PPARγ-Independent Side Effects of Thiazolidinediones on Mitochondrial Redox State in Rat Isolated Hearts
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Heart Isolation
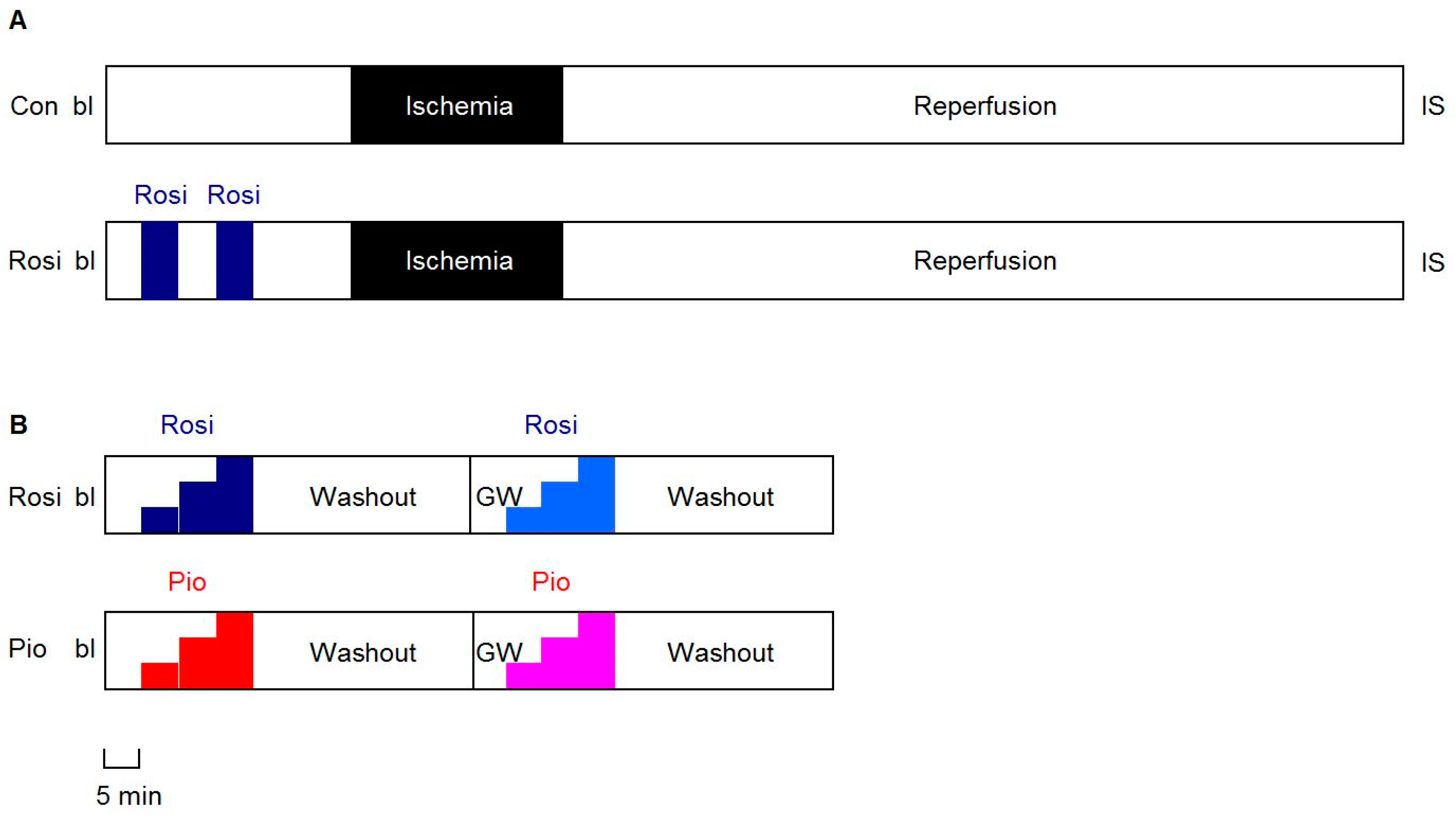
2.3. Protocols
2.3.1. IR Experiments
2.3.2. Dose-Response Experiments
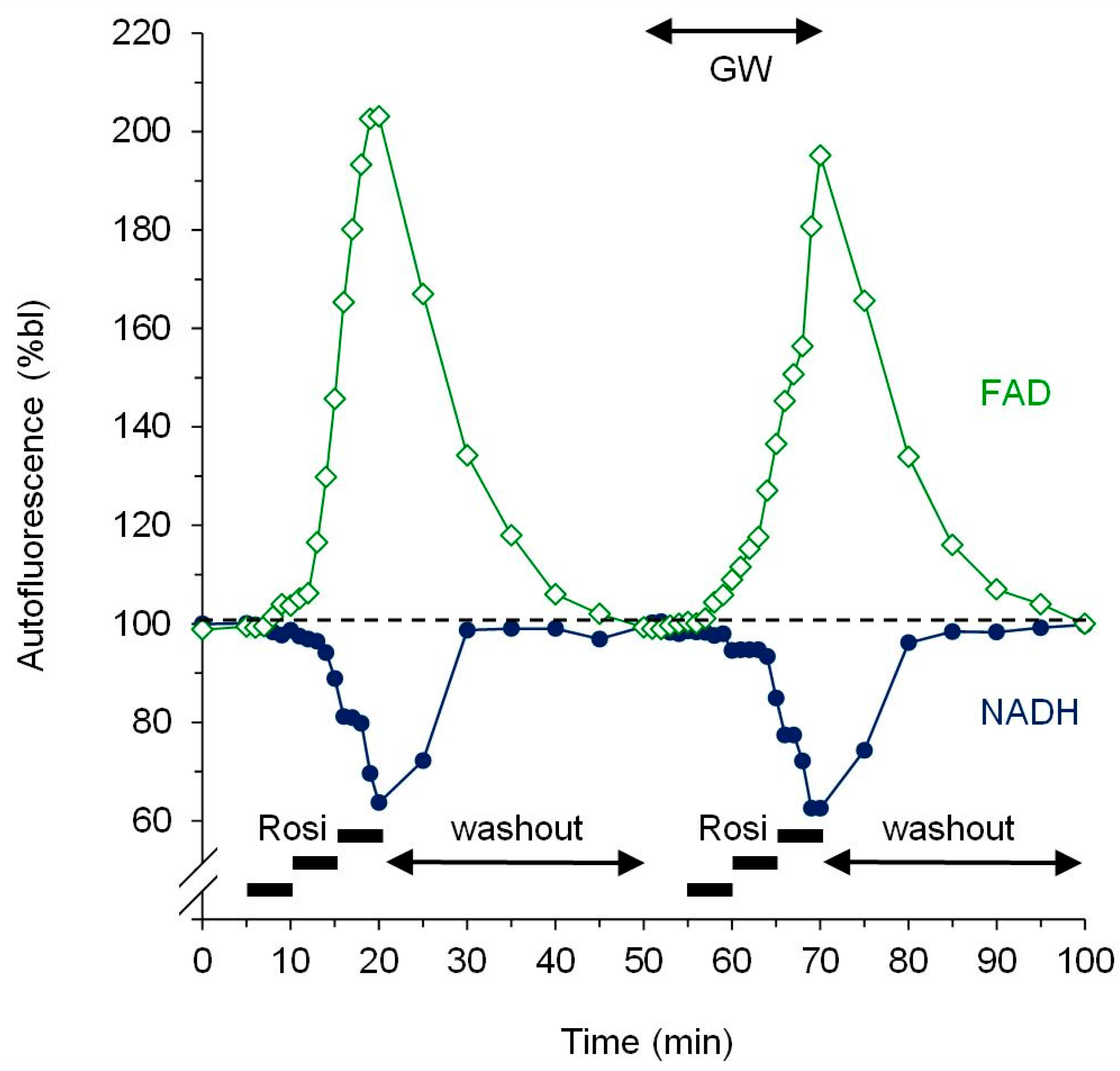
2.4. Fluorescence Measurement of Mitochondrial Redox State
2.5. Statistical Analysis
3. Results
3.1. IR Experiments
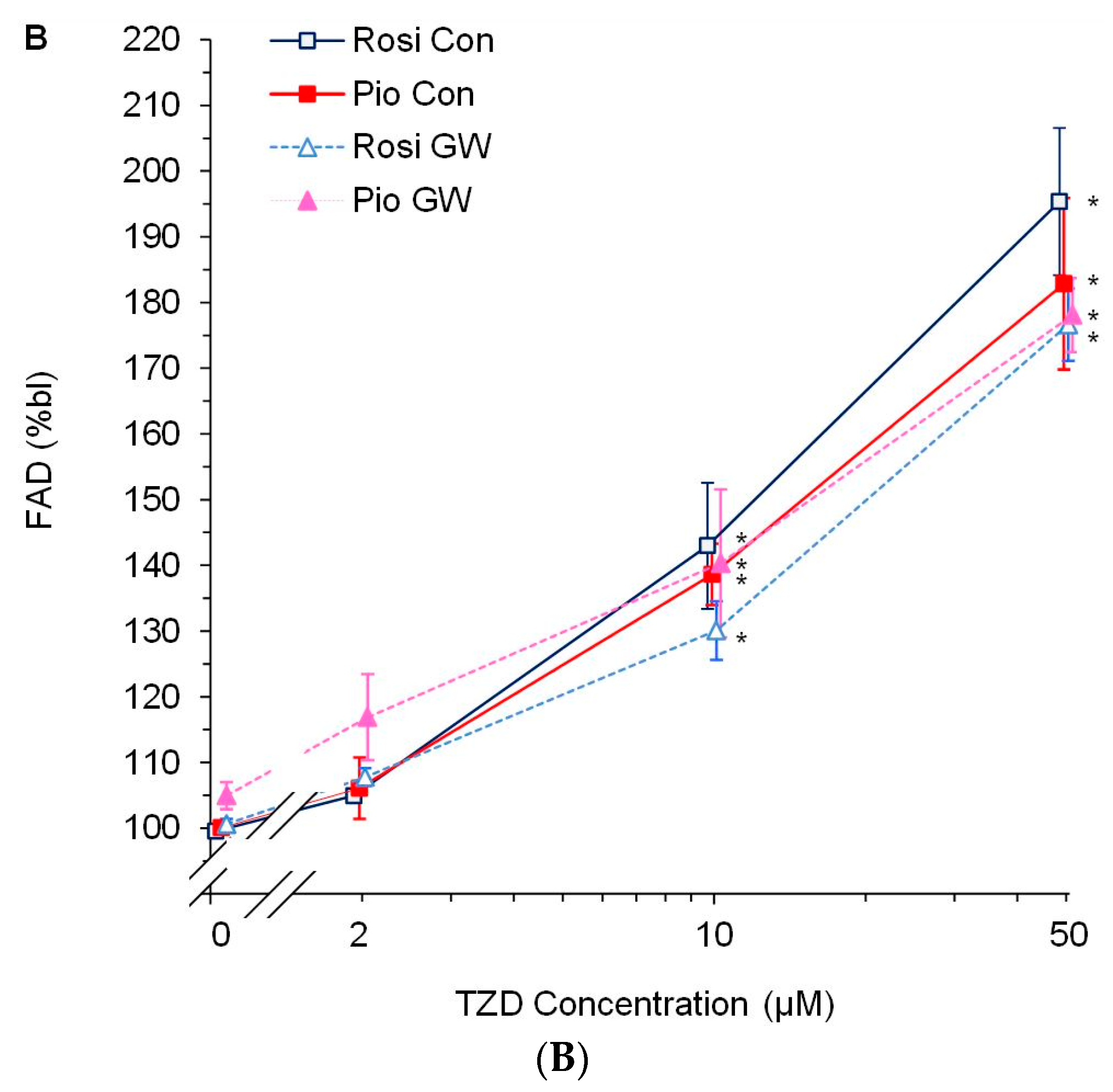
3.2. Dose-Response Experiments
4. Discussion
4.1. PPARγ Activation and Myocardial Protection: Friend or Foe?
4.2. Specificity of TZDs for PPARγ Activation
4.3. TZDs Affect Mitochondrial Function
4.4. Study Limitations and Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eldor, R.; DeFronzo, R.A.; Abdul-Ghani, M. In vivo actions of peroxisome proliferator-activated receptors: Glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 2013, 36, S162–S174. [Google Scholar] [CrossRef]
- Kobayashi, N.; Ohno, T.; Yoshida, K.; Fukushima, H.; Mamada, Y.; Nomura, M.; Hirata, H.; Machida, Y.; Shinoda, M.; Suzuki, N.; et al. Cardioprotective mechanism of telmisartan via PPAR-gamma-eNOS pathway in dahl salt-sensitive hypertensive rats. Am. J. Hypertens. 2008, 21, 576–581. [Google Scholar] [CrossRef]
- Yasuda, S.; Kobayashi, H.; Iwasa, M.; Kawamura, I.; Sumi, S.; Narentuoya, B.; Yamaki, T.; Ushikoshi, H.; Nishigaki, K.; Nagashima, K.; et al. Antidiabetic drug pioglitazone protects the heart via activation of PPAR-gamma receptors, PI3-kinase, Akt, and eNOS pathway in a rabbit model of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1558–H1565. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, L.; Xu, Y.; Schwartz, G.G. Troglitazone improves recovery of left ventricular function after regional ischemia in pigs. Circulation 2000, 101, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.L.; Chen, J.; Bao, W.; Narayanan, P.K.; Bril, A.; Jiang, W.; Lysko, P.G.; Gu, J.L.; Boyce, R.; Zimmerman, D.M.; et al. In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 2001, 104, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Khandoudi, N.; Delerive, P.; Berrebi-Bertrand, I.; Buckingham, R.E.; Staels, B.; Bril, A. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma, inhibits the Jun NH(2)-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes 2002, 51, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Wayman, N.S.; Hattori, Y.; McDonald, M.C.; Mota-Filipe, H.; Cuzzocrea, S.; Pisano, B.; Chatterjee, P.K.; Thiemermann, C. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002, 16, 1027–1040. [Google Scholar] [CrossRef]
- Wynne, A.M.; Mocanu, M.M.; Yellon, D.M. Pioglitazone mimics preconditioning in the isolated perfused rat heart: A role for the prosurvival kinases PI3K and P42/44MAPK. J. Cardiovasc. Pharmacol. 2005, 46, 817–822. [Google Scholar] [CrossRef]
- Li, J.; Lang, M.J.; Mao, X.B.; Tian, L.; Feng, Y.B. Antiapoptosis and mitochondrial effect of pioglitazone preconditioning in the ischemic/reperfused heart of rat. Cardiovasc. Drugs Ther. 2008, 22, 283–291. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, J.; Jiang, C.; Liu, H.F. Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury. Asian Pac. J. Trop. Med. 2014, 7, 271–275. [Google Scholar] [CrossRef]
- Nabor, D.; ElOrbany, R.; Cheng, Q.; Kersten, J.; Stowe, D.; Riess, M. PPARγ Mediates Endogenous and Exogenous Cardioprotection Associated with Rat Chromosome 6. Anesth. Analg. 2013, 116, S93. [Google Scholar]
- Lotz, C.; Lange, M.; Redel, A.; Stumpner, J.; Schmidt, J.; Tischer-Zeitz, T.; Roewer, N.; Kehl, F. Peroxisome-proliferator-activated receptor gamma mediates the second window of anaesthetic-induced preconditioning. Exp. Physiol. 2011, 96, 317–324. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, L.; Greyson, C.; Lee, J.; Gen, M.; Kinugawa, K.; Long, C.S.; Schwartz, G.G. Deleterious effects of acute treatment with a peroxisome proliferator-activated receptor-gamma activator in myocardial ischemia and reperfusion in pigs. Diabetes 2003, 52, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gen, M.; Lu, L.; Fox, J.; Weiss, S.O.; Brown, R.D.; Perlov, D.; Ahmad, H.; Zhu, P.; Greyson, C.; et al. PPAR-gamma activation fails to provide myocardial protection in ischemia and reperfusion in pigs. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1314–H1323. [Google Scholar] [CrossRef] [PubMed]
