Lentiform Nucleus Hyperechogenicity in Parkinsonian Syndromes: A Systematic Review and Meta-Analysis with Consideration of Molecular Pathology
Abstract
1. Introduction
2. Materials and Methods
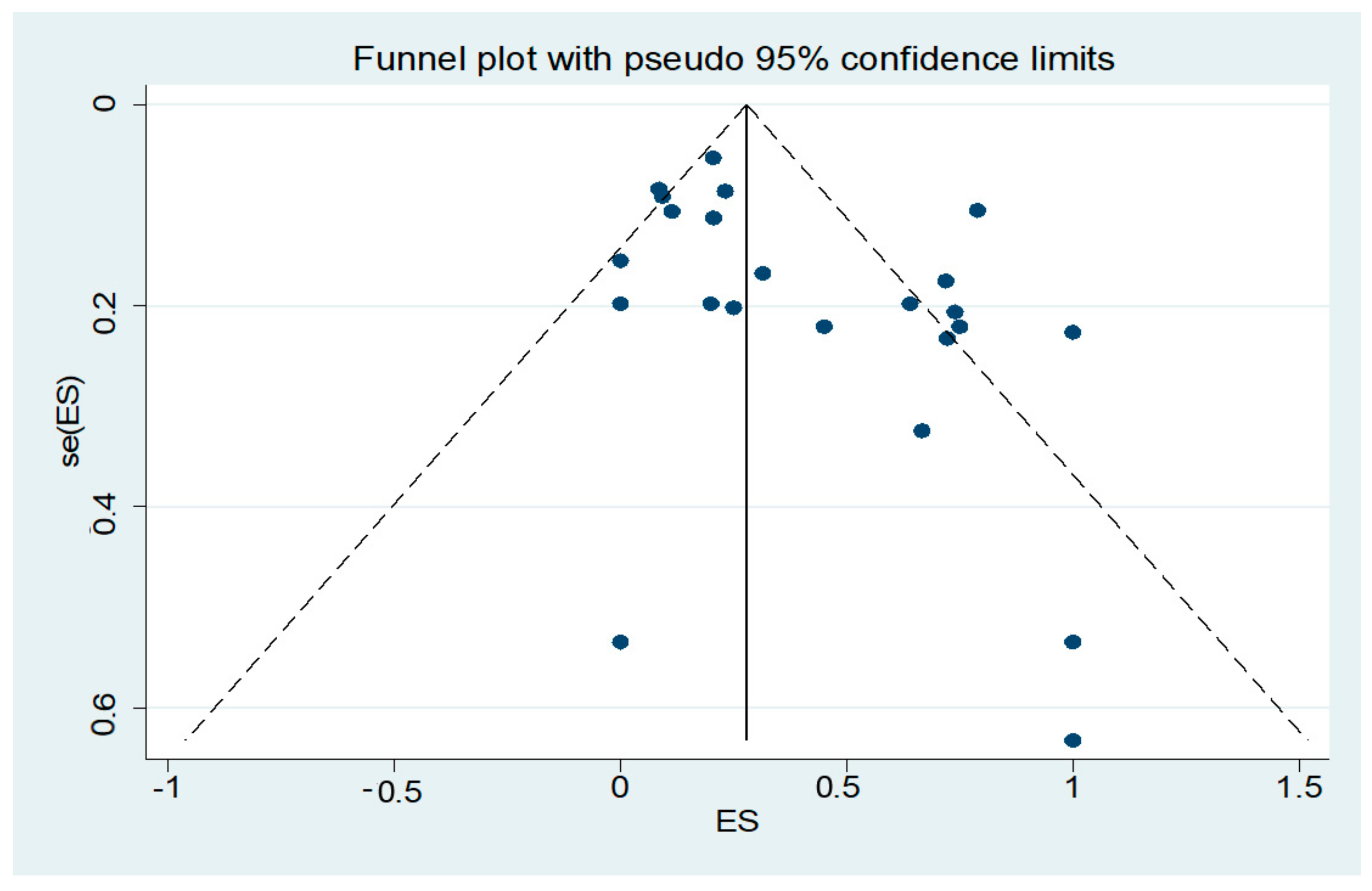
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef]
- Li, D.H.; He, Y.C.; Liu, J.; Chen, S.D. Diagnostic Accuracy of Transcranial Sonography of the Substantia Nigra in Parkinson’s disease: A Systematic Review and Meta-analysis. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Chen, G.; Deng, Y.; Xu, R. Accuracy of Transcranial Sonography of the Substantia Nigra for Detection of Parkinson’s Disease: A Systematic Review and Meta-analysis. Ultrasound Med. Biol. 2019, 3, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Shafieesabet, A.; Fereshtehnejad, S.M.; Shafieesabet, A.; Delbari, A.; Baradaran, H.R.; Postuma, R.B.; Lökk, J. Hyperechogenicity of substantia nigra for differential diagnosis of Parkinson’s disease: A meta-analysis. Parkinsonism Relat. Disord. 2017, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Godau, J.; Walter, U. Transcranial sonography in movement disorders. Lancet Neurol. 2008, 7, 1044–1055. [Google Scholar] [CrossRef]
- Krogias, C.; Eyding, J.; Postert, T. Transcranial sonography in Huntington’s disease. Int. Rev. Neurobiol. 2010, 90, 237–257. [Google Scholar]
- Behnke, S.; Berg, D.; Naumann, M.; Becker, G. Differentiation of Parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. J. Neurol. Neurosurg. Psychiatry 2005, 76, 423–425. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico- pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.G.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson- Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.Y.; Colosimo, C.; Dürr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Niehaus, L.; Probst, T.; Benecke, R.; Meyer, B.U.; Dressler, D. Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology 2003, 60, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Wenning, G.K.; Ben Shlomo, Y.; Magahaes, M.; Daniel, S.E.; Quinn, N.P. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain 1994, 117, 835–845. [Google Scholar] [CrossRef]
- Freeman, M.F.; Tukey, J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950, 21, 607–611. [Google Scholar] [CrossRef]
- Alonso-Cánovas, A.; López-Sendón, J.L.; Buisán, J.; deFelipe-Mimbrera, A.; Guillán, M.; García-Barragán, N.; Corral, I.; Matute-Lozano, M.C.; Masjuan, J.; Martínez-Castrillo, J.C.; et al. Sonography for diagnosis of Parkinson disease-from theory to practice: A study on 300 participants. J. Ultrasound Med. 2014, 33, 2069–2074. [Google Scholar] [CrossRef]
