Parsing the IL-37-Mediated Suppression of Inflammasome Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Experiments
2.3. Generation of Bone Marrow-Derived Macrophages (BMDM)
2.4. Quantification of Cytokine Concentrations
2.5. Inflammasome Activation
2.6. RNA Isolation and Detection of Gene Expression
2.7. Caspase-1 Activation Assay
2.8. ASC Oligomerization Assay
2.9. Immunoblot Analysis
2.10. Production and Purification of Recombinant IL-37b-Y85A (recIL-37)
2.11. ASC Speck Formation
2.12. LDH Assay
2.13. Statistical Analysis
3. Results
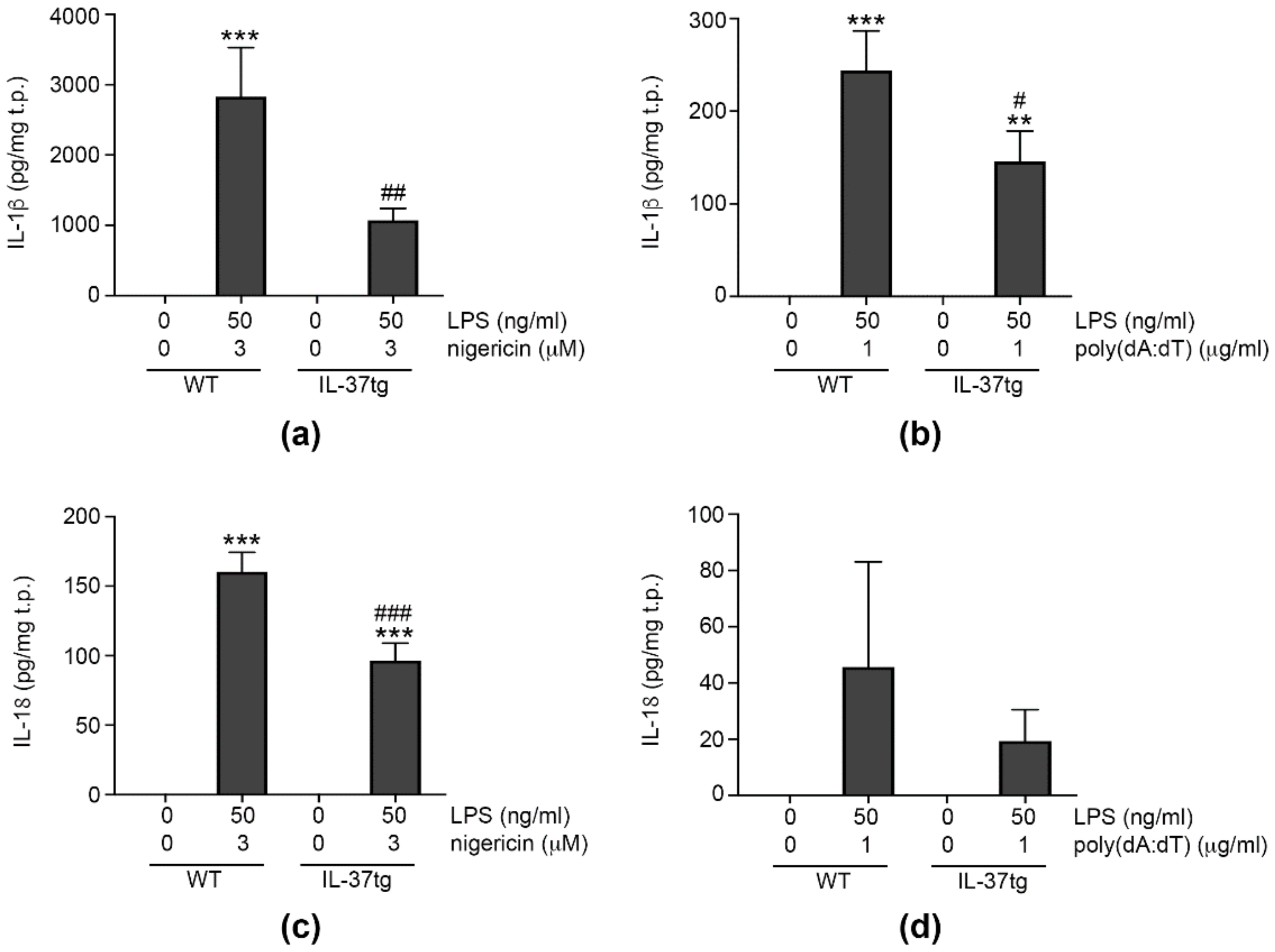
3.1. IL-37 Inhibits Inflammasome-Mediated Production of IL-1β and IL-18
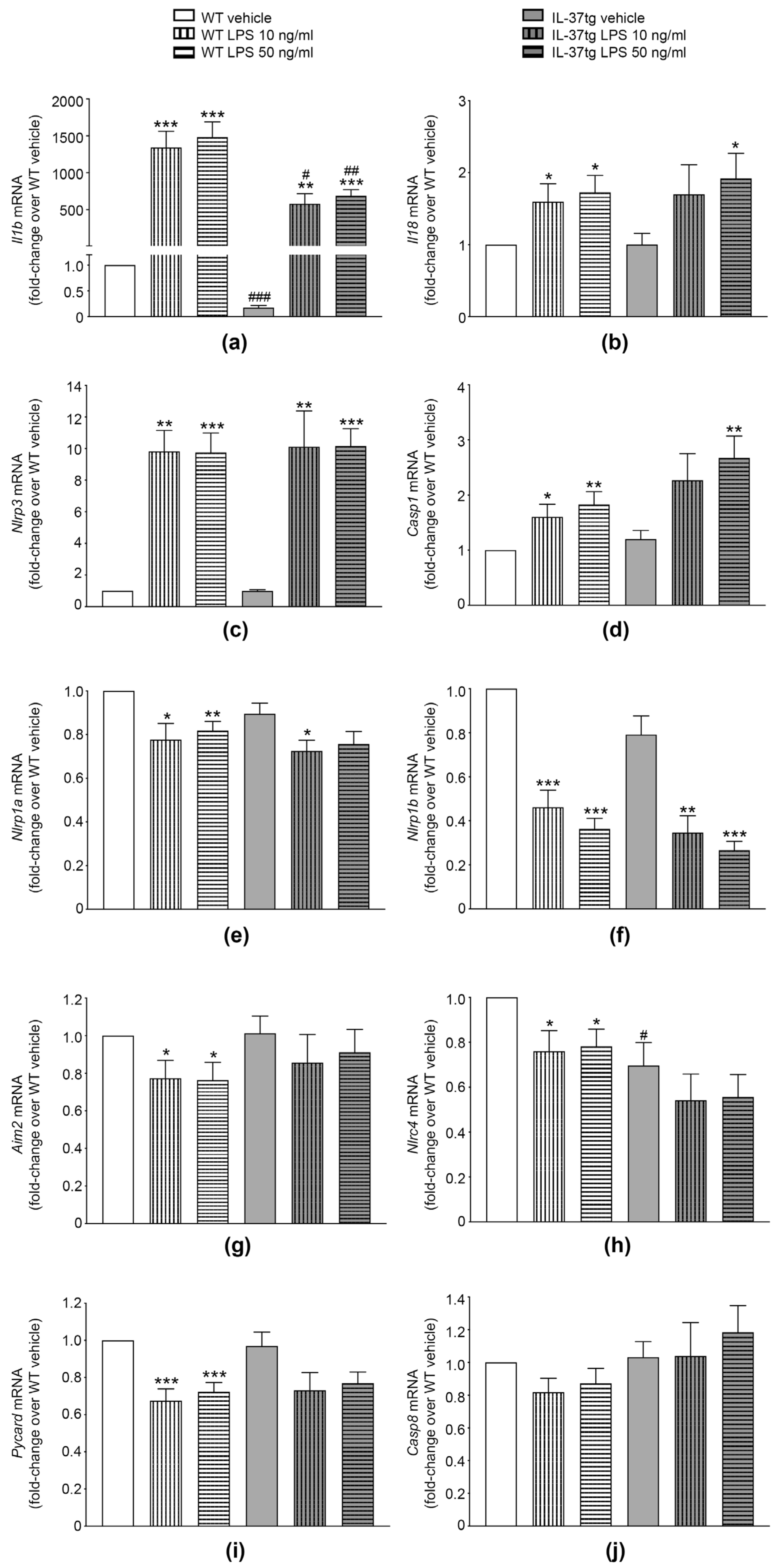
3.2. IL-37 Inhibits the mRNA Expression of Il1b
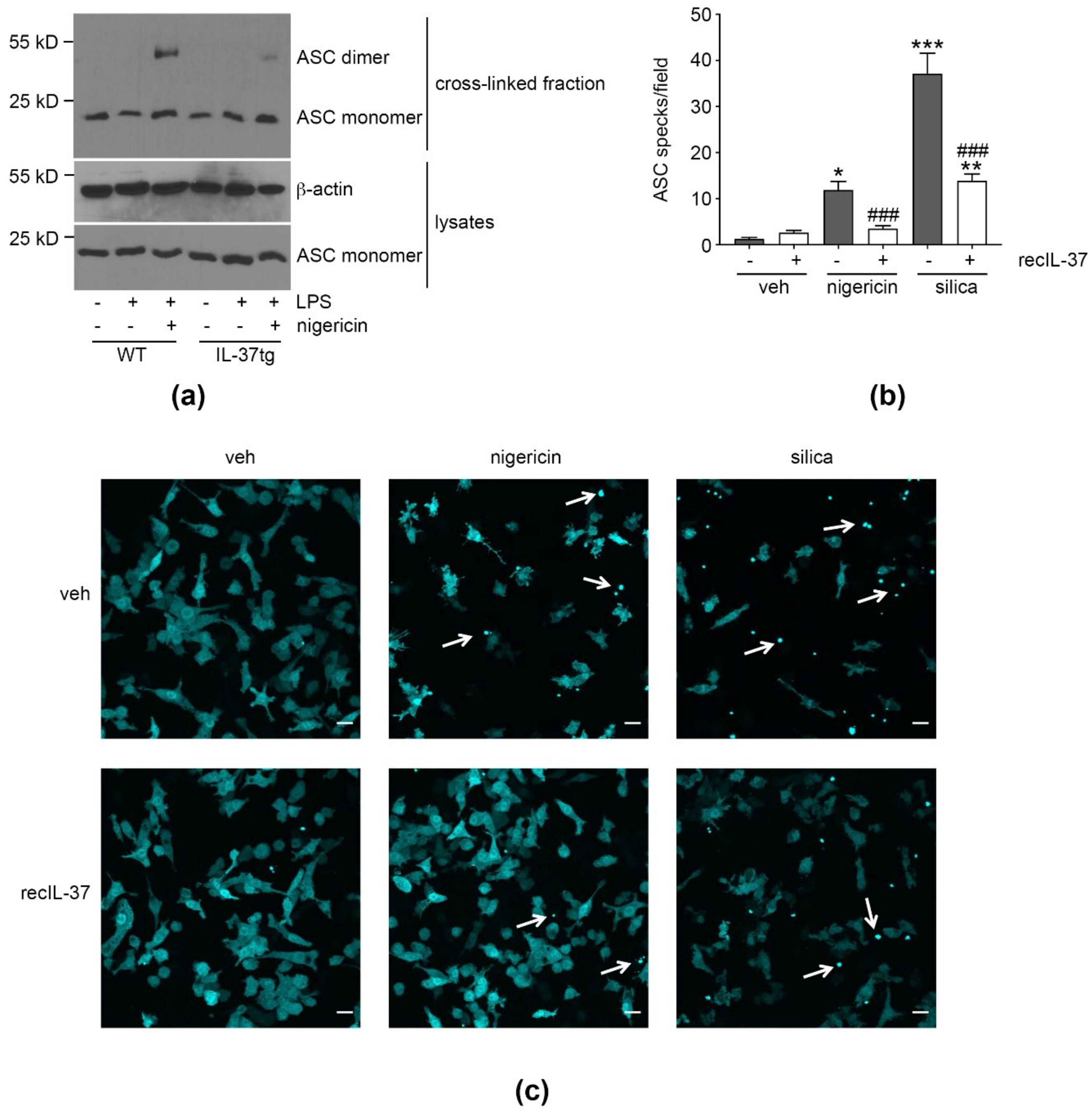
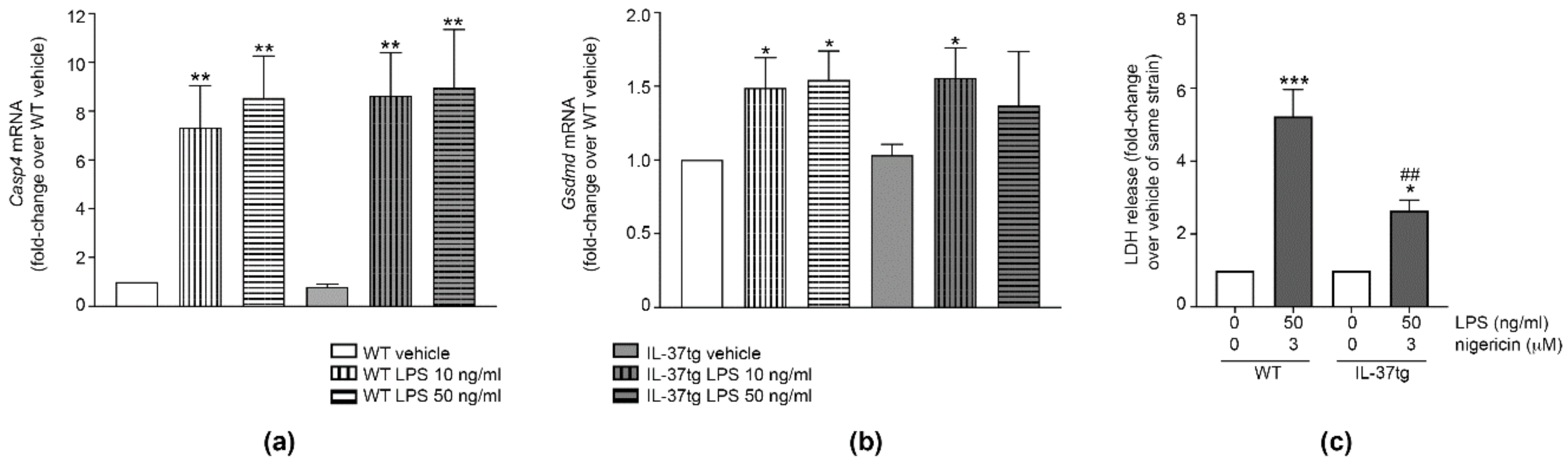
3.3. IL-37 Inhibits ASC Multimerization
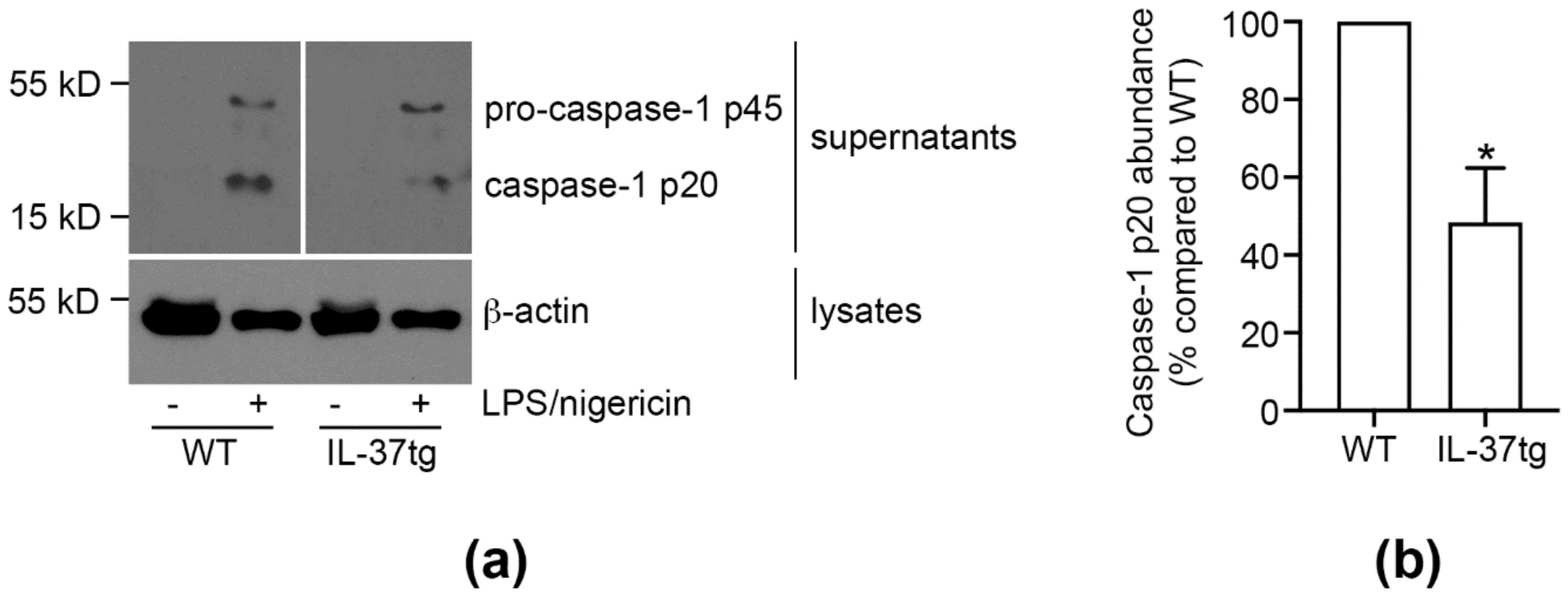
3.4. IL-37 Inhibits Caspase-1 Activation
3.5. IL-37 Inhibits Pyroptosis
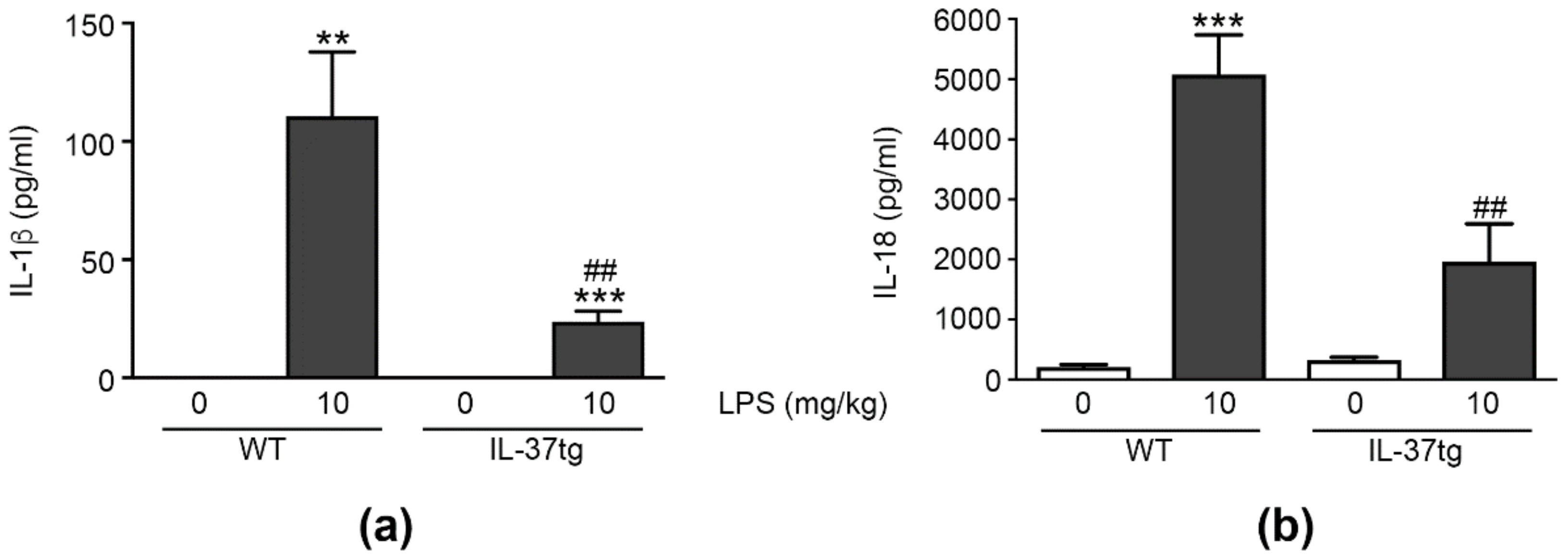
3.6. IL-37 Inhibits the LPS-Induced Production of IL-1β and IL-18 In Vivo
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Rudloff, I.; Godsell, J.; Nold-Petry, C.A.; Harris, J.; Hoi, A.; Morand, E.F.; Nold, M.F. Brief Report: Interleukin-38 Exerts Antiinflammatory Functions and Is Associated With Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 67, 3219–3225. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Stoeckman, A.K.; Wu, G.; Boeckermann, A.N.; Azam, T.; Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Hao, R.; Kalabokis, V.; et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc. Natl. Acad. Sci. USA 2012, 109, 3001–3005. [Google Scholar] [CrossRef]
