Tensin-3 Regulates Integrin-Mediated Proliferation and Differentiation of Tonsil-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Palatine TMSCs, ADSCs, and BMSCs
2.2. Long-Term Passage Culturing of Palatine TMSCs
2.3. Quantitative Reverse Transcription-Polymerase Chain Reaction
2.4. Western Blot Analysis
2.5. Transfection of siRNA
2.6. Cell Proliferation
2.7. Cell Migration Assay
2.8. Senescence-Associated Beta-Galactosidase Staining
2.9. Colony Formation Assay
2.10. Multi-Lineage Differentiation
2.11. Flow Cytometry
2.12. Immunocytochemical Staining
2.13. Statistical Analysis
3. Results
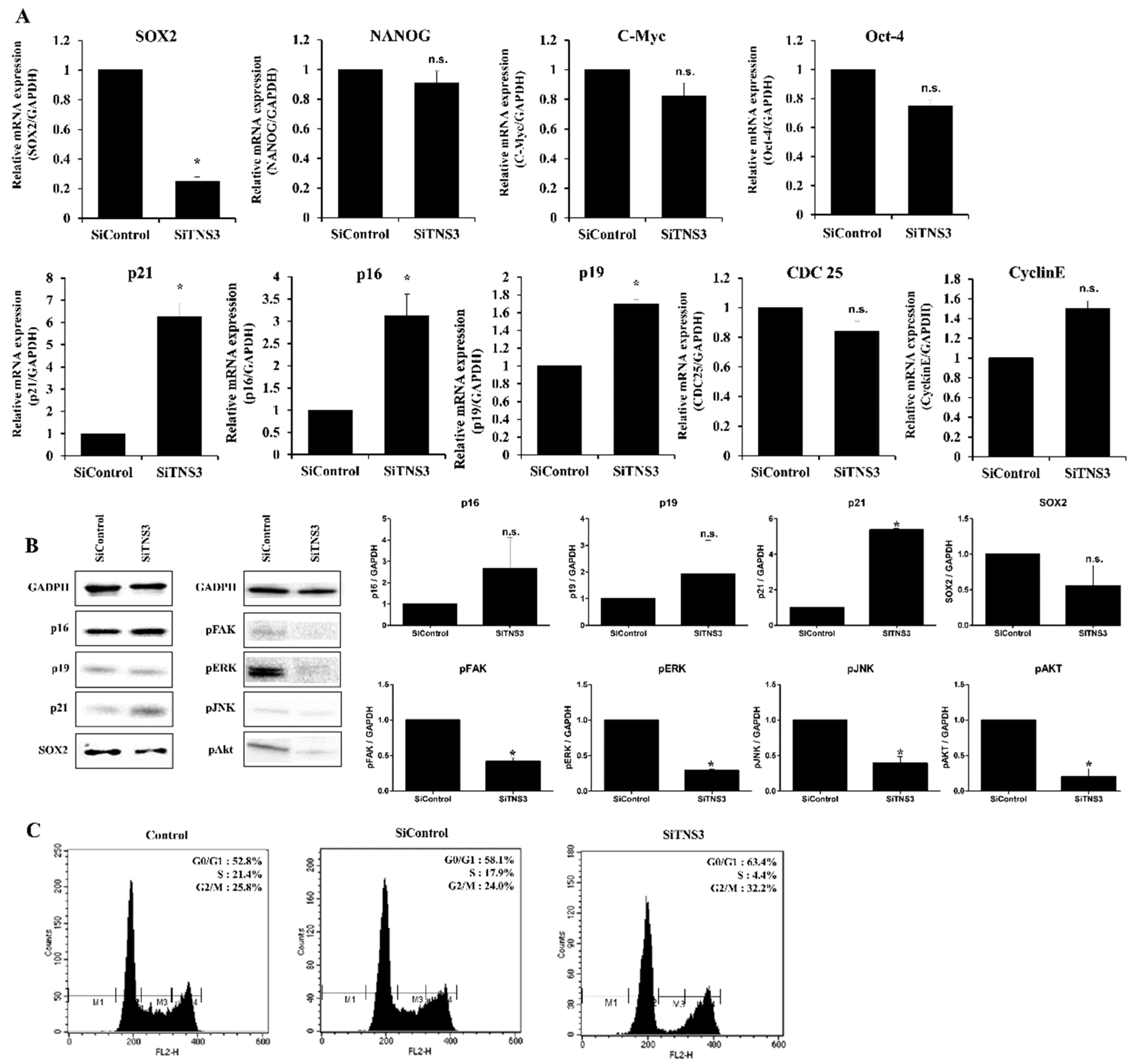
3.1. Tensin-3 Regulates the Proliferation of Palatine TMSCs
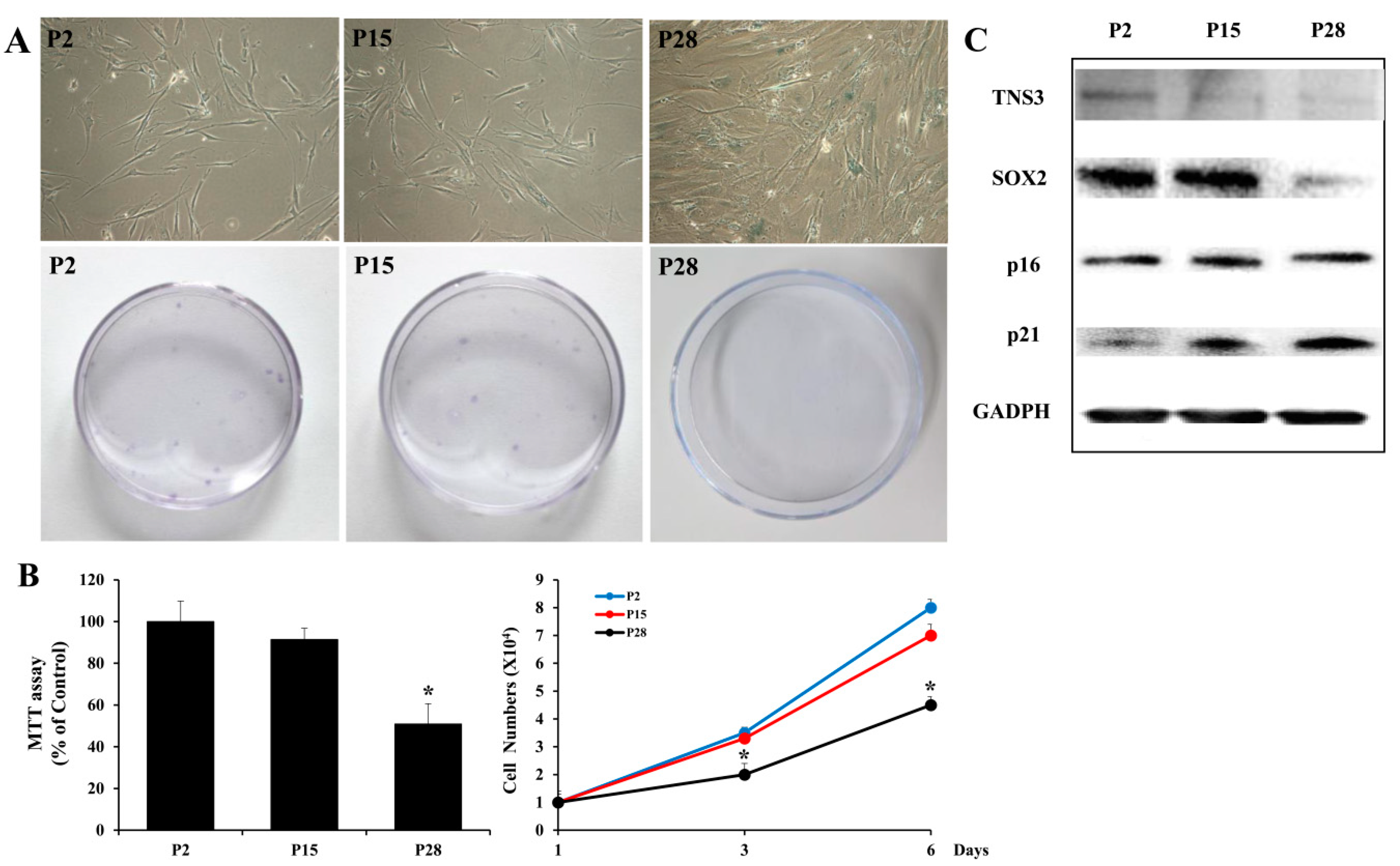
3.2. Replicatively-Senescent TMSCs Exhibit the Senescence Phenotype and Decreased TNS3 Expression
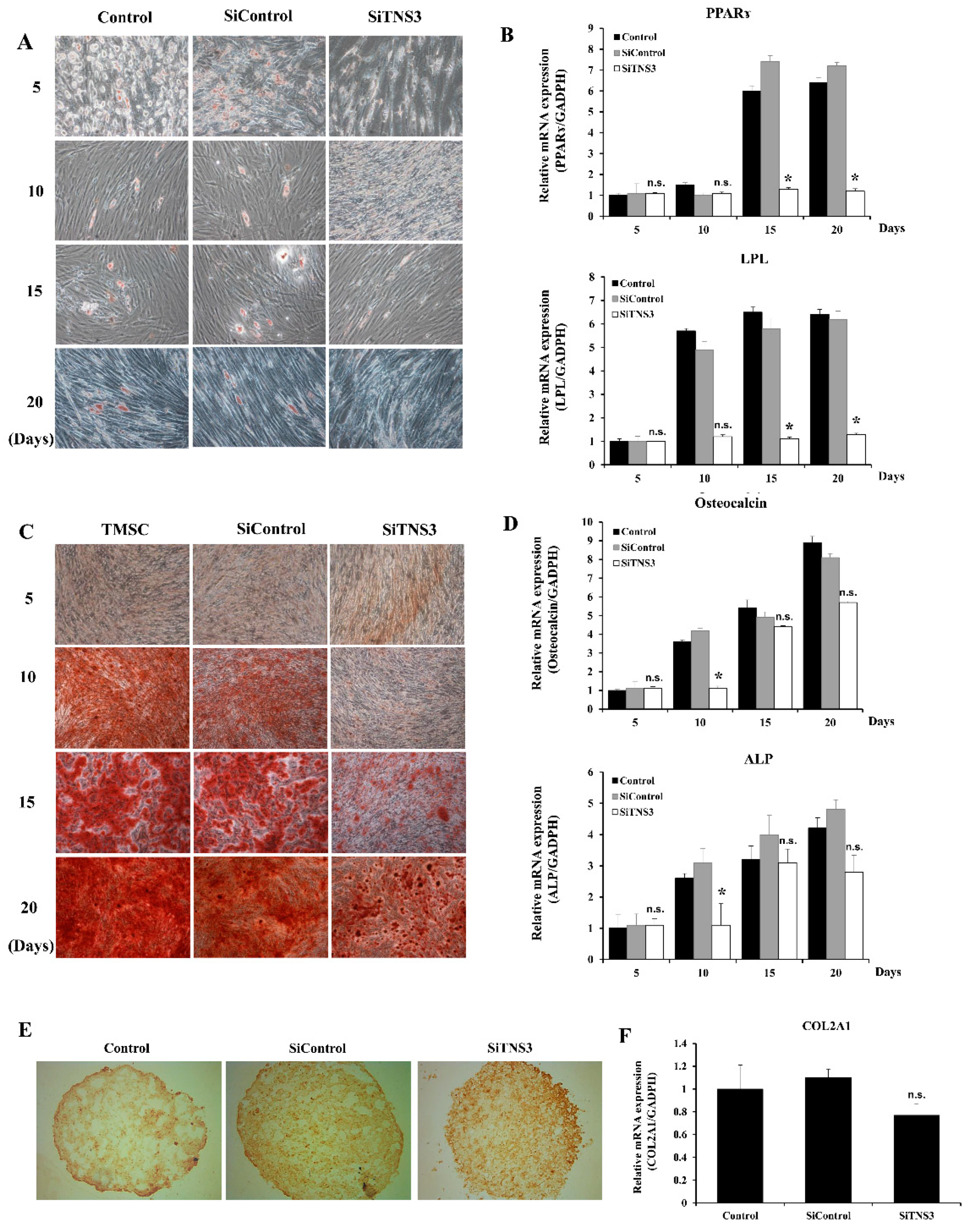
3.3. Tensin-3 Plays a Role in the Differentiation of Palatine TMSCs
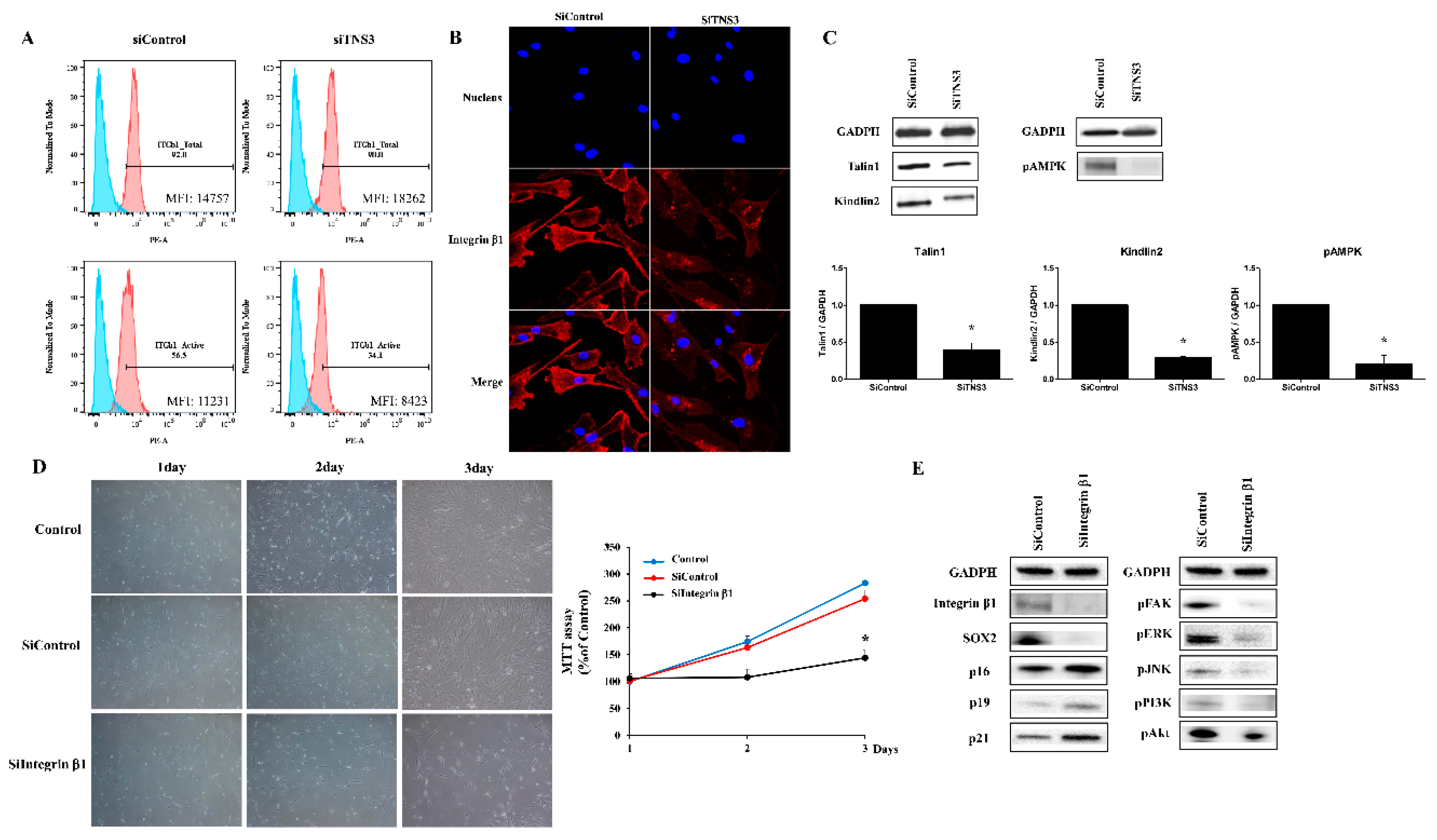
3.4. Tensin-3 Controls Integrin Beta-1 Activity
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Janjanin, S.; Djouad, F.; Shanti, R.M.; Baksh, D.; Gollapudi, K.; Prgomet, D.; Rackwitz, L.; Joshi, A.S.; Tuan, R.S. Human palatine tonsil: A new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis Res. Ther. 2008, 10, R83. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Cho, K.A.; Park, H.S.; Kim, J.Y.; Woo, S.Y.; Jo, I.; Choi, Y.H.; Park, Y.M.; Jung, S.C.; Chung, S.M.; et al. Tonsil-derived mesenchymal stromal cells: Evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy 2012, 14, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, B.J.; Park, H.Y.; Song, J.S.; Shin, S.C.; Lee, J.C.; Wang, S.G.; Jung, J.S. