Shockwaves Suppress Adipocyte Differentiation via Decrease in PPARγ
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Differentiation (3T3L-1 Mouse Preadipocytes and Primary Human Subcutaneous Preadipocytes)
2.3. Shockwave Treatment
2.4. Annexin V Staining
2.5. Adenosine Triphosphate (ATP) Measurement
2.6. cAMP Measurement
2.7. Oil-Red-O Staining
2.8. MTT Assay
2.9. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.10. Immunoblot Analyses
2.11. Statistical Analysis
3. Results
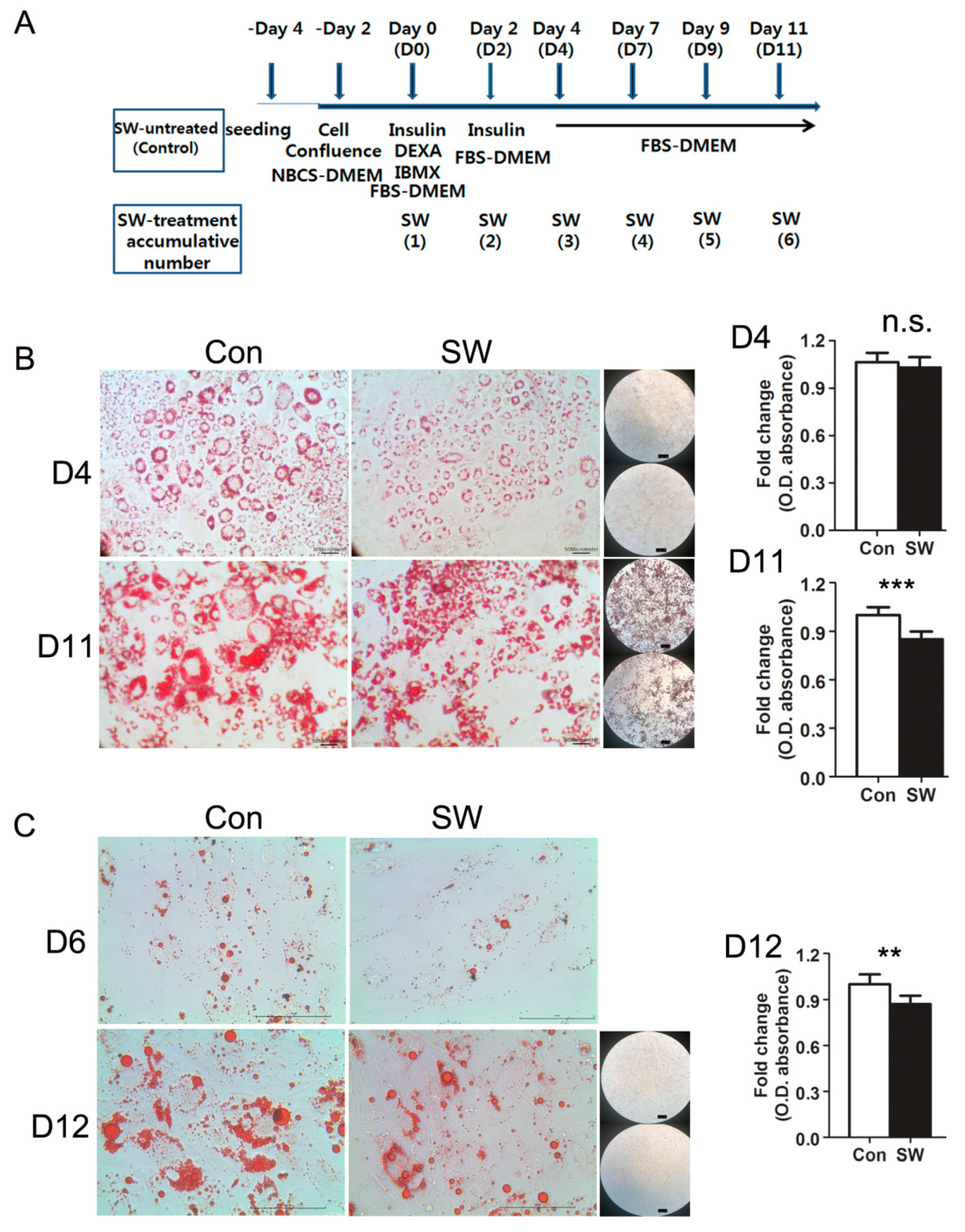
3.1. Shockwaves Suppressed Adipocyte Differentiation of Murine 3T3L-1 and Human Primary Preadipocytes
