TIMP-1 Is Overexpressed and Secreted by Platinum Resistant Epithelial Ovarian Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
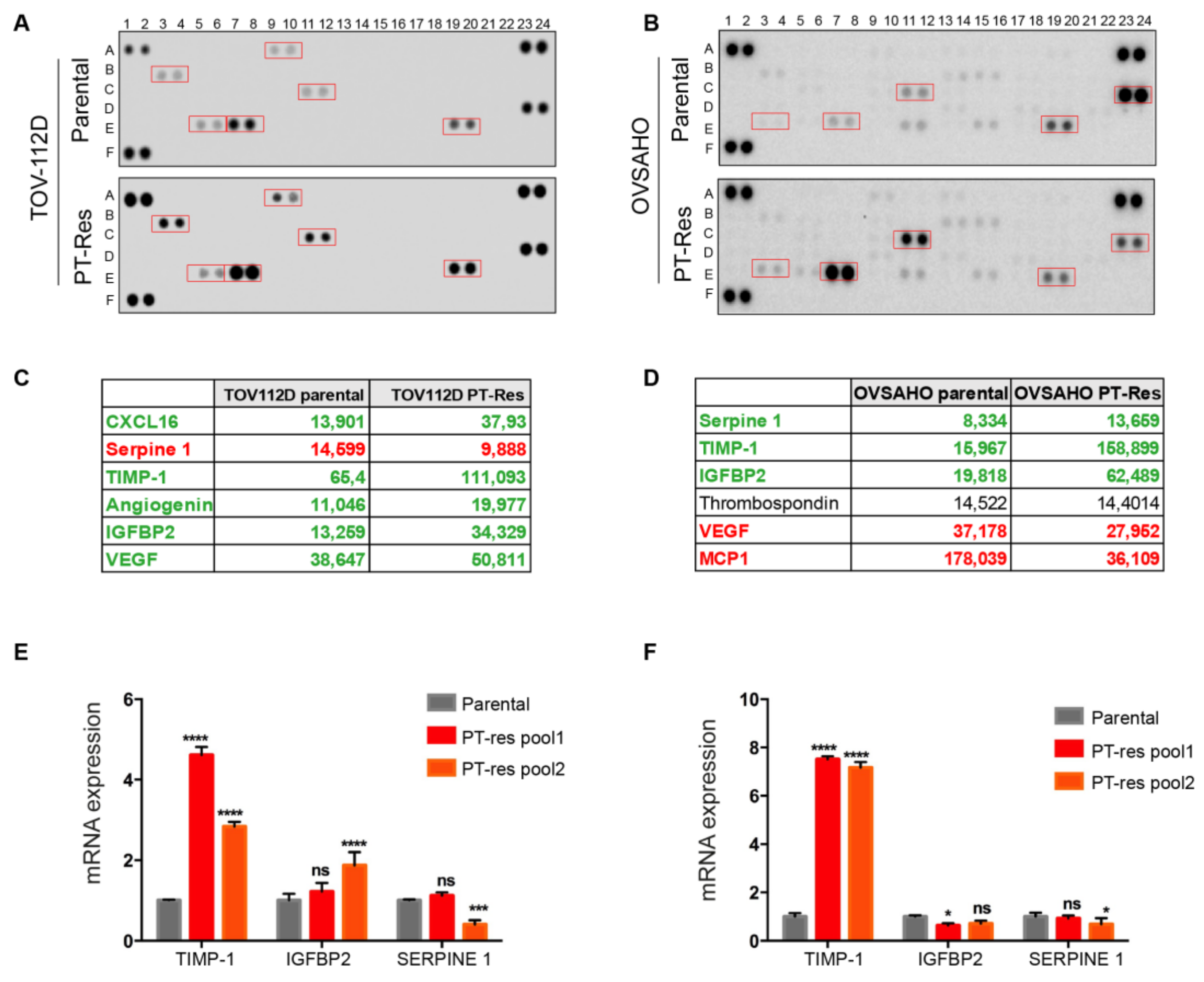
2.2. Cytokines Array
2.3. Proliferation Assay
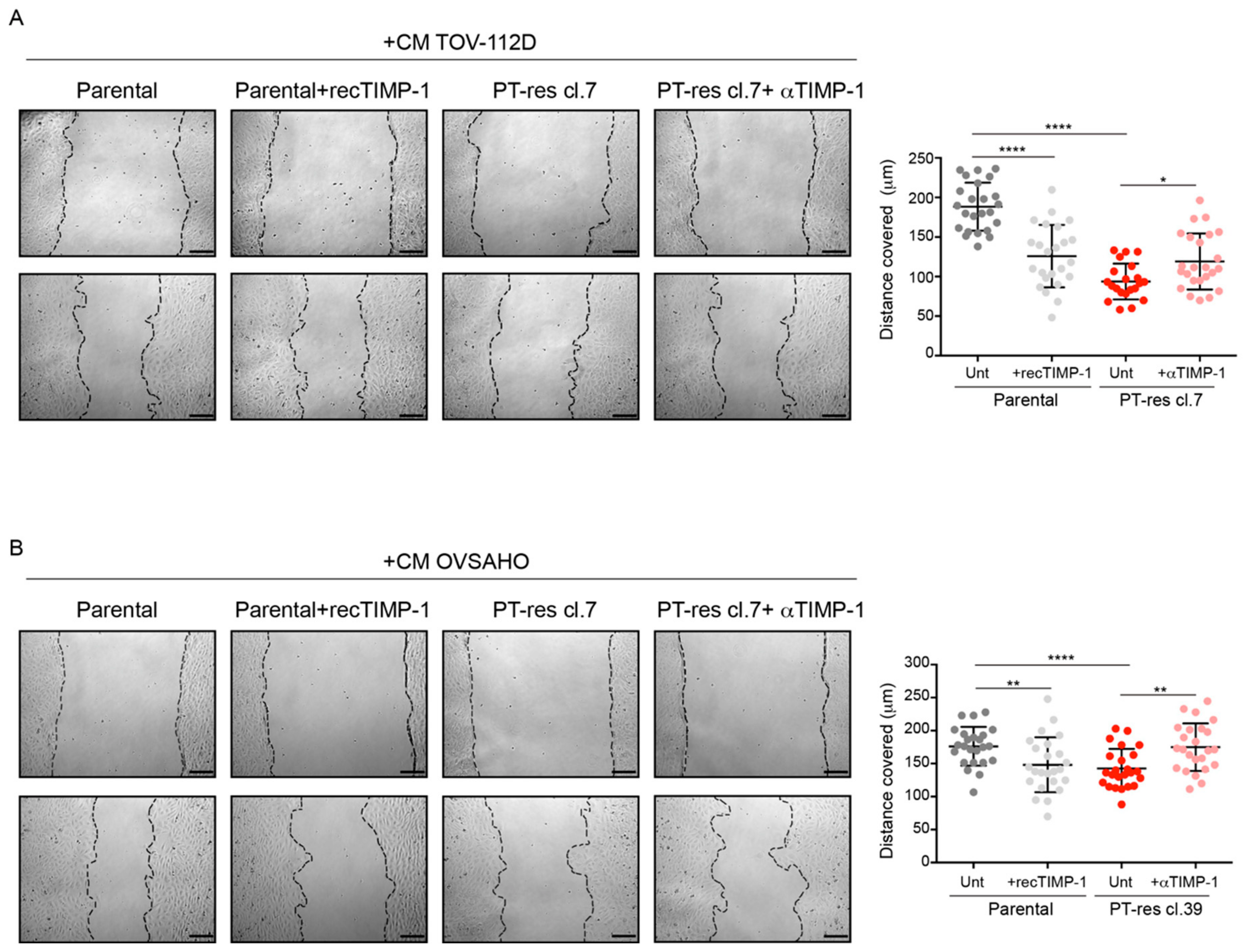
2.4. Scratch Test
2.5. Tube Formation Assay
2.6. Compounds and Drugs Treatment
2.7. Preparation of Conditioned Medium and Immunoblotting
2.8. ELISA
2.9. RNA Isolation and Real-Time PCR
2.10. Patients’ Clinical Data and Prognostic Relationships
2.11. Statistical Analysis
3. Results
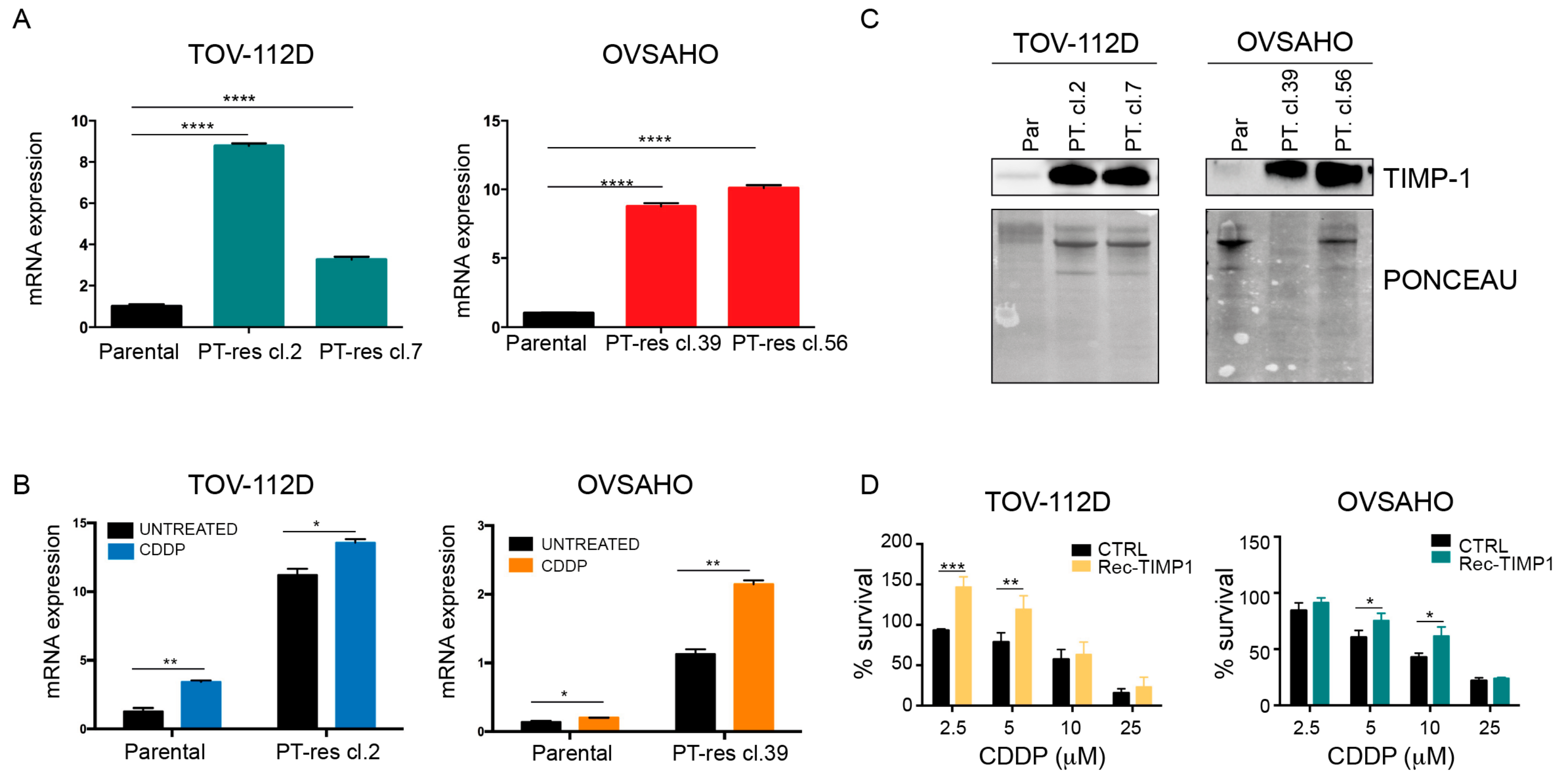
3.1. TIMP-1 is Overexpressed and Secreted by PT-Resistant Cells
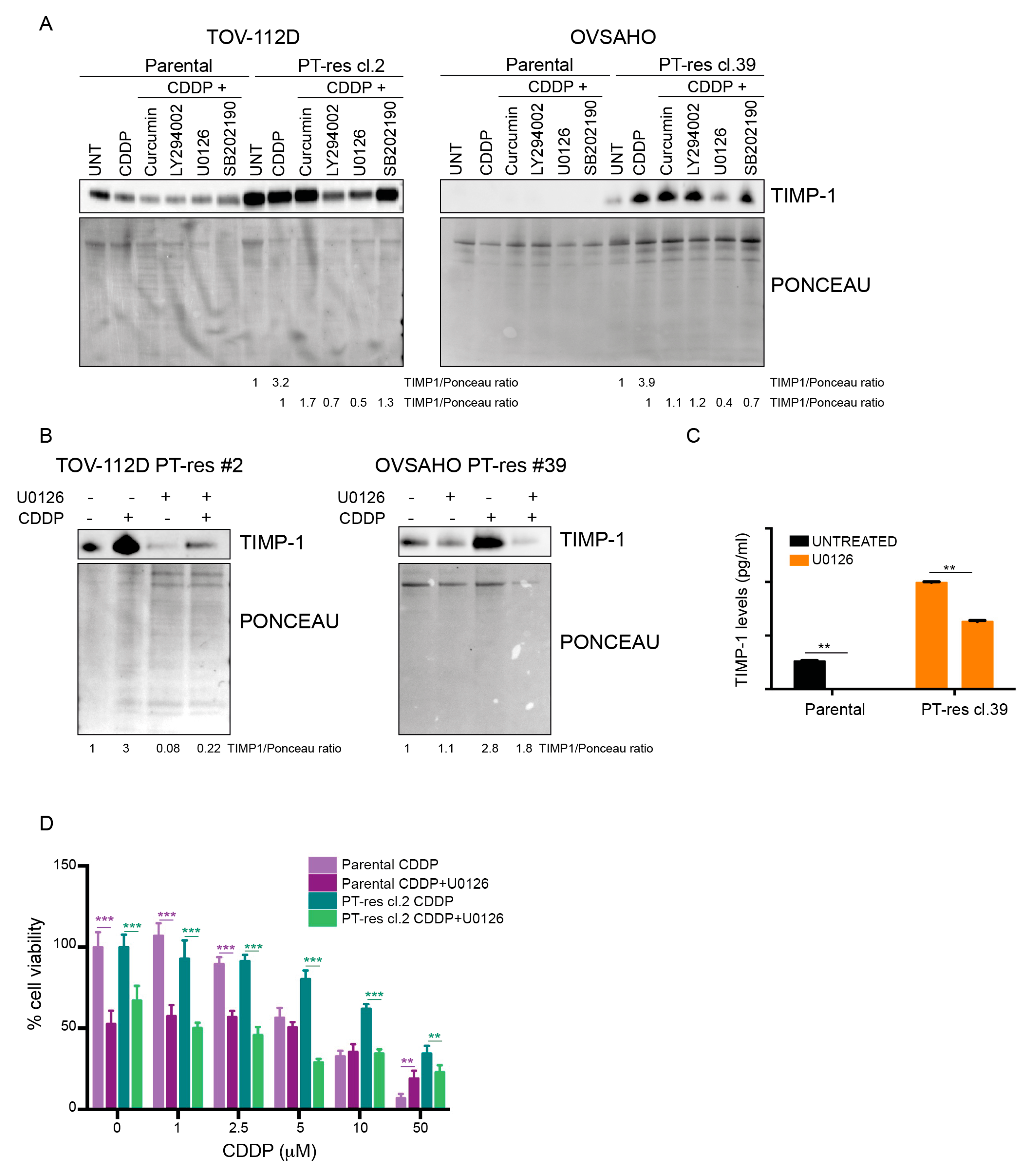
3.2. TIMP-1 Expression is Regulated by PT via the Activation of the MEK/ERK Pathway
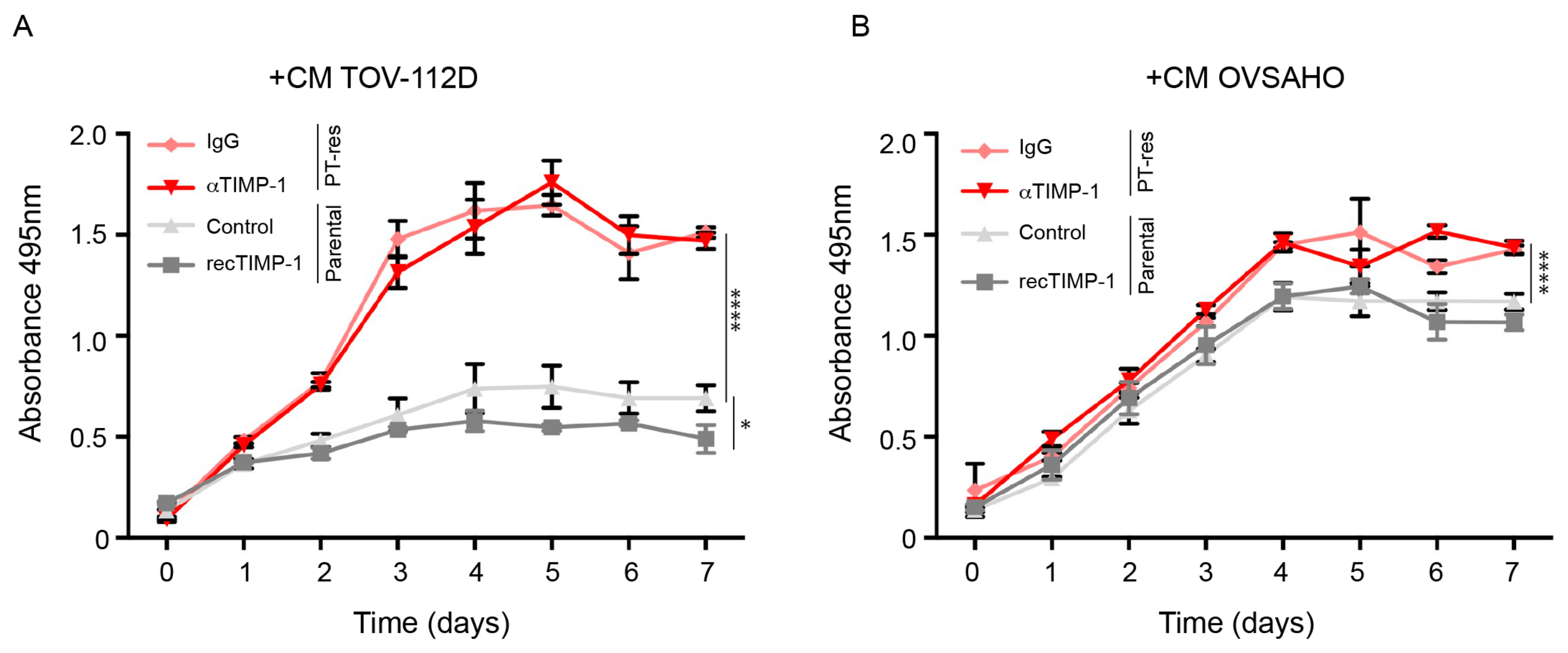
3.3. Conditioned Medium of PT-Resistant is Biologically Active on Endothelial Cells
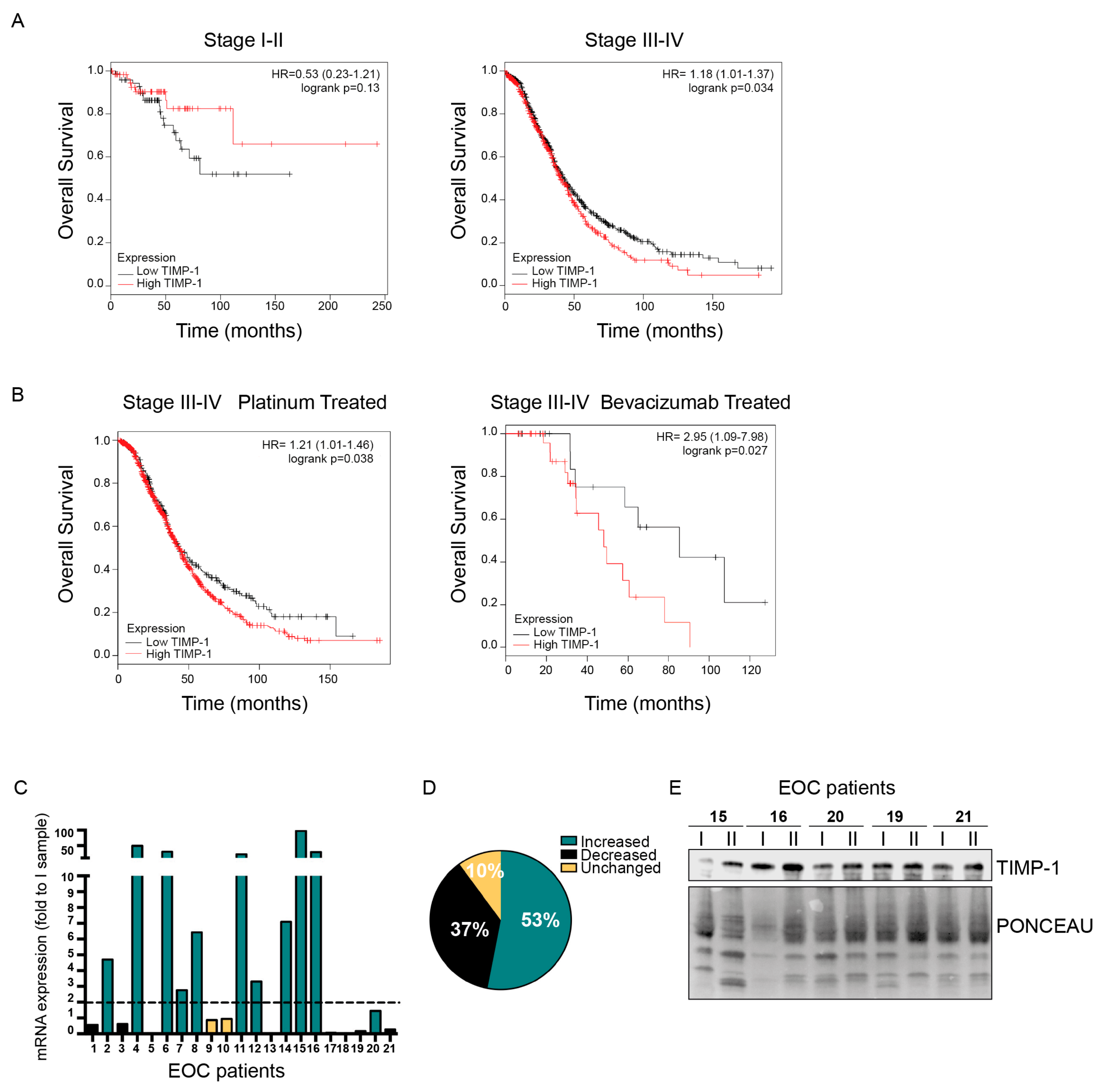
3.4. Role of TIMP-1 Levels in EOC Patients
4. Discussion
- (1)
- PT-resistant tumors usually grow as a myriad of small neoplastic nodules that can completely disrupt the peritoneum (peritoneal carcinomatosis). In this context a further alteration of the vasculature could favor tumor cell survival by impairing the intra-tumoral delivery of the anti-cancer drugs, thus affecting the total tumor burden.
- (2)