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cell. Physiol. Biochem. 2015, 36, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Park, G.C.; Song, J.S.; Park, H.Y.; Shin, S.C.; Jang, J.Y.; Lee, J.C.; Wang, S.G.; Lee, B.J.; Jung, J.S. Role of fibroblast growth factor-5 on the proliferation of human tonsil-derived mesenchymal stem cells. Stem Cells Dev. 2016, 25, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Kang, D.W.; Park, H.Y.; Song, J.S.; Kim, J.M.; Jang, J.Y.; Lee, J.C.; Wang, S.G.; Jung, J.S.; Shin, S.C. Isolation and localization of mesenchymal stem cells in human palatine tonsil by W5C5 (SUSD2). Cell. Physiol. Biochem. 2016, 38, 83–93. [Google Scholar] [CrossRef] [PubMed]
- McCleverty, C.J.; Lin, D.C.; Liddington, R.C. Structure of the PTB domain of tensin1 and a model for its recruitment to fibrillar adhesions. Protein Sci. 2007, 16, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Blangy, A. Tensins are versatile regulators of Rho GTPase signalling and cell adhesion. Biol. Cell 2017, 109, 115–126. [Google Scholar] [CrossRef]
- Lo, S.H. Tensin. Int. J. Biochem. Cell Biol. 2004, 36, 31–34. [Google Scholar] [CrossRef]
- Lee, R.H.; Kim, B.; Choi, I.; Kim, H.; Choi, H.S.; Suh, K.; Bae, Y.C.; Jung, J.S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 2004, 14, 311–324. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell. Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef]
