical studies using tissue engineering.
9. Conclusions
In summary, the available literature demonstrates that MSCs appear to be safe and effective in producing superior quality meniscal repairs. There is compelling pre-clinical data that has studied the effects of various cell sources, scaffolds, and even growth factor additives. Despite this, presently there is no consensus on the ideal cell source and scaffold for meniscus regeneration. Current limitations of the data include a lack of long-term follow-up, control groups, and objective outcome endpoints. In comparison to articular cartilage regeneration, where there have been more clinical studies that have reported on repair tissue histology, second-look arthroscopy, and radiologic imaging, robust outcomes are still lacking for meniscal stem cell therapy studies.
At the end of our review, we do note that each therapy and mode of delivery has its own advantages and disadvantages and at present, we cannot identify or recommend a certain intervention as a standard of care. We do however encourage the use of stem cell therapies as an investigational agent in the setting of meniscal injuries in order to increase the available literature and evidence for or against its use.
The solution to meniscal tissue regeneration is a particularly elusive one and appears far more complex than that of cartilage regeneration due to the complex phenotype and function of meniscal tissue. We anticipate that stem cell therapies will become more effective in the near future in order to aid meniscal repair modalities, thereby adding another weapon to retard dreaded OA progression in the knee.
Author Contributions
G.J.: Writing of the manuscript and illustrations; K.S.: Review and editing; A.J.K.: Review and editing; N.N.: Conceptualization, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
NIL.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDermott, I.D.; Amis, A.A. The consequences of meniscectomy. J. Bone Jt. Surg. 2006, 88, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Human Knee Menisci: Structure, Composition, and Function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P.; Warren, R.F. Microvasculature of the human meniscus. Am. J. Sports Med. 1982, 10, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, S.; Lotito, K.; Rodeo, S.A. Biomechanics and healing response of the meniscus. Oper. Tech. Sports Med. 2003, 11, 68–76. [Google Scholar] [CrossRef]
- McNeill Love, R.J. Prognosis after removal of semilunar cartilages. Br. Med. J. 1923, 2, 324–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seil, R.; Becker, R. Time for a paradigm change in meniscal repair: Save the meniscus! Knee Surgery, Sport. Traumatol. Arthrosc. 2016, 24, 1421–1423. [Google Scholar] [CrossRef]
- Zellner, J.; Pattappa, G.; Koch, M.; Lang, S.; Weber, J.; Pfeifer, C.G.; Mueller, M.B.; Kujat, R.; Nerlich, M.; Angele, P. Autologous mesenchymal stem cells or meniscal cells: What is the best cell source for regenerative meniscus treatment in an early osteoarthritis situation? Stem Cell Res. Ther. 2017, 8, 225. [Google Scholar] [CrossRef]
- Vaquero, J.; Forriol, F. Meniscus tear surgery and meniscus replacement. Muscles. Ligaments Tendons J. 2016, 6, 71–89. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Grassi, A.; Marcheggiani Muccioli, G.M.; Bonanzinga, T.; Nitri, M.; Raggi, F.; Ravazzolo, G.; Marcacci, M. MRI evaluation of a collagen meniscus implant: A systematic review. Knee Surgery, Sport. Traumatol. Arthrosc. 2015, 23, 3228–3237. [Google Scholar] [CrossRef]