- Dinarello, C.A. Keep up the heat on IL-1. Blood 2012, 120, 2538–2539. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- McGeough, M.D.; Wree, A.; Inzaugarat, M.E.; Haimovich, A.; Johnson, C.D.; Pena, C.A.; Goldbach-Mansky, R.; Broderick, L.; Feldstein, A.E.; Hoffman, H.M. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J. Clin. Investig. 2017, 127, 4488–4497. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of inflammasome signaling. Nat. Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Niepmann, S.; Knolle, P.A.; Hornung, V. Aging-Associated TNF Production Primes Inflammasome Activation and NLRP3-Related Metabolic Disturbances. J. Immunol. 2016, 197, 2900–2908. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Xiao, T.S. Post-translational regulation of inflammasomes. Cell. Mol. Immunol. 2017, 14, 65–79. [Google Scholar] [CrossRef]
- Dowling, J.K.; O’Neill, L.A. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 424–443. [Google Scholar] [CrossRef]
- Gurung, P.; Kanneganti, T.D. Novel roles for caspase-8 in IL-1beta and inflammasome regulation. Am. J. Pathol. 2015, 185, 17–25. [Google Scholar] [CrossRef]
- Kono, H.; Kimura, Y.; Latz, E. Inflammasome activation in response to dead cells and their metabolites. Curr. Opin. Immunol. 2014, 30, 91–98. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Nold, M.F.; Mangan, N.E.; Rudloff, I.; Cho, S.X.; Shariatian, N.; Samarasinghe, T.D.; Skuza, E.M.; Pedersen, J.; Veldman, A.; Berger, P.J.; et al. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc. Natl. Acad. Sci. USA 2013, 110, 14384–14389. [Google Scholar] [CrossRef]