- Lee, S.; Yu, K.R.; Ryu, Y.S.; Oh, Y.S.; Hong, I.S.; Kim, H.S.; Lee, J.Y.; Kim, S.; Seo, K.W.; Kang, K.S. miR-543 and miR-590-3p regulate human mesenchymal stem cell aging via direct targeting of AIMP3/p18. Age 2014, 36, 9724. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yu, K.R.; Kim, H.S.; Kang, I.; Kim, J.J.; Lee, B.C.; Choi, S.W.; Shin, J.H.; Seo, Y.; Kang, K.S. BMI1 inhibits senescence and enhances the immunomodulatory properties of human mesenchymal stem cells via the direct suppression of MKP-1/DUSP1. Aging 2016, 8, 1670–1689. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.R.; Lee, J.Y.; Kim, H.S.; Hong, I.S.; Choi, S.W.; Seo, Y.; Kang, I.; Kim, J.J.; Lee, B.C.; Kultz, A.; et al. A p38 MAPK-mediated alteration of COX-2/PGE2 regulates immunomodulatory properties in human mesenchymal stem cell aging. PLoS ONE 2014, 9, e102426. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.R.; Lee, S.; Jung, J.W.; Hong, I.S.; Kim, H.S.; Seo, Y.; Shin, T.H.; Kang, K.S. MicroRNA-141-3p plays a role in human mesenchymal stem cell aging by directly targeting ZMPSTE24. J. Cell. Sci. 2013, 126, 5422–5431. [Google Scholar] [CrossRef]
- Yu, K.R.; Park, S.B.; Jung, J.W.; Seo, M.S.; Hong, I.S.; Kim, H.S.; Seo, Y.; Kang, T.W.; Lee, J.Y.; Kurtz, A.; et al. HMGA2 regulates the in vitro aging and proliferation of human umbilical cord blood-derived stromal cells through the mTOR/p70S6K signaling pathway. Stem Cell Res. 2013, 10, 156–165. [Google Scholar] [CrossRef]