- Krych, A.J.; Lorich, D.G.; Kelly, B.T. Treatment of Focal Osteochondral Defects of the Acetabulum with Osteochondral Allograft Transplantation. Orthopedics 2011, 34, e307–e311. [Google Scholar] [CrossRef]
- Monllau, J.C.; Gelber, P.E.; Abat, F.; Pelfort, X.; Abad, R.; Hinarejos, P.; Tey, M. Outcome after partial medial meniscus substitution with the collagen meniscal implant at a minimum of 10 years’ follow-up. Arthroscopy 2011, 27, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed]
- King, D. The healing of semilunar cartilages. 1936. Clin. Orthop. Relat. Res. 1990, 252, 4–7. [Google Scholar]
- Mauck, R.L.; Burdick, J.A. From Repair to Regeneration: Biomaterials to Reprogram the Meniscus Wound Microenvironment. Ann. Biomed. Eng. 2015, 43, 529–542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osawa, A.; Harner, C.D.; Gharaibeh, B.; Matsumoto, T.; Mifune, Y.; Kopf, S.; Ingham, S.J.M.; Schreiber, V.; Usas, A.; Huard, J. The use of blood vessel-derived stem cells for meniscal regeneration and repair. Med. Sci. Sports Exerc. 2013, 45, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Kusayama, T.; Harner, C.D.; Carlin, G.J.; Xerogeanes, J.W.; Smith, B.A. Anatomical and biomechanical characteristics of human meniscofemoral ligaments. Knee Surgery, Sport. Traumatol. Arthrosc. 1994, 2, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Laprade, C.M.; Ellman, M.B.; Rasmussen, M.T.; James, E.W.; Wijdicks, C.A.; Engebretsen, L.; Laprade, R.F. Anatomy of the anterior root attachments of the medial and lateral menisci: A quantitative analysis. Am. J. Sports Med. 2014, 42, 2386–2392. [Google Scholar] [CrossRef]
- Andrews, S.H.J.; Adesida, A.B.; Abusara, Z.; Shrive, N.G. Current concepts on structure–function relationships in the menisci. Connect. Tissue Res. 2017, 58, 271–281. [Google Scholar] [CrossRef]
- Andrews, S.H.J.; Ronsky, J.L.; Rattner, J.B.; Shrive, N.G.; Jamniczky, H.A. An evaluation of meniscal collagenous structure using optical projection tomography. BMC Med. Imaging 2013, 13, 21. [Google Scholar] [CrossRef]
- Melrose, J.; Smith, S.; Cake, M.; Read, R.; Whitelock, J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: An ageing study. Histochem. Cell Biol. 2005, 124, 225–235. [Google Scholar] [CrossRef]
- Doral, M.N.; Bilge, O.; Huri, G.; Turhan, E.; Verdonk, R. Modern treatment of meniscal tears. EFORT Open Rev. 2018, 3, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Evans, E.; Donthineni Rao, R.; Findlay, J.; Pepberton, D. Quantitative differences in the histology of the attachemnt zones of tghe mesiscal horns in the knee joint of man. J. Anat. 1991, 177, 127–134. [Google Scholar] [PubMed]
- Herwig, J.; Egner, E.; Buddecke, E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann. Rheum. Dis. 1984, 43, 635–640. [Google Scholar] [CrossRef]
- Cheung, H.S. Distribution of type I, II, III and v in the pepsin solubilized collagens in bovine menisci. Connect. Tissue Res. 1987, 16, 343–356. [Google Scholar] [CrossRef]
- Nakano, T.; Dodd, C.M.; Scott, P.G. Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J. Orthop. Res. 1997, 15, 213–220. [Google Scholar] [CrossRef]
- Swann, D.A.; Silver, F.H.; Slayter, H.S.; Stafford, W.; Shore, E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem. J. 1985, 225, 195–201. [Google Scholar] [CrossRef]
- Peters, T.J.; Smillie, I.S. Studies on the chemical composition of the menisci of the knee joint with special reference to the horizontal cleavage lesion. Clin. Orthop. Relat. Res. 1972, 86, 245–252. [Google Scholar] [CrossRef]