- EOC metastasize mainly as small nodules transported by the peritoneal fluids, a plasma ultrafiltrate that moves clockwise in the abdomen and adhere to the mesothelium. Hence, for this type of tumor, the inhibition of MMPs would have less impact on the metastatic abilities of EOC spheroids.
- (3)
- Local release of TIMP-1 could conversely impact the ability of immune cells to infiltrate the tumor nodules, eventually impairing the anticancer immune response of the host. Certainly, all these speculations must be specifically tested in appropriate in vivo models and verified in human pathological samples.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Lheureux, S.; Bruce, J.P.; Burnier, J.V.; Karakasis, K.; Shaw, P.A.; Clarke, B.A.; Yang, S.Y.C.; Quevedo, R.; Li, T.; Dowar, M.; et al. Somatic BRCA1/2 Recovery as a Resistance Mechanism After Exceptional Response to Poly (ADP-ribose) Polymerase Inhibition. JCO 2017, 35, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Patch, A.-M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.K.; Harrell, M.I.; Oza, A.M.; Oaknin, A.; Ray-Coquard, I.; Tinker, A.V.; Helman, E.; Radke, M.R.; Say, C.; Vo, L.-T.; et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2019, 9, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kreuzinger, C.; Gamperl, M.; Wolf, A.; Heinze, G.; Geroldinger, A.; Lambrechts, D.; Boeckx, B.; Smeets, D.; Horvat, R.; Aust, S.; et al. Molecular characterization of 7 new