- Auger, K.R.; Songyang, Z.; Lo, S.H.; Roberts, T.M.; Chen, L.B. Platelet-derived growth factor-induced formation of tensin and phosphoinositide 3-kinase complexes. J. Biol. Chem. 1996, 271, 23452–23457. [Google Scholar] [CrossRef]
- Davis, S.; Lu, M.L.; Lo, S.H.; Lin, S.; Butler, J.A.; Druker, B.J.; Roberts, T.M.; An, Q.; Chen, L.B. Presence of an SH2 domain in the actin-binding protein tensin. Science 1991, 252, 712–715. [Google Scholar] [CrossRef]
- Chen, H.; Lo, S.H. Regulation of tensin-promoted cell migration by its focal adhesion binding and Src homology domain 2. Biochem. J. 2003, 370, 1039–1045. [Google Scholar] [CrossRef]
- Touaitahuata, H.; Morel, A.; Urbach, S.; Mateos-Langerak, J.; de Rossi, S.; Blangy, A. Tensin 3 is a new partner of Dock5 that controls osteoclast podosome organization and activity. J. Cell Sci. 2016, 129, 3449–3461. [Google Scholar] [CrossRef]
- Qian, X.; Li, G.; Vass, W.C.; Papageorge, A.; Walker, R.C.; Asnaghi, L.; Steinbach, P.J.; Tosato, G.; Hunter, K.; Lowy, D.R. The Tensin-3 protein, including its SH2 domain, is phosphorylated by Src and contributes to tumorigenesis and metastasis. Cancer Cell 2009, 16, 246–258. [Google Scholar] [CrossRef]
- Shinchi, Y.; Hieda, M.; Nishioka, Y.; Matsumoto, A.; Yokoyama, Y.; Kimura, H.; Matsuura, S.; Matsuura, N. SUV420H2 suppresses breast cancer cell invasion through down regulation of the SH2 domain-containing focal adhesion protein tensin-3. Exp. Cell Res. 2015, 334, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Manero, J.M.; Gil, J.; Kessler, H.; Mas-Moruno, C. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf. B Biointerfaces 2015, 128, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Cheah, M.; Andrews, M.R. Integrin activation: Implications for axon regeneration. Cells 2018, 7, 20. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin signaling in cancer: Mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Qu, J.; He, L.; Peng, H.; Chen, P.; Zhou, Y. alpha6-Integrin alternative splicing: Distinct cytoplasmic variants in stem cell fate specification and niche interaction. Stem Cell Res. Ther. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Hamidouche, Z.; Fromigue, O.; Ringe, J.; Haupl, T.; Vaudin, P.; Pages, J.C.; Srouji, S.; Livne, E.; Marie, P.J. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 18587–18591. [Google Scholar] [CrossRef]
- Wilschut, K.J.; van Tol, H.T.; Arkesteijn, G.J.; Haagsman, H.P.; Roelen, B.A. Alpha 6 integrin is important for myogenic stem cell differentiation. Stem Cell Res. 2011, 7, 112–123. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Krebsbach, P.H.; Villa-Diaz, L.G. The role of integrin alpha6 (CD49f) in stem cells: More than a conserved biomarker. Stem Cells Dev. 2017, 26, 1090–1099. [Google Scholar] [CrossRef]
- Suresh, A.; Biswas, A.; Perumal, S.; Khurana, S. Periostin and integrin signaling in stem cell regulation. Adv. Exp. Med. Biol. 2019, 1132, 163–176. [Google Scholar] [PubMed]