- McDevitt, C.A.; Mukherjee, S.; Kambic, H.; Parker, R. Emerging concepts of the cell biology of the meniscus. Curr. Opin. Orthop. 2002, 13, 345–350. [Google Scholar] [CrossRef]
- Verdonk, P.C.M.; Forsyth, R.G.; Wang, J.; Almqvist, K.F.; Verdonk, R.; Veys, E.M.; Verbruggen, G. Characterisation of human knee meniscus cell phenotype. Osteoarthr. Cartil. 2005, 13, 548–560. [Google Scholar] [CrossRef]
- Le Graverand, M.P.H.; Ou, Y.C.; Schield-Yee, T.; Barclay, L.; Hart, D.; Natsume, T.; Rattner, J.B. The cells of the rabbit meniscus: Their arrangement, interrelationship, morphological variations and cytoarchitecture. J. Anat. 2001, 198, 525–535. [Google Scholar] [CrossRef]
- Van der Bracht, H.; Verdonk, R.; Verbruggen, D.; Elewaut, D.; Verdonk, P. Cell-based meniscus tissue engineering. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R.L., Chiellini, E., Eds.; Biomaterials and Tissue Engineering Group (BTE): London, UK, 2007; Volume 3. [Google Scholar]
- Cameron, H.U.; Macnab, I. The structure of the meniscus of the human knee joint. Clin. Orthop. Relat. Res. 1972, 89, 215–219. [Google Scholar] [CrossRef]
- Proctor, C.S.; Schmidt, M.B.; Whipple, R.R.; Kelly, M.A.; Mow, V.C. Material properties of the normal medial bovine meniscus. J. Orthop. Res. 1989, 7, 771–782. [Google Scholar] [CrossRef]
- Voloshin, A.S.; Wosk, J. Shock absorption of meniscectomized and painful knees: A comparative in vivo study. J. Biomed. Eng. 1983, 5, 157–161. [Google Scholar] [CrossRef]
- Walker, P.S.; Erkman, M.J. The role of the menisci in force transmission across the knee. Clin. Orthop. 1975, 109, 184–192. [Google Scholar] [CrossRef]
- Hoshino, A.; Wallace, W.A. Impact-absorbing properties of the human knee. J. Bone Jt. Surg. 1987, 69, 807–811. [Google Scholar] [CrossRef]
- Markolf, K.L.; Mensch, J.S.; Amstutz, H.C. Stiffness and laxity of the knee—The contributions of the supporting structures. J. Bone Jt. Surg. Am. 1976, 58, 583–594. [Google Scholar] [CrossRef]
- Bird, M.D.T.; Sweet, M.B.E. Canals in the semilunar meniscus: Brief report. J. Bone Jt. Surg. 1988, 70, 839. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Warren, R.F.; Spivak, J.M. Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J. Bone Jt. Surg. 1988, 70, 1209–1217. [Google Scholar] [CrossRef]
- Skinner, H.B.; Barrack, R.L.; Cook, S.D. Age related decline in proprioception. Clin. Orthop. Relat. Res. 1984, 184, 208–211. [Google Scholar] [CrossRef]
- Reider, B.; Arcand, M.A.; Diehl, L.H.; Mroczek, K.; Abulencia, A.; Stroud, C.C.; Palm, M.; Gilbertson, J.; Staszak, P. Proprioception of the knee before and after anterior cruciate ligament reconstruction. Arthroscopy 2003, 19, 2–12. [Google Scholar] [CrossRef]
- Kennedy, J.C.; Alexander, I.J.; Hayes, K.C. Nerve supply of the human knee and its functional importance. Am. J. Sports Med. 1982, 10, 329–335. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Gale, D.; Dewire, P.; Totterman, S.; Gale, M.E.; McLaughlin, S.; Einhorn, T.A.; Felson, D.T. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J. Bone Jt. Surg. 2003, 85, 4–9. [Google Scholar] [CrossRef]
- Link, T.M.; Steinbach, L.S.; Ghosh, S.; Ries, M.; Lu, Y.; Lane, N.; Majumdar, S. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology 2003, 226, 373–381. [Google Scholar] [CrossRef]
- Boks, S.S.; Vroegindeweij, D.; Koes, B.W.; Hunink, M.M.G.M.; Bierma-Zeinstra, S.M.A. Magnetic resonance imaging abnormalities in symptomatic and contralateral knees: Prevalence and associations with traumatic history in general practice. Am. J. Sports Med. 2006, 34, 1984–1991. [Google Scholar] [CrossRef]