- Rudloff, I.; Cho, S.X.; Bui, C.B.; McLean, C.; Veldman, A.; Berger, P.J.; Nold, M.F.; Nold-Petry, C.A. Refining anti-inflammatory therapy strategies for bronchopulmonary dysplasia. J. Cell. Mol. Med. 2017, 21, 1128–1138. [Google Scholar] [CrossRef]
- Busfield, S.J.; Comrack, C.A.; Yu, G.; Chickering, T.W.; Smutko, J.S.; Zhou, H.; Leiby, K.R.; Holmgren, L.M.; Gearing, D.P.; Pan, Y. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics 2000, 66, 213–216. [Google Scholar] [CrossRef]
- Kumar, S.; McDonnell, P.C.; Lehr, R.; Tierney, L.; Tzimas, M.N.; Griswold, D.E.; Capper, E.A.; Tal-Singer, R.; Wells, G.I.; Doyle, M.L.; et al. Identification and initial characterization of four novel members of the interleukin-1 family. J. Biol. Chem. 2000, 275, 10308–10314. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E.; Renshaw, B.R.; Ketchem, R.R.; Kubin, M.; Garka, K.E.; Sims, J.E. Four new members expand the interleukin-1 superfamily. J. Biol. Chem. 2000, 275, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Riva, F.; Bonavita, E.; Gentile, S.; Mantovani, A. Decoys and Regulatory “Receptors” of the IL-1/Toll-Like Receptor Superfamily. Front. Immunol. 2013, 4, 180. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cai, X.; Liu, S.; Wang, S.; Nold-Petry, C.A.; Nold, M.F.; Bufler, P.; Norris, D.; Dinarello, C.A.; Fujita, M. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15178–15183. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef]