- Palee, S.; Weerateerangkul, P.; Surinkeaw, S.; Chattipakorn, S.; Chattipakorn, N. Effect of rosiglitazone on cardiac electrophysiology, infarct size and mitochondrial function in ischaemia and reperfusion of swine and rat heart. Exp. Physiol. 2011, 96, 778–789. [Google Scholar] [CrossRef]
- Sarraf, M.; Lu, L.; Ye, S.; Reiter, M.J.; Greyson, C.R.; Schwartz, G.G. Thiazolidinedione drugs promote onset, alter characteristics, and increase mortality of ischemic ventricular fibrillation in pigs. Cardiovasc. Drugs Ther. 2012, 26, 195–204. [Google Scholar] [CrossRef][Green Version]
- Palee, S.; Weerateerangkul, P.; Chinda, K.; Chattipakorn, S.C.; Chattipakorn, N. Mechanisms responsible for beneficial and adverse effects of rosiglitazone in a rat model of acute cardiac ischaemia-reperfusion. Exp. Physiol. 2013, 98, 1028–1037. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Zhai, S.D. Risk of cardiovascular disease and all-cause mortality among diabetic patients prescribed rosiglitazone or pioglitazone: A meta-analysis of retrospective cohort studies. Chin. Med. J. 2012, 125, 4301–4306. [Google Scholar]
- Nissen, S.E. Rosiglitazone: A case of regulatory hubris. BMJ 2013, 347, f7428. [Google Scholar] [CrossRef]
- Hickson, R.P.; Cole, A.L.; Dusetzina, S.B. Implications of Removing Rosiglitazone’s Black Box Warning and Restricted Access Program on the Uptake of Thiazolidinediones and Dipeptidyl Peptidase-4 Inhibitors Among Patients with Type 2 Diabetes. J. Manag. Care Spec. Pharm. 2019, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Wolski, K.; Nicholls, S.J.; Nissen, S.E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA 2007, 298, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Colmers, I.N.; Bowker, S.L.; Majumdar, S.R.; Johnson, J.A. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: A meta-analysis. CMAJ 2012, 184, E675–E683. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M.; Kwok, C.S.; Chen-Turner, C.; Maduakor, C.A.; Singh, S.; Loke, Y.K. Thiazolidinediones and associated risk of bladder cancer: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2014, 78, 258–273. [Google Scholar] [CrossRef]
- Tang, H.; Shi, W.; Fu, S.; Wang, T.; Zhai, S.; Song, Y.; Han, J. Pioglitazone and bladder cancer risk: A systematic review and meta-analysis. Cancer Med. 2018, 7, 1070–1080. [Google Scholar] [CrossRef]
- Stone, J.C.; Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A. Was there really any evidence that rosiglitazone increased the risk of myocardial infarction or death from cardiovascular causes? Pharmacoepidemiol. Drug Saf. 2015, 24, 223–227. [Google Scholar] [CrossRef]
- Loke, Y.K.; Kwok, C.S.; Singh, S. Comparative cardiovascular effects of thiazolidinediones: Systematic review and meta-analysis of observational studies. BMJ 2011, 342, d1309. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Hear. Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef]
- Camara, A.K.; Bienengraeber, M.; Stowe, D.F. Mitochondrial approaches to protect against cardiac ischemia and reperfusion injury. Front. Physiol. 2011, 2, 13. [Google Scholar] [CrossRef]
- Riess, M.L.; Camara, A.K.S.; Chen, Q.; Novalija, E.; Rhodes, S.S.; Stowe, D.F. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H53–H60. [Google Scholar] [CrossRef]
- Riess, M.L.; Kevin, L.G.; Camara, A.K.S.; Heisner, J.S.; Stowe, D.F. Dual exposure to sevoflurane improves anesthetic preconditioning in intact hearts. Anesthesiology 2004, 100, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Nabbi, R.; Gadicherla, A.K.; Kersten, J.R.; Stowe, D.F.; Lazar, J.; Riess, M.L. Genetically determined mitochondrial preservation and cardioprotection against myocardial ischemia-reperfusion injury in a consomic rat model. Physiol. Genom. 