- Gaenslen, A.; Unmuth, B.; Godau, J.; Liepelt, I.; Di Santo, A.; Schweitzer, K.J.; Gasser, T.; Machulla, H.J.; Reimold, M.; Marek, K.; et al. The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson’s disease: A prospective blinded study. Lancet Neurol. 2008, 7, 417–424. [Google Scholar] [CrossRef]
- Laučkaitė, K.; Rastenytė, D.; Šurkienė, D.; Vaidelytė, B.; Dambrauskaitė, G.; Sakalauskas, A.; Vaitkus, A.; Gleiznienė, R. Ultrasonographic (TCS) and clinical findings in overlapping phenotype of essential tremor and Parkinson’s disease (ET-PD). BMC Neurol. 2014, 14, 54. [Google Scholar] [CrossRef]
- Laučkaitė, K.; Rastenytė, D.; Šurkienė, D.; Vaitkus, A.; Sakalauskas, A.; Lukoševičius, A.; Gleiznienė, R. Specificity of transcranial sonography in parkinson spectrum disorders in comparison to degenerative cognitive syndromes. BMC Neurol. 2012, 12, 12. [Google Scholar] [CrossRef]
- Monaco, D.; Berg, D.; Thomas, A.; Di Stefano, V.; Barbone, F.; Vitale, M.; Ferrante, C.; Bonanni, L.; Di Nicola, M.; Garzarella, T.; et al. The predictive power of transcranial sonography in movement disorders: A longitudinal cohort study. Neurol. Sci. 2018, 39, 1887–1894. [Google Scholar] [CrossRef]
- Prati, P.; Bignamini, A.; Coppo, L.; Naldi, A.; Comi, C.; Cantello, R.; Gusmaroli, G.; Walter, U. The measuring of substantia nigra hyperechogenicity in an Italian cohort of Parkinson disease patients: A case/control study (NOBIS Study). J. Neural Transm. (Vienna) 2017, 124, 869–879. [Google Scholar] [CrossRef]
- Sheng, A.Y.; Zhang, Y.C.; Sheng, Y.J.; Wang, C.S.; Zhang, Y.; Hu, H.; Luo, W.F.; Liu, C.F. Transcranial sonography image characteristics in different Parkinson’s disease subtypes. Neurol. Sci. 2017, 38, 1805–1810. [Google Scholar] [CrossRef]
- Smajlovic, D.; Ibrahimagic, O.C. Transcranial Brain Sonography in Parkinson’s Disease and Other Parkinsonian Disorders: A Hospital Study from Tuzla, Bosnia and Herzegovina. Med. Arch. 2017, 71, 261–264. [Google Scholar] [CrossRef]
- Walter, U.; Wittstock, M.; Benecke, R.; Dressler, D. Substantia nigra echogenicity is normal in non-extrapyramidal cerebral disorders but increased in Parkinson’s disease. J. Neural Transm. (Vienna) 2002, 109, 191–196. [Google Scholar] [CrossRef]
- Walter, U.; Dressler, D.; Wolters, A.; Wittstock, M.; Greim, B.; Benecke, R. Sonographic discrimination of dementia with Lewy bodies and Parkinson’s disease with dementia. J. Neurol. 2006, 253, 448–454. [Google Scholar] [CrossRef]