- Nold-Petry, C.A.; Lo, C.Y.; Rudloff, I.; Elgass, K.D.; Li, S.; Gantier, M.P.; Lotz-Havla, A.S.; Gersting, S.W.; Cho, S.X.; Lao, J.C.; et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 2015, 16, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Bufler, P.; Gamboni-Robertson, F.; Azam, T.; Kim, S.H.; Dinarello, C.A. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem. J. 2004, 381, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.E.; Smith, D.E. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Ellisdon, A.M.; Nold-Petry, C.A.; D’Andrea, L.; Cho, S.X.; Lao, J.C.; Rudloff, I.; Ngo, D.; Lo, C.Y.; Soares da Costa, T.P.; Perugini, M.A.; et al. Homodimerization attenuates the anti-inflammatory activity of interleukin-37. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, I.; Cho, S.X.; Lao, J.C.; Ngo, D.; McKenzie, M.; Nold-Petry, C.A.; Nold, M.F. Monocytes and dendritic cells are the primary sources of interleukin 37 in human immune cells. J. Leukoc. Biol. 2017, 101, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Hanning, C.R.; Brigham-Burke, M.R.; Rieman, D.J.; Lehr, R.; Khandekar, S.; Kirkpatrick, R.B.; Scott, G.F.; Lee, J.C.; Lynch, F.J.; et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 2002, 18, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Bulau, A.M.; Nold, M.F.; Li, S.; Nold-Petry, C.A.; Fink, M.; Mansell, A.; Schwerd, T.; Hong, J.; Rubartelli, A.; Dinarello, C.A.; et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc. Natl. Acad. Sci. USA 2014, 111, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulk, N.; Nold, M.F.; Graf, R.; Kim, S.H.; Reinhardt, D.; Dinarello, C.A.; Bufler, P. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J. Immunol. 2008, 180, 5477–5482. [Google Scholar] [CrossRef]