- Kummer, D.; Ebnet, K. Junctional adhesion molecules (JAMs): The JAM-integrin connection. Cells 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, M.; Lilja, J.; Jacquemet, G.; Guzman, C.; Rafaeva, M.; Alibert, C.; Yan, Y.; Sahgal, P.; Lerche, M.; Manneville, J.B.; et al. AMPK negatively regulates tensin-dependent integrin activity. J. Cell Biol. 2017, 216, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Amit, I.; Citri, A.; Shay, T.; Carvalho, S.; Lavi, S.; Milanezi, F.; Lyass, L.; Amariglio, N.; Jacob-Hirsch, J.; et al. A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat. Cell Biol. 2007, 9, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Boosani, C.S.; Gunasekar, P.; Agrawal, D.K. An update on PTEN modulators—A patent review. Expert Opin. Ther. Pat. 2019, 29, 881–889. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Wang, Y.; Chan, A.M. Multifaceted regulation of PTEN subcellular distributions and biological functions. Cancers 2019, 11, 1247. [Google Scholar] [CrossRef]

| Gene | Primer Sequences |
|---|---|
| TNS3 | Forward 5′-GGACGCATAGGAGTGGTGAT-3′ Reverse 5′-GGGAGAGGCATTCATTTTCA-3′ |
| SOX2 | Forward 5′-GGGAAATGGGAGGGGTGCAAAAGAGG-3′ Reverse 5′-TTGCGTGAGTGTGGATGGGATTGGTG-3′ |
| NANOG | Forward 5′-AGTCCCAAAGGCAAACAACCCACTTC-3′ Reverse 5′-TGCTGGAGGCTGAGGTATTTCTGTCTC-3′ |
| Oct-4 | Forward 5′-GACAGGGGGAGGGGAGGAGCTAGG-3′ Reverse 5′-CTTCCCTCCAACCAGTTGCCCCAAAC-3′ |
| C-myc | Forward 5′-AAAGGCCCCCAAGGTAGTTA-3′ Reverse 5′-GCACAAGAGTTCCGTAGCTG-3′ |
| p16 | Forward 5′-GGGGAGAGTAGATAGCGGGC-3′ Reverse 5′-AACCAATCAACCGAAAATTCCATA-3′ |
| p19 | Forward 5′-CTCTGCTCCCTGATAGCCCT-3′ Reverse 5′-TGCGAAGGATTTTGAAGCGG-3′ |