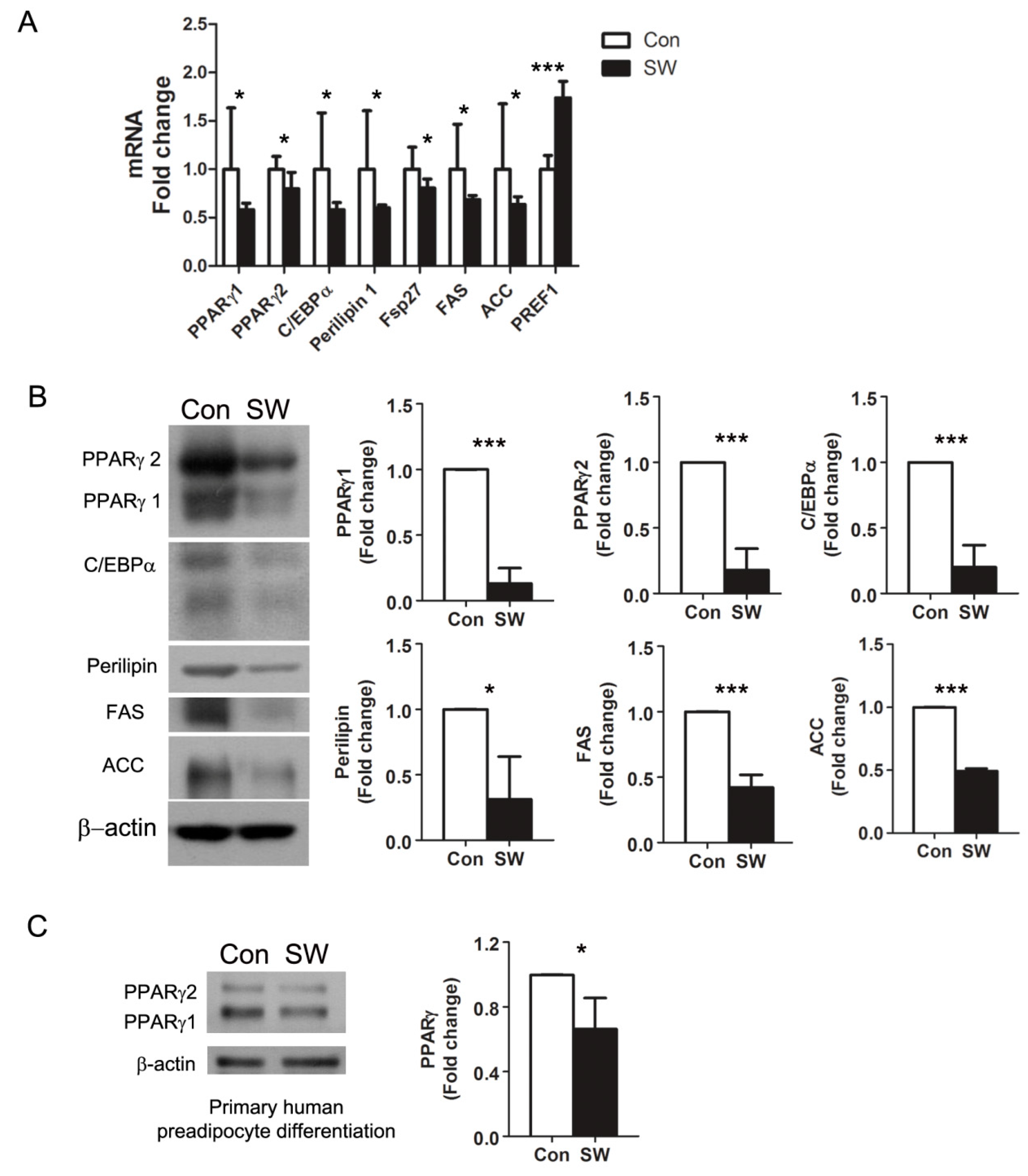
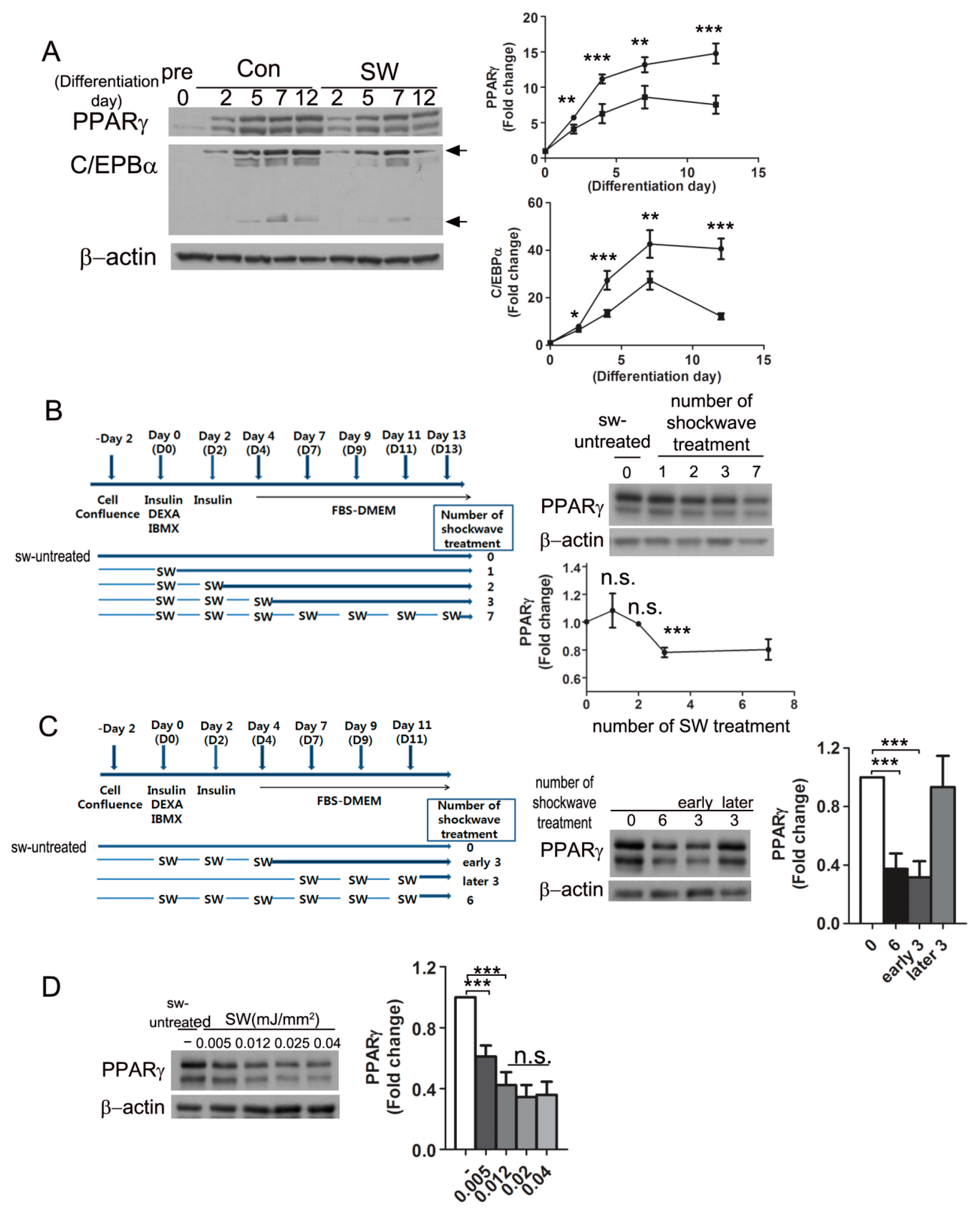
3.2. Shockwaves Decreased PPARγ, A Key Adipogenic Transcriptional Factor, in 3T3-L1 and Human Preadipocytes