2014, 46, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Salzman, M.M.; Cheng, Q.; Deklotz, R.J.; Dulai, G.K.; Douglas, H.F.; Dikalova, A.E.; Weihrauch, D.; Barnes, B.M.; Riess, M.L. Lipid emulsion enhances cardiac performance after ischemia-reperfusion in isolated hearts from summer-active arctic ground squirrels. J. Comp. Physiol. B 2017, 187, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.E.; Konorev, E.A.; Gross, G.J.; Chilian, W.M.; Jacob, H.J. Resistance to myocardial ischemia in five rat strains: Is there a genetic component of cardioprotection? Am. J. Physiol. 2000, 278, H1395–H1400. [Google Scholar] [CrossRef] [PubMed]
- Kevin, L.G.; Novalija, E.; Riess, M.L.; Camara, A.K.; Rhodes, S.S.; Stowe, D.F. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth. Analg. 2003, 96, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Kevin, L.G.; Katz, P.; Camara, A.K.; Novalija, E.; Riess, M.L.; Stowe, D.F. Anesthetic preconditioning: Effects on latency to ischemic injury in isolated hearts. Anesthesiology 2003, 99, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.; Diaz, R.J.; Mao, G.D.; Wilson, G.J. Ischemic preconditioning: Differences in protection and susceptibility to blockade with single-cycle versus multicycle transient ischemia. Circulation 1997, 96, 984–995. [Google Scholar] [CrossRef]
- Altman, F.P. Tetrazolium salts and formazans. Prog. Histochem. Cytochem. 1976, 9, 1–56. [Google Scholar] [CrossRef]
- Riess, M.L.; Rhodes, S.S.; Stowe, D.F.; Aldakkak, M.; Camara, A.K. Comparison of cumulative planimetry versus manual dissection to assess experimental infarct size in isolated hearts. J. Pharmacol. Toxicol. Methods 2009, 60, 275–280. [Google Scholar] [CrossRef]
- Shidham, S.V.; Nabbi, R.; Camara, A.K.S.; Riess, M.L. Development of automated infarct size measurement in TTC stained rat isolated hearts after global ischemia/reperfusion. FASEB J. 2011, 25, 1130–1132. [Google Scholar]
- Peng, X.; Chen, R.; Wu, Y.; Huang, B.; Tang, C.; Chen, J.; Wang, Q.; Wu, Q.; Yang, J.; Qiu, H.; et al. PPARgamma-PI3K/AKT-NO signal pathway is involved in cardiomyocyte hypertrophy induced by high glucose and insulin. J. Diabetes Complicat. 2015, 29, 755–760. [Google Scholar] [CrossRef]