- Walter, U.; Dressler, D.; Probst, T.; Wolters, A.; Abu-Mugheisib, M.; Wittstock, M.; Benecke, R. Transcranial brain sonography findings in discriminating between parkinsonism and idiopathic Parkinson disease. Arch. Neurol. 2007, 64, 1635–1640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadowski, K.; Serafin-Król, M.; Szlachta, K.; Friedman, A. Basal ganglia echogenicity in tauopathies. J. Neural Transm. (Vienna) 2015, 122, 863–865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanzaro, E.; Lemolo, F. Transcranial sonography in movement disorders: An interesting tool for diagnostic perspectives. Neurol. Sci. 2016, 37, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Hagenah, J.; König, I.R.; Kötter, C.; Seidel, G.; Klein, C.; Brüggemann, N. Basal ganglia hyperechogenicity does not distinguish between patients with primary dystonia and healthy individuals. J. Neurol. 2011, 258, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Movement Disorder Society-endorsed PSP Study Group; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, V.C.; Paraskevas, G.P.; Velonakis, G.; Toulas, P.; Stamboulis, E.; Kapaki, E. MRI Planimetry and Magnetic Resonance Parkinsonism Index in the Differential Diagnosis of Patients with Parkinsonism. Am. J. Neuroradiol. 2018, 39, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Merz, B.; Reiners, K.; Naumann, M.; Becker, G. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov. Disord. 2005, 20, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Boxer, A.L.; Yu, J.T.; Golbe, L.I.; Litvan, I.; Lang, A.E.; Höglinger, G.U. Advances in progressive supranuclear palsy: New diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 2017, 16, 552–563. [Google Scholar] [CrossRef]
- Berg, D.; Grote, C.; Rausch, W.D.; Mäurer, M.; Wesemann, W.; Riederer, P.; Becker, G. Iron accumulation in the substantia nigra in rats visualized by ultrasound. Ultrasound Med. Biol. 1999, 25, 901–904. [Google Scholar] [CrossRef]
- Berg, D.; Roggendorf, W.; Schroder, U.; Klein, R.; Tatschner, T.; Benz, P.; Tucha, O.; Preier, M.; Lange, K.W.; Reiners, K.; et al. Echogenicity of the substantia nigra: Association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch. Neurol. 2002, 59, 999–1005. [Google Scholar] [CrossRef]
- Berg, D. In vivo detection of iron and neuromelanin by transcranial sonography—A new approach for early detection of substantia nigra damage. J. Neural Transm. (Vienna) 2006, 113, 775–780. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, B.; Tao, K.; Yang, H.; Wang, Y.; Zhou, T.; Yang, Y.; Yuan, L.; Liu, X.; Duan, Y. Iron accumulation and microglia activation contribute to substantia nigra hyperechogenicity in the 6-OHDA-induced rat model of Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 36, 76–82. [Google Scholar] [CrossRef]
- Gerlach, M.; Ben-Shachar, D.; Riederer, P.; Youdim, M.B. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J. Neurochem. 1994, 63, 793–807. [Google Scholar] [CrossRef]