established cell lines from high grade serous ovarian cancer. Cancer Lett. 2015, 362, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Kreuzinger, C.; Von der Decken, I.; Wolf, A.; Gamperl, M.; Koller, J.; Karacs, J.; Pfaffinger, S.; Bartl, T.; Reinthaller, A.; Grimm, C.; et al. Patient-derived cell line models revealed therapeutic targets and molecular mechanisms underlying disease progression of high grade serous ovarian cancer. Cancer Lett. 2019, 459, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stronach, E.A.; Chen, M.; Maginn, E.N.; Agarwal, R.; Mills, G.B.; Wasan, H.; Gabra, H. DNA-PK mediates AKT activation and apoptosis inhibition in clinically acquired platinum resistance. Neoplasia 2011, 13, 1069–1080. [Google Scholar] [CrossRef]
- Stronach, E.A.; Alfraidi, A.; Rama, N.; Datler, C.; Studd, J.B.; Agarwal, R.; Guney, T.G.; Gourley, C.; Hennessy, B.T.; Mills, G.B.; et al. HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Res. 2011, 71, 4412–4422. [Google Scholar] [CrossRef]
- Sonego, M.; Pellizzari, I.; Dall’Acqua, A.; Pivetta, E.; Lorenzon, I.; Benevol, S.; Bomben, R.; Spessotto, P.; Sorio, R.; Gattei, V.; et al. Common biological phenotypes characterize the acquisition of platinum-resistance in epithelial ovarian cancer cells. Sci. Rep. 2017, 7, 7104. [Google Scholar] [CrossRef]