- Ding, C.; Martel-Pelletier, J.; Pelletier, J.P.; Abram, F.; Raynauld, J.P.; Cicuttini, F.; Jones, G. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: A cross-sectional study. J. Rheumatol. 2007, 34, 776–784. [Google Scholar]
- Noble, J.; Hamblen, D.L. The pathology of the degenerate meniscus lesion. J. Bone Jt. Surg. 1975, 57, 180–186. [Google Scholar] [CrossRef]
- Englund, M.; Roemer, F.W.; Hayashi, D.; Crema, M.D.; Guermazi, A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat. Rev. Rheumatol. 2012, 22, 412–419. [Google Scholar] [CrossRef]
- Kenny, C. Radial displacement of the medial meniscus and Fairbank’s signs. Clin. Orthop. Relat. Res. 1997, 339, 163–173. [Google Scholar] [CrossRef]
- Rauscher, I.; Stahl, R.; Cheng, J.; Li, X.; Huber, M.B.; Luke, A.; Majumdar, S.; Link, T.M. Meniscal measurements of T1 p and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology 2008, 249, 591–600. [Google Scholar] [CrossRef]
- Hunter, D.J.; Buck, R.; Vignon, E.; Eckstein, F.; Brandt, K.; Mazzuca, S.A.; Wyman, B.T.; Otterness, I.; Hellio Le Graverand, M.P. Relation of regional articular cartilage morphometry and meniscal position by MRI to joint space width in knee radiographs. Osteoarthr. Cartil. 2009, 17, 1170–1176. [Google Scholar] [CrossRef][Green Version]
- Forriol, F. Growth factors in cartilage and meniscus repair. Injury 2009, 40, S12–S16. [Google Scholar] [CrossRef]
- Hunter, D.J.; Zhang, Y.Q.; Niu, J.B.; Tu, X.; Amin, S.; Clancy, M.; Guermazi, A.; Grigorian, M.; Gale, D.; Felson, D.T. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006, 54, 795–801. [Google Scholar] [CrossRef]
- Berthiaume, M.J.; Raynauld, J.P.; Martel-Pelletier, J.; Labonté, F.; Beaudoin, G.; Bloch, D.A.; Choquette, D.; Haraoui, B.; Altman, R.D.; Hochberg, M.; et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann. Rheum. Dis. 2005, 64, 556–563. [Google Scholar] [CrossRef]
- Kraus, V.B.; Feng, S.; Wang, S.C.; White, S.; Ainslie, M.; Brett, A.; Holmes, A.; Charles, H.C. Trabecular morphometry by fractal signature analysis is a novel marker of osteoarthritis progression. Arthritis Rheum. 2009, 60, 3711–3722. [Google Scholar] [CrossRef]
- Wolski, M.; Podsiadlo, P.; Stachowiak, G.W.; Lohmander, L.S.; Englund, M. Differences in trabecular bone texture between knees with and without radiographic osteoarthritis detected by directional fractal signature method. Osteoarthr. Cartil. 2010, 18, 684–690. [Google Scholar] [CrossRef]
- Hunter, D.J.; Zhang, Y.Q.; Tu, X.; LaValley, M.; Niu, J.B.; Amin, S.; Guermazi, A.; Genant, H.; Gale, D.; Felson, D.T. Change in joint space width: Hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006, 54, 2488–2495. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Roemer, F.W.; Yang, M.; Zhang, Y.; Nevitt, M.C.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Felson, D.T. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: The MOST study. Ann. Rheum. Dis. 2010, 69, 1796–1802. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 25, 663–676. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 2015, 28, 35. [Google Scholar] [CrossRef]
- Fan, J.; Varshney, R.R.; Ren, L.; Cai, D.; Wang, D.A. Synovium-derived mesenchymal stem cells: A new cell source for musculoskeletal regeneration. Tissue Eng. 2009, 15, 75–86. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Gorskaja, U.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Cao, C.; Dong, Y.; Dong, Y. Study on culture and in vitro osteogenesis of blood-derived human mesenchymal stem cells. Chin. J. Repar. Reconstruct. Surg. 2005, 19, 642–647. [Google Scholar]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef]