- Peymani, M.; Ghaedi, K.; Irani, S.; Nasr-Esfahani, M.H. Peroxisome Proliferator-Activated Receptor gamma Activity is Required for Appropriate Cardiomyocyte Differentiation. Cell J. 2016, 18, 221–228. [Google Scholar] [PubMed]
- Chance, B.; Williamson, J.R.; Jamieson, D.; Schoenner, B. Properties and kinetics of reduced pyridine nucleotide fluorescence of the isolated and in vivo rat heart. Biochem. Zeit 1965, 341, 357–377. [Google Scholar]
- Camara, A.K.; Aldakkak, M.; Heisner, J.S.; Rhodes, S.S.; Riess, M.L.; An, J.; Heinen, A.; Stowe, D.F. ROS scavenging before 27 degrees C ischemia protects hearts and reduces mitochondrial ROS, Ca2+ overload, and changes in redox state. Am. J. Physiol. Cell Physiol. 2007, 292, C2021–C2031. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Gao, H.; Li, W. Long-term risk of rosiglitazone on cardiovascular events—A systematic review and meta-analysis. Endokrynol. Pol. 2018, 69, 381–394. [Google Scholar] [PubMed]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef]
- Sivarajah, A.; McDonald, M.C.; Thiemermann, C. The cardioprotective effects of preconditioning with endotoxin, but not ischemia, are abolished by a peroxisome proliferator-activated receptor-gamma antagonist. J. Pharmacol. Exp. Ther. 2005, 313, 896–901. [Google Scholar] [CrossRef]
- Morrison, A.; Yan, X.; Tong, C.; Li, J. Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, and JNK signaling in nondiabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H895–H902. [Google Scholar] [CrossRef]
- Chen, T.; Jin, X.; Crawford, B.H.; Cheng, H.; Saafir, T.B.; Wagner, M.B.; Yuan, Z.; Ding, G. Cardioprotection from oxidative stress in the newborn heart by activation of PPARgamma is mediated by catalase. Free Radic. Biol. Med. 2012, 53, 208–215. [Google Scholar] [CrossRef]
- Nagashima, A.; Watanabe, R.; Ogawa, M.; Suzuki, J.; Masumura, M.; Hishikari, K.; Shimizu, T.; Takayama, K.; Hirata, Y.; Nagai, R.; et al. Different roles of PPAR-gamma activity on physiological and pathological alteration after myocardial ischemia. J. Cardiovasc. Pharmacol. 2012, 60, 158–164. [Google Scholar] [CrossRef]