- Vecchione, A.; Belletti, B.; Lovat, F.; Volinia, S.; Chiappetta, G.; Giglio, S.; Sonego, M.; Cirombella, R.; Onesti, E.C.; Pellegrini, P.; et al. A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 9845–9850. [Google Scholar] [CrossRef]
- Giusti, I.; Di Francesco, M.; D’Ascenzo, S.; Palmerini, M.G.; Macchiarelli, G.; Carta, G.; Dolo, V. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer Biol. 2018, 19, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.-N.; Wang, G.; Hood, B.L.; Conrads, K.A.; Hamilton, C.A.; Maxwell, G.L.; Darcy, K.M.; Conrads, T.P. Identification of candidate circulating cisplatin-resistant biomarkers from epithelial ovarian carcinoma cell secretomes. Br. J. Cancer 2014, 110, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Faça, V.M.; Hanash, S.M. In-Depth Proteomics to Define the Cell Surface and Secretome of Ovarian Cancer Cells and Processes of Protein Shedding. Cancer Res. 2009, 69, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Makridakis, M.; Vlahou, A. Secretome proteomics for discovery of cancer biomarkers. J. Proteom. 2010, 73, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.A.; Hoyer, L.W.; Nachman, R.L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J. Clin. Invest. 1973, 52, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, A.; Sonego, M.; Pellizzari, I.; Pellarin, I.; Canzonieri, V.; D’Andrea, S.; Benevol, S.; Sorio, R.; Giorda, G.; Califano, D.; et al. CDK6 protects epithelial ovarian cancer from platinum-induced death via FOXO3 regulation. Embo Mol. Med. 2017, 9, 1415–1433. [Google Scholar] [CrossRef]