- Moretti, S.; Bozza, S.; Oikonomou, V.; Renga, G.; Casagrande, A.; Iannitti, R.G.; Puccetti, M.; Garlanda, C.; Kim, S.; Li, S.; et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014, 10, e1004462. [Google Scholar] [CrossRef] [PubMed]
- Ballak, D.B.; van Diepen, J.A.; Moschen, A.R.; Jansen, H.J.; Hijmans, A.; Groenhof, G.J.; Leenders, F.; Bufler, P.; Boekschoten, M.V.; Muller, M.; et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat. Commun. 2014, 5, 4711. [Google Scholar] [CrossRef] [PubMed]
- McNamee, E.N.; Masterson, J.C.; Jedlicka, P.; McManus, M.; Grenz, A.; Collins, C.B.; Nold, M.F.; Nold-Petry, C.; Bufler, P.; Dinarello, C.A.; et al. Interleukin 37 expression protects mice from colitis. Proc. Natl. Acad. Sci. USA 2011, 108, 16711–16716. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Van Sweringen, H.L.; Belizaire, R.M.; Quillin, R.C.; Schuster, R.; Blanchard, J.; Burns, J.M.; Tevar, A.D.; Edwards, M.J.; Lentsch, A.B. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J. Gastroenterol. Hepatol. 2012, 27, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Roberson, S.M.; Walker, W.S. Immortalization of cloned mouse splenic macrophages with a retrovirus containing the v-raf/mil and v-myc oncogenes. Cell. Immunol. 1988, 116, 341–351. [Google Scholar] [CrossRef]
- De Nardo, D.; Kalvakolanu, D.V.; Latz, E. Immortalization of Murine Bone Marrow-Derived Macrophages. Methods Mol. Biol. 2018, 1784, 35–49. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Petry, C.; Fritz, G.; Pfeilschifter, J.; Huwiler, A. Inhibition of Rho modulates cytokine-induced prostaglandin E2 formation in renal mesangial cells. Biochim. Biophys. Acta 2004, 1636, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Lee, J.P.W.; Elgass, K.; Pinar, A.A.; Tate, M.D.; Aitken, E.H.; Fan, H.; Creed, S.J.; Deen, N.S.; Traore, D.A.K.; et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat. Commun. 