- Han, J.; Wang, D.; Ye, L.; Li, P.; Hao, W.; Chen, X.; Ma, J.; Wang, B.; Shang, J.; Li, D.; et al. Rosmarinic Acid Protects against Inflammation and Cardiomyocyte Apoptosis during Myocardial Ischemia/Reperfusion Injury by Activating Peroxisome Proliferator-Activated Receptor Gamma. Front. Pharmacol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Ravingerova, T.; Adameova, A.; Carnicka, S.; Nemcekova, M.; Kelly, T.; Matejikova, J.; Galatou, E.; Barlaka, E.; Lazou, A. The role of PPAR in myocardial response to ischemia in normal and diseased heart. Gen. Physiol. Biophys. 2011, 30, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.R.; El-Mansy, M.F.; Sem, D.S.; Greene, A.S. Chemical proteomics-based analysis of off-target binding profiles for rosiglitazone and pioglitazone: Clues for assessing potential for cardiotoxicity. J. Med. Chem. 2012, 55, 8260–8271. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, D.L.; Spagnolo, A.; Akar, C.; Weinberg, G.; Murphy, P.; Gavrilyuk, V.; Dello Russo, C. Receptor-independent actions of PPAR thiazolidinedione agonists: Is mitochondrial function the key? Biochem. Pharmacol. 2005, 70, 177–188. [Google Scholar] [CrossRef]
- Gardner, O.S.; Shiau, C.W.; Chen, C.S.; Graves, L.M. Peroxisome proliferator-activated receptor gamma-independent activation of p38 MAPK by thiazolidinediones involves calcium/calmodulin-dependent protein kinase II and protein kinase R: Correlation with endoplasmic reticulum stress. J. Biol. Chem. 2005, 280, 10109–10118. [Google Scholar] [CrossRef] [PubMed]
- Mughal, R.S.; Warburton, P.; O’Regan, D.J.; Ball, S.G.; Turner, N.A.; Porter, K.E. Peroxisome proliferator-activated receptor gamma-independent effects of thiazolidinediones on human cardiac myofibroblast function. Clin. Exp. Pharmacol. Physiol. 2009, 36, 478–486. [Google Scholar] [CrossRef]
- He, H.; Tao, H.; Xiong, H.; Duan, S.Z.; McGowan, F.X., Jr.; Mortensen, R.M.; Balschi, J.A. Rosiglitazone causes cardiotoxicity via peroxisome proliferator-activated receptor gamma-independent mitochondrial oxidative stress in mouse hearts. Toxicol. Sci. 2014, 138, 468–481. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef]