- Sonego, M.; Pellarin, I.; Costa, A.; Vinciguerra, G.L.R.; Coan, M.; Kraut, A.; D’Andrea, S.; Dall’Acqua, A.; Castillo-Tong, D.C.; Califano, D.; et al. USP1 links platinum resistance to cancer cell dissemination by regulating Snail stability. Sci. Adv. 2019, 5, eaav3235. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Ries, C. Cytokine functions of TIMP-1. Cell. Mol. Life Sci. 2014, 71, 659–672. [Google Scholar] [CrossRef]
- Ramer, R.; Schmied, T.; Wagner, C.; Haustein, M.; Hinz, B. The antiangiogenic action of cisplatin on endothelial cells is mediated through the release of tissue inhibitor of matrix metalloproteinases-1 from lung cancer cells. Oncotarget 2018, 9, 34038–34055. [Google Scholar] [CrossRef]
- Reis, P.P.; Waldron, L.; Perez-Ordonez, B.; Pintilie, M.; Galloni, N.N.; Xuan, Y.; Cervigne, N.K.; Warner, G.C.; Makitie, A.A.; Simpson, C.; et al. A gene signature in histologically normal surgical margins is predictive of oral carcinoma recurrence. BMC Cancer 2011, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Eichele, K.; Hinz, B. Upregulation of tissue inhibitor of matrix metalloproteinases-1 confers the anti-invasive action of cisplatin on human cancer cells. Oncogene 2007, 26, 5822–5827. [Google Scholar] [CrossRef] [PubMed]