- Shirasawa, S.; Sekiya, I.; Sakaguchi, Y.; Yagishita, K.; Ichinose, S.; Muneta, T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: Optimal condition and comparison with bone marrow-derived cells. J. Cell. Biochem. 2006, 97, 84–97. [Google Scholar] [CrossRef]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006, 54, 848–853. [Google Scholar] [CrossRef]
- Banfi, A.; Muraglia, A.; Dozin, B.; Mastrogiacomo, M.; Cancedda, R.; Quarto, R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 2000, 28, 707–715. [Google Scholar] [CrossRef]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.C.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.D.; Gimble, J.M. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Im, G.I.; Shin, Y.W.; Lee, K.B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr. Cartil. 2005, 13, 845–853. [Google Scholar] [CrossRef]
- De Albornoz, P.M.; Forriol, F. The meniscal healing process. Muscles Ligaments Tendons J. 2012, 2, 10–18. [Google Scholar] [PubMed]
- Guisasola, I.; Vaquero, J.; Forriol, F. Knee immobilization on meniscal healing after suture: An experimental study in sheep. Clin. Orthop. Relat. Res. 2002, 395, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Dehaven, K.E.; Lohrer, W.A.; Lovelock, J.E. Long-Term Results of Open Meniscal Repair. Am. J. Sports Med. 1995, 23, 524–530. [Google Scholar] [CrossRef]
- Matsukura, Y.; Muneta, T.; Tsuji, K.; Koga, H.; Sekiya, I. Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clin. Orthop. Relat. Res. 2014, 472, 1357–1364. [Google Scholar] [CrossRef]
- King, T.V.; Vallee, B.L. Neovascularisation of the meniscus with angiogenin: An experimental study in rabbits. J. Bone Jt. Surg. 1991, 73, 587–590. [Google Scholar] [CrossRef]
- Zhongran, Z.; Kaiyuan, T.; Yinkan, X.; Wenming, Z.; Zhentian, L.; Shihuan, O. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trepanation. Arthrosc. J. Arthrosc. Relat. Surg. 1988, 4, 151–159. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Warren, R.F. The microvasculature of the meniscus and its response to injury: An experimental study in the dog. Am. J. Sports Med. 1983, 11, 131–141. [Google Scholar] [CrossRef]
- Cabaud, H.E.; Rodkey, W.G.; Fitzwater, J.E. Medial meniscus repairs. An experimental and morphologic study. Am. J. Sports Med. 1981, 9, 129–134. [Google Scholar] [CrossRef]
- Heatley, F.W. The meniscus—Can it be repaired? An experimental investigation in rabbits. J. Bone Jt. Surg. 1980, 62, 397–402. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Fortier, L.A.; Mao, J.J.; Lee, C.H.; Goodale, M.B.; Koff, M.F.; Uppstrom, T.J.; Croen, B.; Wada, S.; Carballo, C.B.; et al. Long-term Evaluation of Meniscal Tissue Formation in 3-dimensional–Printed Scaffolds with Sequential Release of Connective Tissue Growth Factor and TGF-β3 in an Ovine Model. Am. J. Sports Med. 2019, 47, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Rodeo, S.A.; Fortier, L.A.; Lu, C.; Erisken, C.; Mao, J.J. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 2014, 6, 266ra171. [Google Scholar] [CrossRef]
- Bray, R.C.; Smith, J.A.; Eng, M.K.; Leonard, C.A.; Sutherland, C.A.; Salo, P.T. Vascular response of the meniscus to injury: Effects of immobilization. J. Orthop. Res. 2001, 19, 384–390. [Google Scholar] [CrossRef]