- Berg, D.; Gerlach, M.; Youdim, M.B.; Double, K.L.; Zecca, L.; Riederer, P.; Becker, G. Brain iron pathways and their relevance to Parkinson’s disease. J. Neurochem. 2001, 79, 225–236. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Zecca, L.; Berg, D.; Arzberger, T.; Ruprecht, P.; Rausch, W.D.; Musicco, M.; Tampellini, D.; Riederer, P.; Gerlach, M.; Becker, G. In vivo detection of iron and neuromelanin by transcranial sonography: A new approach for early detection of substantia nigra damage. Mov. Disord. 2005, 20, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Connor, D.J.; Hentz, J.G.; Sabbagh, M.N.; Caviness, J.N.; Shill, H.A.; Noble, B.; Beach, T.G. Incidental Lewy body disease: Clinical comparison to a control cohort. Mov. Disord. 2010, 25, 642–646. [Google Scholar] [CrossRef]
- Zecca, L.; Gallorini, M.; Schunemann, V.; Trautwein, A.X.; Gerlach, M.; Riederer, P.; Vezzoni, P.; Tampellini, D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: Consequences for iron storage and neurodegenerative processes. J. Neurochem. 2001, 76, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Fariello, R.; Riederer, P.; Sulzer, D.; Gatti, A.; Tampellini, D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002, 510, 216–220. [Google Scholar] [CrossRef]
- Double, K.L.; Gerlach, M.; Schunemann, V.; Trautwein, A.X.; Zecca, L.; Gallorini, M.; Youdim, M.B.; Riederer, P.; Ben-Shachar, D. Iron binding characteristics of neuromelanin of the human substantia nigra. Biochem. Pharmacol. 2003, 66, 489–494. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, M.S. Brain Iron Accumulation in Atypical Parkinsonian Syndromes: In vivo MRI Evidences for Distinctive Patterns. Front. Neurol. 2019, 10, 74. [Google Scholar] [CrossRef]
- Dexter, D.T.; Carayon, A.; Javoy-Agid, F.; Agid, Y.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991, 114, 1953–1975. [Google Scholar] [CrossRef] [PubMed]
- Kaindlstorfer, C.; Jellinger, K.A.; Eschlböck, S.; Stefanova, N.; Weiss, G.; Wenning, G.K. The relevance of iron in the pathogenesis of multiple system atrophy: A viewpoint. J. Alzheimers Dis. 2018, 61, 1253–1273. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Skowrońska, M.; Litwin, T.; Szpak, G.M.; Jabłonka-Salach, K.; Skoloudík, D.; Bulska, E.; Członkowska, A. Lenticular nucleus hyperechogenicity in Wilson’s disease reflects local copper, but not iron accumulation. J. Neural Transm. (Vienna) 2014, 121, 1273–1279. [Google Scholar] [CrossRef] [PubMed]


| Authors | Year | Country | Center | TCS Device (MHz) | Ultrasound System | PD Cases | aPS Cases (MSA-P/ PSP) | Mean Age (PD/ aPS) |
|---|---|---|---|---|---|---|---|---|
| Monaco et al. | 2018 | Italy | Mono | 2–3.5 | Sonos 750, Philipps | 119 | 90 (-/-) | 66/62 |
| Prati et al. | 2017 | Italy | Multi | 2.5 | APLIO 400 Platinum, Toshiba | 25 | - | - |