- Karam, A.K.; Santiskulvong, C.; Fekete, M.; Zabih, S.; Eng, C.; Dorigo, O. Cisplatin and PI3kinase inhibition decrease invasion and migration of human ovarian carcinoma cells and regulate matrix-metalloproteinase expression. Cytoskelet. (Hoboken) 2010, 67, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, O.; Munk, S.; Fogh, L.; Yadav, R.; Francavilla, C.; Horn, H.; Würtz, S.Ø.; Schrohl, A.-S.; Damsgaard, B.; Rømer, M.U.; et al. TIMP-1 increases expression and phosphorylation of proteins associated with drug resistance in breast cancer cells. J. Proteome Res. 2013, 12, 4136–4151. [Google Scholar] [CrossRef]
- Botelho, F.M.; Edwards, D.R.; Richards, C.D. Oncostatin M stimulates c-Fos to bind a transcriptionally responsive AP-1 element within the tissue inhibitor of metalloproteinase-1 promoter. J. Biol. Chem. 1998, 273, 5211–5218. [Google Scholar] [CrossRef]
- Hall, M.-C.; Young, D.A.; Waters, J.G.; Rowan, A.D.; Chantry, A.; Edwards, D.R.; Clark, I.M. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-beta 1. J. Biol. Chem. 2003, 278, 10304–10313. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeon, O.-H.; Lee, H.D.; Yoo, K.H.; Kim, D.-S. Integrin alphavbeta3-mediated transcriptional regulation of TIMP-1 in a human ovarian cancer cell line. Biochem. Biophys. Res. Commun. 2008, 377, 479–483. [Google Scholar] [CrossRef]