2018, 9, 2223. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, M.; Zhang, Y.; Miao, E.A. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods Mol. Biol. 2013, 1040, 85–90. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Stutz, A.; Horvath, G.L.; Monks, B.G.; Latz, E. ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol. 2013, 1040, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Hett, E.C.; Slater, L.H.; Mark, K.G.; Kawate, T.; Monks, B.G.; Stutz, A.; Latz, E.; Hung, D.T. Chemical genetics reveals a kinase-independent role for protein kinase R in pyroptosis. Nat. Chem. Biol. 2013, 9, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Aachoui, Y.; Sagulenko, V.; Miao, E.A.; Stacey, K.J. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr. Opin. Microbiol. 2013, 16, 319–326. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Awad, F.; Assrawi, E.; Jumeau, C.; Georgin-Lavialle, S.; Cobret, L.; Duquesnoy, P.; Piterboth, W.; Thomas, L.; Stankovic-Stojanovic, K.; Louvrier, C.; et al. Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation. PLoS ONE 2017, 12, e0175336. [Google Scholar] [CrossRef]
- Stoll, S.; Jonuleit, H.; Schmitt, E.; Muller, G.; Yamauchi, H.; Kurimoto, M.; Knop, J.; Enk, A.H. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur. J. Immunol. 1998, 28, 3231–3239. [Google Scholar] [CrossRef]
- Zhu, Q.; Kanneganti, T.D. Cutting Edge: Distinct Regulatory Mechanisms Control Proinflammatory Cytokines IL-18 and IL-1beta. J. Immunol. 2017, 198, 4210–4215. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [PubMed]