| Sheng et al. | 2017 | China | Mono | 2.5 | Sequoia 512, Siemens | 356 | - | 64/- |
| Smaljovic et al. | 2017 | Bosnia and Herzegovina | Mono | 2.5 | EnVisor C HD, Philips | 41 | - | 65/- |
| Sadowski et al. | 2015 | Poland | Mono | 1–4 | Esaote, MyLab 70XVision | - | 20 (0/20) | -/60 |
| Sanzaro et al. | 2015 | Italy | Mono | 2.5 | General Electric, Logiq 7 Pro | - | 5 (2/3) | -/- |
| Alonso-C. et al. | 2014 | Spain | Mono | 2.5 | Xario, Toshiba | 78 | - | 73/- |
| Laučkaitė et al. | 2014 | Lithuania | Mono | 2-5 | Voluson 730, General Electrics Healthcare | 141 | - | 64/- |
| Laučkaitė et al. | 2012 | Lithuania | Mono | 1, 3–4 | Voluson 730, General Electrics Healthcare | - | 3 (-/-) | 67 |
| Gaenslen et al. | 2008 | Germany | Mono | 2.5 | Elegra, Siemens | 35 | 9 (-/-) | -/- |
| Walter et al. | 2007 | Germany | Mono | 2.5 | Elegra, Siemens | 134 | 39 (20/19) | 67/68 |
| Walter et al. | 2006 | Germany | Mono | 2.5 | Elegra, Siemens | 25 | - | 71/- |
| Behnke et al. | 2005 | Germany | Multi | 2.5 | Elegra, Siemens | 88 | 50 (32/18) | 67/66 |
| Walter et al. | 2003 | Germany | Mono | 2.5 | Elegra, Siemens | 25 | 23 (-/-) | 68/69 |
| Walter et al. | 2002 | Germany | Mono | 2.5 | Elegra, Siemens | 24 | - | 69/- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, D.; Katsanos, A.H.; Schroeder, C.; Tsivgoulis, G.; Paraskevas, G.P.; Müller, T.; Alexandrov, A.V.; Gold, R.; Tönges, L.; Krogias, C. Lentiform Nucleus Hyperechogenicity in Parkinsonian Syndromes: A Systematic Review and Meta-Analysis with Consideration of Molecular Pathology. Cells 2020, 9, 2. https://doi.org/10.3390/cells9010002
Richter D, Katsanos AH, Schroeder C, Tsivgoulis G, Paraskevas GP, Müller T, Alexandrov AV, Gold R, Tönges L, Krogias C. Lentiform Nucleus Hyperechogenicity in Parkinsonian Syndromes: A Systematic Review and Meta-Analysis with Consideration of Molecular Pathology. Cells. 2020; 9(1):2. https://doi.org/10.3390/cells9010002
Chicago/Turabian StyleRichter, Daniel, Aristeidis H. Katsanos, Christoph Schroeder, Georgios Tsivgoulis, George P. Paraskevas, Thomas Müller, Andrei V. Alexandrov, Ralf Gold, Lars Tönges, and Christos Krogias. 2020. "Lentiform Nucleus Hyperechogenicity in Parkinsonian Syndromes: A Systematic Review and Meta-Analysis with Consideration of Molecular Pathology" Cells 9, no. 1: 2. https://doi.org/10.3390/cells9010002
APA StyleRichter, D., Katsanos, A. H., Schroeder, C., Tsivgoulis, G., Paraskevas, G. P., Müller, T., Alexandrov, A. V., Gold, R., Tönges, L., & Krogias, C. (2020). Lentiform Nucleus Hyperechogenicity in Parkinsonian Syndromes: A Systematic Review and Meta-Analysis with Consideration of Molecular Pathology. Cells, 9(1), 2. https://doi.org/10.3390/cells9010002