- Mahner, S.; Woelber, L.; Eulenburg, C.; Schwarz, J.; Carney, W.; Jaenicke, F.; Milde-Langosch, K.; Mueller, V. TIMP-1 and VEGF-165 serum concentration during first-line therapy of ovarian cancer patients. BMC Cancer 2010, 10, 139. [Google Scholar] [CrossRef]
- Steffensen, K.D.; Waldstrøm, M.; Christensen, R.K.; Bartels, A.; Brünner, N.; Jakobsen, A. Lack of relationship between TIMP-1 tumour cell immunoreactivity, treatment efficacy and prognosis in patients with advanced epithelial ovarian cancer. BMC Cancer 2010, 10, 185. [Google Scholar] [CrossRef][Green Version]
- Ikenaka, Y.; Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Noguchi, R.; Tsujinoue, H.; Yanase, K.; Namisaki, T.; Imazu, H.; Masaki, T.; et al. Tissue inhibitor of metalloproteinases-1 (TIMP-1) inhibits tumor growth and angiogenesis in the TIMP-1 transgenic mouse model. Int. J. Cancer 2003, 105, 340–346. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
| Patient Number | Age | Tumor Histotype * | Tumor Stage |
|---|---|---|---|
| 1 | 67 | HGSOC | IV |
| 2 | 55 | HGSOC | IIIC |
| 3 | 72 | HGSOC | IV |
| 4 | 57 | HGSOC | IV |
| 5 | 70 | HGSOC | IV |
| 6 | 54 | HGSOC | IIIC |
| 7 | 69 | HGSOC | IV |
| 8 | 43 | HGSOC | IV |
| 9 | 82 | HGSOC | IIIC |
| 10 | 36 | LGSOC | IV |
| 11 | 81 | HGSOC | IIIC |
| 12 | 64 | HGSOC | IIIC |
| 13 | 72 | HGSOC | IIIC |
| 14 | 68 | HGSOC | IIIC |
| 15 | 70 | HGSOC | IIIC |
| 16 | 41 | HGSOC | IIIC |
| 17 | 57 | HGSOC | IIIC |
| 18 | 65 | HGSOC | IV |