- Kupz, A.; Guarda, G.; Gebhardt, T.; Sander, L.E.; Short, K.R.; Diavatopoulos, D.A.; Wijburg, O.L.; Cao, H.; Waithman, J.C.; Chen, W.; et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8(+) T cells. Nat. Immunol. 2012, 13, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Puren, A.J.; Fantuzzi, G.; Dinarello, C.A. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. USA 1999, 96, 2256–2261. [Google Scholar] [CrossRef]
- Robinson, N.; Ganesan, R.; Hegedus, C.; Kovacs, K.; Kufer, T.A.; Virag, L. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019, 26, 101239. [Google Scholar] [CrossRef]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, B. Neutrophil pyroptosis: New perspectives on sepsis. Cell. Mol. Life Sci. 2019, 76, 2031–2042. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, R.; Tan, H. Role of Pyroptosis in Cardiovascular Diseases and its Therapeutic Implications. Int. J. Biol. Sci. 2019, 15, 1345–1357. [Google Scholar] [CrossRef]
- Van Gorp, H.; Van Opdenbosch, N.; Lamkanfi, M. Inflammasome-Dependent Cytokines at the Crossroads of Health and Autoinflammatory Disease. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Russo, V.; Alikhan, A. Failure of Anakinra in a Case of Severe Hidradenitis Suppurativa. J. Drugs Dermatol. 2016, 15, 772–774. [Google Scholar] [PubMed]
- Buch, M.H.; Bingham, S.J.; Seto, Y.; McGonagle, D.; Bejarano, V.; White, J.; Emery, P. Lack of response to anakinra in rheumatoid arthritis following failure of tumor necrosis factor alpha blockade. Arthritis Rheum. 