- Dowdy, P.A.; Miniaci, A.; Arnoczky, S.P.; Fowler, P.J.; Boughner, D.R. The Effect of Cast Immobilization on Meniscal Healing: An Experimental Study in the Dog. Am. J. Sports Med. 1995, 23, 721–728. [Google Scholar] [CrossRef]
- McNulty, A.L.; Guilak, F. Mechanobiology of the meniscus. J. Biomech. 2015, 48, 1469–1478. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef]
- Nimura, A.; Muneta, T.; Koga, H.; Mochizuki, T.; Suzuki, K.; Makino, H.; Umezawa, A.; Sekiya, I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: Comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008, 58, 501–510. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Muneta, T.; Kondo, S.; Mizuno, M.; Takakuda, K.; Ichinose, S.; Tabuchi, T.; Koga, H.; Tsuji, K.; Sekiya, I. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthr. Cartil. 2015, 23, 1007–1017. [Google Scholar] [CrossRef]
- Hatsushika, D.; Muneta, T.; Nakamura, T.; Horie, M.; Koga, H.; Nakagawa, Y.; Tsuji, K.; Hishikawa, S.; Kobayashi, E.; Sekiya, I. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthr. Cartil. 2014, 22, 941–950. [Google Scholar] [CrossRef]
- Ruiz-Ibán, M.N.; Díaz-Heredia, J.; García-Gómez, I.; Gonzalez-Lizán, F.; Elías-Martín, E.; Abraira, V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: An experimental study in rabbits. Arthroscopy 2011, 27, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.J.; Frisbie, D.D.; Kisiday, J.D.; Mcilwraith, C.W.; Hague, B.A.; Major, M.D.; Schneider, R.K.; Zubrod, C.J.; Kawcak, C.E.; Goodrich, L.R. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet. Surg. 2014, 43, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Hussein, M.R.; Ahmad, A.F.; Elgezawi, E.M. Enhancement of the repair of meniscal wounds in the red-white zone (middle third) by the injection of bone marrow cells in canine animal model. Int. J. Exp. Pathol. 2005, 86, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo Perea, S.; Lyons, L.P.; Nishimuta, J.F.; Weinberg, J.B.; McNulty, A.L. Evaluation of culture conditions for in vitro meniscus repair model systems using bone marrow-derived mesenchymal stem cells. Connect. Tissue Res. 2019, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Thomas Vangsness, J.; Burke, W.S.; Narvy, S.J.; MacPhee, R.D.; Fedenko, A.N. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis: A pilot study. Bull. NYU Hosp. Jt. Dis. 2011, 69, 122–127. [Google Scholar]
- Hennerbichler, A.; Moutos, F.T.; Hennerbichler, D.; Weinberg, J.B.; Guilak, F. Interleukin-1 and tumor necrosis factor alpha inhibit repair of the porcine meniscus in vitro. Osteoarthr. Cartil. 2007, 15, 1053–1060. [Google Scholar] [CrossRef]
- McNulty, A.L.; Moutos, F.T.; Weinberg, J.B.; Guilak, F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor α. Arthritis Rheum. 2007, 56, 3033–3042. [Google Scholar] [CrossRef]
- Upton, M.L.; Guilak, F.; Laursen, T.A.; Setton, L.A. Finite element modeling predictions of region-specific cell-matrix mechanics in the meniscus. Biomech. Model. Mechanobiol. 2006, 5, 140–149. [Google Scholar] [CrossRef]
- Zhang, H.; Leng, P.; Zhang, J. Enhanced meniscal repair by overexpression of hIGF-1 in a full-thickness model. Clin. Orthop. Relat. Res. 2009, 467, 3165–3174. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Tateishi, K.; Ando, W.; Shimomura, K.; Yonetani, Y.; Tanaka, Y.; Kita, K.; Hart, D.A.; Gobbi, A.; Shino, K.; et al. Repair of meniscal lesions using a scaffold-free tissue-engineered construct derived from allogenic synovial MSCs in a miniature swine model. Biomaterials 2013, 34, 2185–2193. [Google Scholar] [CrossRef]