| 19 | 67 | HGSOC | IV |
| 20 | 82 | HGSOC | IIIC |
| 21 | 56 | HGSOC | IIIC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonego, M.; Poletto, E.; Pivetta, E.; Nicoloso, M.S.; Pellicani, R.; Rampioni Vinciguerra, G.L.; Citron, F.; Sorio, R.; Mongiat, M.; Baldassarre, G. TIMP-1 Is Overexpressed and Secreted by Platinum Resistant Epithelial Ovarian Cancer Cells. Cells 2020, 9, 6. https://doi.org/10.3390/cells9010006
Sonego M, Poletto E, Pivetta E, Nicoloso MS, Pellicani R, Rampioni Vinciguerra GL, Citron F, Sorio R, Mongiat M, Baldassarre G. TIMP-1 Is Overexpressed and Secreted by Platinum Resistant Epithelial Ovarian Cancer Cells. Cells. 2020; 9(1):6. https://doi.org/10.3390/cells9010006
Chicago/Turabian StyleSonego, Maura, Evelina Poletto, Eliana Pivetta, Milena S. Nicoloso, Rosanna Pellicani, Gian Luca Rampioni Vinciguerra, Francesca Citron, Roberto Sorio, Maurizio Mongiat, and Gustavo Baldassarre. 2020. "TIMP-1 Is Overexpressed and Secreted by Platinum Resistant Epithelial Ovarian Cancer Cells" Cells 9, no. 1: 6. https://doi.org/10.3390/cells9010006
APA StyleSonego, M., Poletto, E., Pivetta, E., Nicoloso, M. S., Pellicani, R., Rampioni Vinciguerra, G. L., Citron, F., Sorio, R., Mongiat, M., & Baldassarre, G. (2020). TIMP-1 Is Overexpressed and Secreted by Platinum Resistant Epithelial Ovarian Cancer Cells. Cells, 9(1), 6. https://doi.org/10.3390/cells9010006