- Krenz, M.; Oldenburg, O.; Wimpee, H.; Cohen, M.V.; Garlid, K.D.; Critz, S.D.; Downey, J.M.; Benoit, J.N. Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res. Cardiol. 2002, 97, 365–373. [Google Scholar] [CrossRef]

| During Application | 120 min Reperfusion | |||
|---|---|---|---|---|
| Con | Rosi | Con | Rosi | |
| sysLVP (%bl) | 95.5 ± 2.0 | 101.9 ± 2.8 | 70.5 ± 4.9 | 60.3 ± 3.9 |
| diaLVP (mmHg) | 10.5 ± 2.1 | 9.9 ± 0.8 | 32.3 ± 4.3 | 31.8 ± 2.7 |
| devLVP (%bl) | 92.6 ± 3.8 | 102.4 ± 3.6 | 33.8 ± 3.7 | 29.7 ± 1.0 |
| RPP (%bl) | 96.4 ± 5.5 | 101.3 ± 4.4 | 33.5 ± 3.7 | 30.9 ± 0.7 |
| dLVP/dtmax (%bl) | 94.4 ± 3.5 | 106.6 ± 4.7 | 35.9 ± 2.6 | 32.8 ± 1.3 |
| dLVP/dtmin (%bl) | 97.2 ± 2.4 | 100.6 ± 4.5 | 38.9 ± 5.0 | 30.4 ± 1.1 |
| HR (%bl) | 105.3 ± 2.8 | 98.8 ± 2.2 | 98.4 ± 2.7 | 104.3 ± 1.6 |
| CF (%bl) | 100.0 ± 1.0 | * 124.0 ± 6.1 | 64.6 ± 5.3 | 64.5 ± 3.0 |
| IS (%) | 36.2 ± 3.4 | * 45.3 ± 0.7 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riess, M.L.; Elorbany, R.; Weihrauch, D.; Stowe, D.F.; Camara, A.K.S. PPARγ-Independent Side Effects of Thiazolidinediones on Mitochondrial Redox State in Rat Isolated Hearts. Cells 2020, 9, 252. https://doi.org/10.3390/cells9010252
Riess ML, Elorbany R, Weihrauch D, Stowe DF, Camara AKS. PPARγ-Independent Side Effects of Thiazolidinediones on Mitochondrial Redox State in Rat Isolated Hearts. Cells. 2020; 9(1):252. https://doi.org/10.3390/cells9010252
Chicago/Turabian StyleRiess, Matthias L., Reem Elorbany, Dorothee Weihrauch, David F. Stowe, and Amadou K.S. Camara. 2020. "PPARγ-Independent Side Effects of Thiazolidinediones on Mitochondrial Redox State in Rat Isolated Hearts" Cells 9, no. 1: 252. https://doi.org/10.3390/cells9010252
APA StyleRiess, M. L., Elorbany, R., Weihrauch, D., Stowe, D. F., & Camara, A. K. S. (2020). PPARγ-Independent Side Effects of Thiazolidinediones on Mitochondrial Redox State in Rat Isolated Hearts. Cells, 9(1), 252. https://doi.org/10.3390/cells9010252