2004, 50, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, C.; Ridene, M.; Lequerre, T.; Costedoat Chalumeau, N.; Amoura, Z.; Sellam, J.; Sibilia, J.; Bourgeois, P.; Fautrel, B.; CRI. Anakinra in adult-onset Still’s disease: Long-term treatment in patients resistant to conventional therapy. Arthritis Care Res. 2013, 65, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xue, Y.; Zhu, Y.; Xuan, D.; Yang, X.; Liang, M.; Wang, J.; Zhu, X.; Zhang, J.; Zou, H. Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout. Arthritis Res. Ther. 2016, 18, 268. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudloff, I.; Ung, H.K.; Dowling, J.K.; Mansell, A.; D’Andrea, L.; Ellisdon, A.M.; Whisstock, J.C.; Berger, P.J.; Nold-Petry, C.A.; Nold, M.F. Parsing the IL-37-Mediated Suppression of Inflammasome Function. Cells 2020, 9, 178. https://doi.org/10.3390/cells9010178
Rudloff I, Ung HK, Dowling JK, Mansell A, D’Andrea L, Ellisdon AM, Whisstock JC, Berger PJ, Nold-Petry CA, Nold MF. Parsing the IL-37-Mediated Suppression of Inflammasome Function. Cells. 2020; 9(1):178. https://doi.org/10.3390/cells9010178
Chicago/Turabian StyleRudloff, Ina, Holly K. Ung, Jennifer K. Dowling, Ashley Mansell, Laura D’Andrea, Andrew M. Ellisdon, James C. Whisstock, Philip J. Berger, Claudia A. Nold-Petry, and Marcel F. Nold. 2020. "Parsing the IL-37-Mediated Suppression of Inflammasome Function" Cells 9, no. 1: 178. https://doi.org/10.3390/cells9010178
APA StyleRudloff, I., Ung, H. K., Dowling, J. K., Mansell, A., D’Andrea, L., Ellisdon, A. M., Whisstock, J. C., Berger, P. J., Nold-Petry, C. A., & Nold, M. F. (2020). Parsing the IL-37-Mediated Suppression of Inflammasome Function. Cells, 9(1), 178. https://doi.org/10.3390/cells9010178