| p21 | Forward 5′-GTCACTGTCTTGTACCCTTGTG-3′ Reverse 5′-CGGCGTTTGGAGTGGTAGAAA-3′ |
| CDC25 | Forward 5′-CTTCCTTTACCGTCTGTC-3′ Reverse 5′-AAACCATTCGGAGTGCTA-3′ |
| Cyclin E | Forward 5′-GGATGTTGACTGCCTTAG-3′ Reverse 5′-CACCACTGATACCCTGAAA-3′ |
| GAPDH | Forward 5′-CCTACACCACCAACTGCTTA-3′ Reverse 5′-GGCCATCCACAGTCTTCTGAG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.C.; Kim, H.-S.; Park, H.-Y.; Seo, Y.; Kim, J.M.; Shin, S.-C.; Kwon, H.-K.; Sung, E.-S.; Lee, J.-C.; Lee, B.-J. Tensin-3 Regulates Integrin-Mediated Proliferation and Differentiation of Tonsil-Derived Mesenchymal Stem Cells. Cells 2020, 9, 89. https://doi.org/10.3390/cells9010089
Park GC, Kim H-S, Park H-Y, Seo Y, Kim JM, Shin S-C, Kwon H-K, Sung E-S, Lee J-C, Lee B-J. Tensin-3 Regulates Integrin-Mediated Proliferation and Differentiation of Tonsil-Derived Mesenchymal Stem Cells. Cells. 2020; 9(1):89. https://doi.org/10.3390/cells9010089
Chicago/Turabian StylePark, Gi Cheol, Hyung-Sik Kim, Hee-Young Park, Yoojin Seo, Ji Min Kim, Sung-Chan Shin, Hyun-Keun Kwon, Eui-Suk Sung, Jin-Choon Lee, and Byung-Joo Lee. 2020. "Tensin-3 Regulates Integrin-Mediated Proliferation and Differentiation of Tonsil-Derived Mesenchymal Stem Cells" Cells 9, no. 1: 89. https://doi.org/10.3390/cells9010089
APA StylePark, G. C., Kim, H.-S., Park, H.-Y., Seo, Y., Kim, J. M., Shin, S.-C., Kwon, H.-K., Sung, E.-S., Lee, J.-C., & Lee, B.-J. (2020). Tensin-3 Regulates Integrin-Mediated Proliferation and Differentiation of Tonsil-Derived Mesenchymal Stem Cells. Cells, 9(1), 89. https://doi.org/10.3390/cells9010089