3.3. Shockwave Treatment at the Initial Stage of Adipocyte Differentiation Suppressed PPARγ Expression
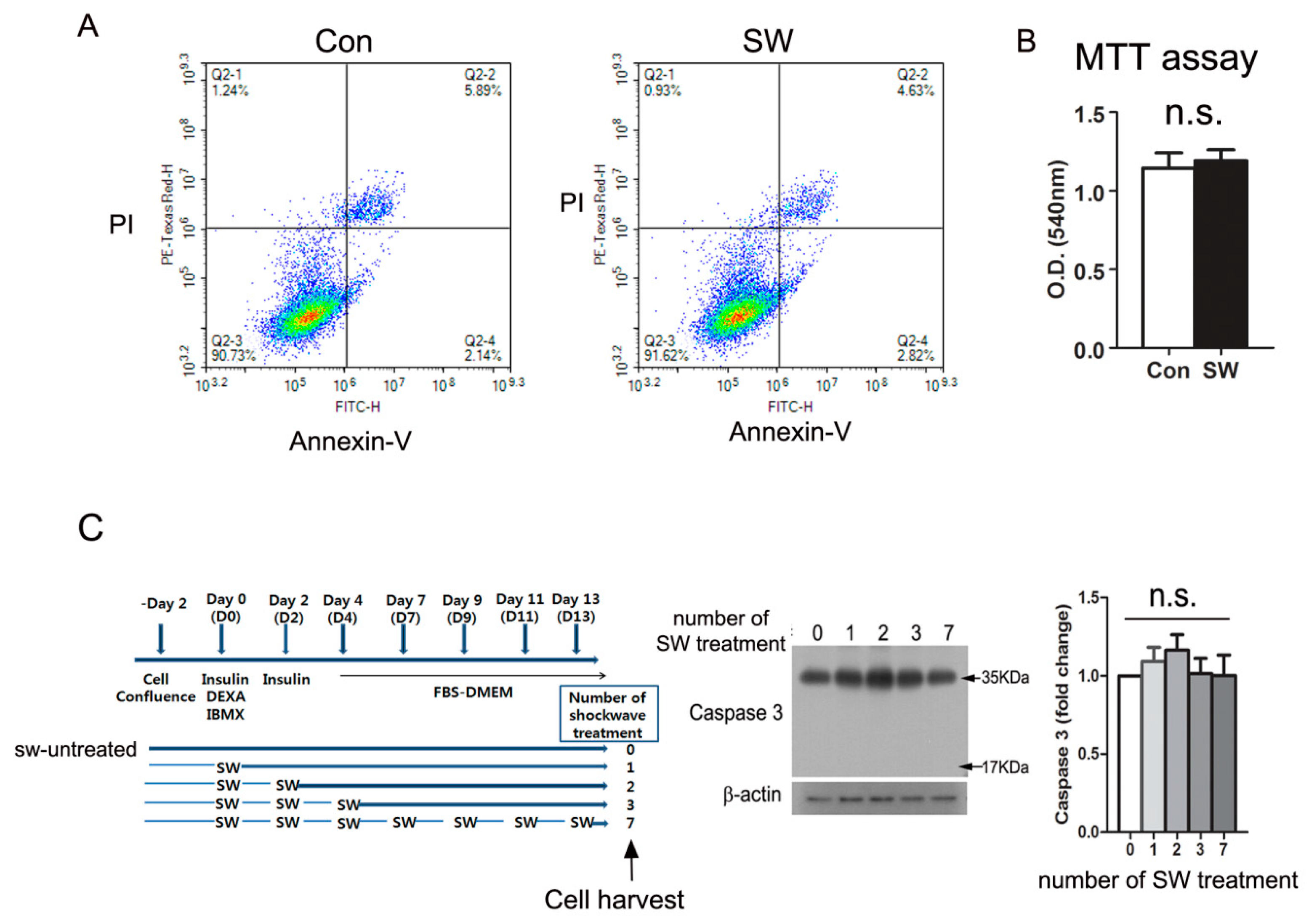
3.4. Shockwave Treatment Did Not Affect Cell Viability
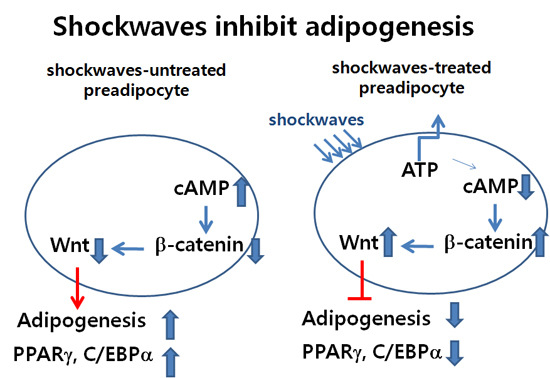