- Kondo, S.; Muneta, T.; Nakagawa, Y.; Koga, H.; Watanabe, T.; Tsuji, K.; Sotome, S.; Okawa, A.; Kiuchi, S.; Ono, H.; et al. Transplantation of autologous synovial mesenchymal stem cells promotes meniscus regeneration in aged primates. J. Orthop. Res. 2017, 35, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Desando, G.; Giavaresi, G.; Cavallo, C.; Bartolotti, I.; Sartoni, F.; Nicoli Aldini, N.; Martini, L.; Parrilli, A.; Mariani, E.; Fini, M.; et al. Autologous Bone Marrow Concentrate in a Sheep Model of Osteoarthritis: New Perspectives for Cartilage and Meniscus Repair. Tissue Eng. 2016, 22, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Berruto, M.; Brocchetta, D.; Delcogliano, A.; Ghinelli, D.; Gobbi, A.; Kon, E.; Pederzini, L.; Rosa, D.; Sacchetti, G.L.; et al. Articular cartilage engineering with Hyalograft® C: 3-Year clinical results. Clin. Orthop. Relat. Res. 2005, 435, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.G.; Magna, H.A.; Reeves, L.M.; Lopresti-Morrow, L.L.; Yocum, S.A.; Rosner, P.J.; Geoghegan, K.F.; Hambor, J.E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996, 97, 761–768. [Google Scholar] [CrossRef]
- Mengshol, J.A. IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: Requirement for Runx-2 and activation by p38 MAPK and JNK pathways. Nucleic Acids Res. 2001, 29, 4361–4372. [Google Scholar] [CrossRef]
- Massoud, D.; Jian, Y.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar]
- Vangsness, C.T.; Farr, J.; Boyd, J.; Dellaero, D.T.; Mills, C.R.; LeRoux-Williams, M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy A Randomized, Double-Blind, Controlled Study. J. Bone Jt. Surg. 2014, 96, 90–98. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Lee, S.H. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. BioMed Res. Int. 2014, 2014, 436029. [Google Scholar] [CrossRef]
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M.; Karli, D. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med. Hypotheses 2008, 71, 900–908. [Google Scholar] [CrossRef]
- Onoi, Y.; Hiranaka, T.; Nishida, R.; Takase, K.; Fujita, M.; Hida, Y.; Fujishiro, T.; Okamoto, K. Second-look arthroscopic findings of cartilage and meniscus repair after injection of adipose-derived regenerative cells in knee osteoarthrits: Report of two cases. Regen. Ther. 2019, 11, 212–216. [Google Scholar] [CrossRef]
- Sekiya, I.; Koga, H.; Otabe, K.; Nakagawa, Y.; Katano, H.; Ozeki, N.; Mizuno, M.; Horie, M.; Kohno, Y.; Katagiri, K.; et al. Additional Use of Synovial Mesenchymal Stem Cell Transplantation Following Surgical Repair of a Complex Degenerative Tear of the Medial Meniscus of the Knee: A Case Report. Cell Transplant. 2019, 28, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, M.R.; Howells, N.R.; Parry, M.C.; Austin, E.; Kafienah, W.; Brady, K.; Goodship, A.E.; Eldridge, J.D.; Blom, A.W.; Hollander, A.P. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: From in vitro optimization to a first-in-human study. Stem Cells Transl. Med. 2017, 6, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Olivos-Meza, A.; Pérez Jiménez, F.J.; Granados-Montiel, J.; Landa-Solís, C.; Cortés González, S.; Jiménez Aroche, C.A.; Valdez Chávez, M.; Renán León, S.; Gomez-Garcia, R.; Martínez-López, V.; et al. First Clinical Application of Polyurethane Meniscal Scaffolds with Mesenchymal Stem Cells and Assessment of Cartilage Quality with T2 Mapping at 12 Months. Cartilage 2019. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).