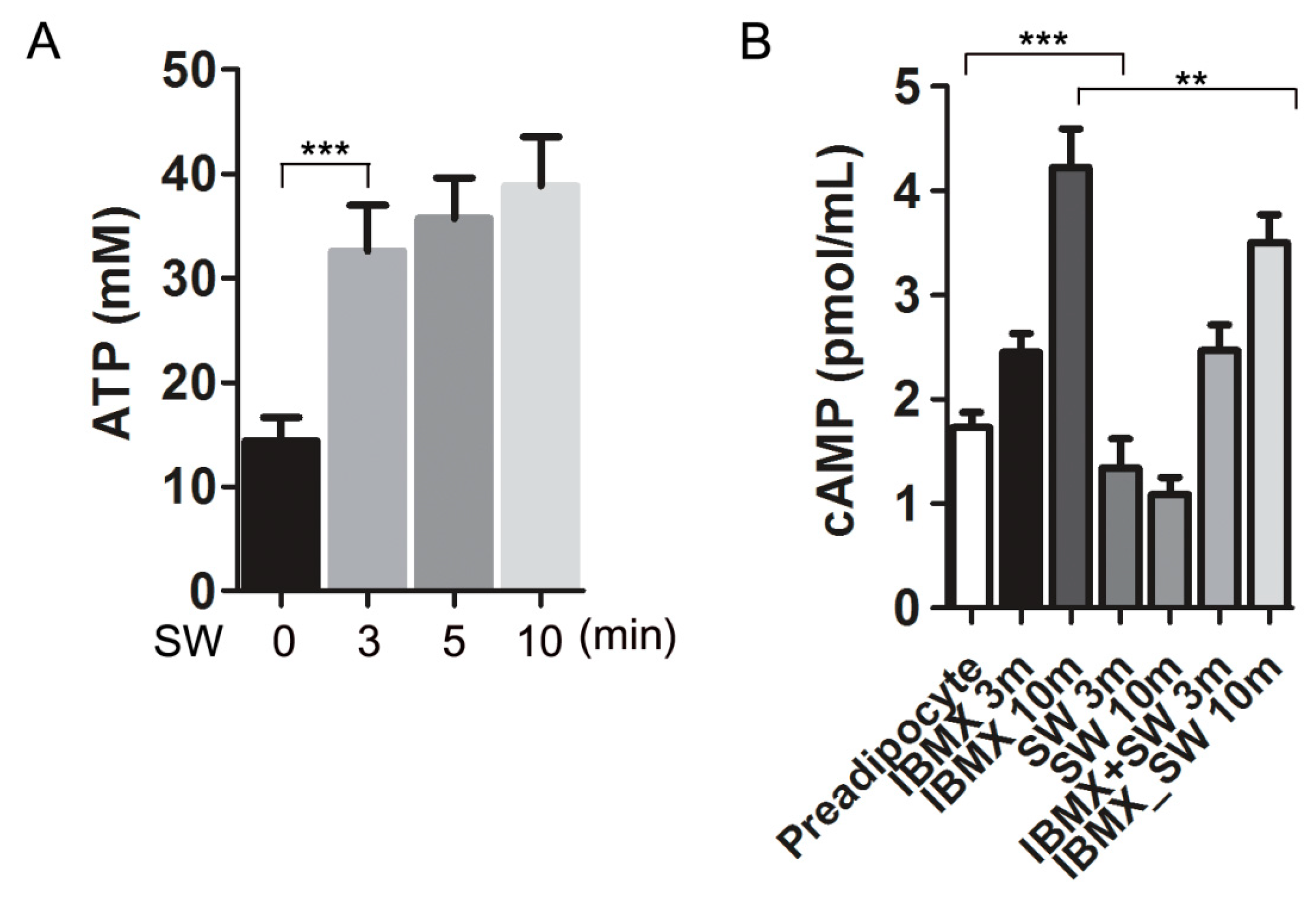
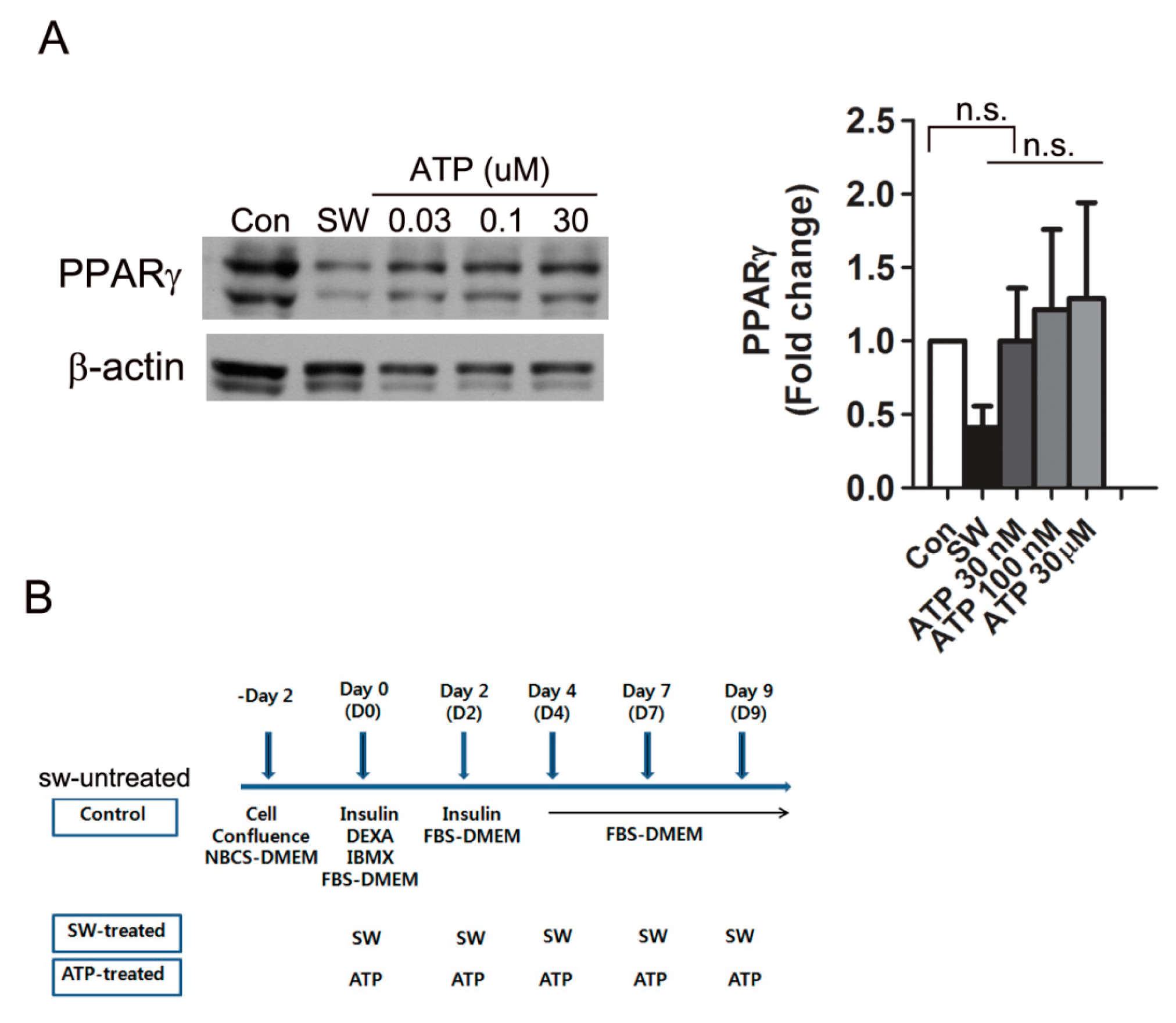
3.5. Shockwaves Promoted Extracellular ATP Release from Preadipocytes and Decreased Intracellular cAMP in 3T3L-1 Cells
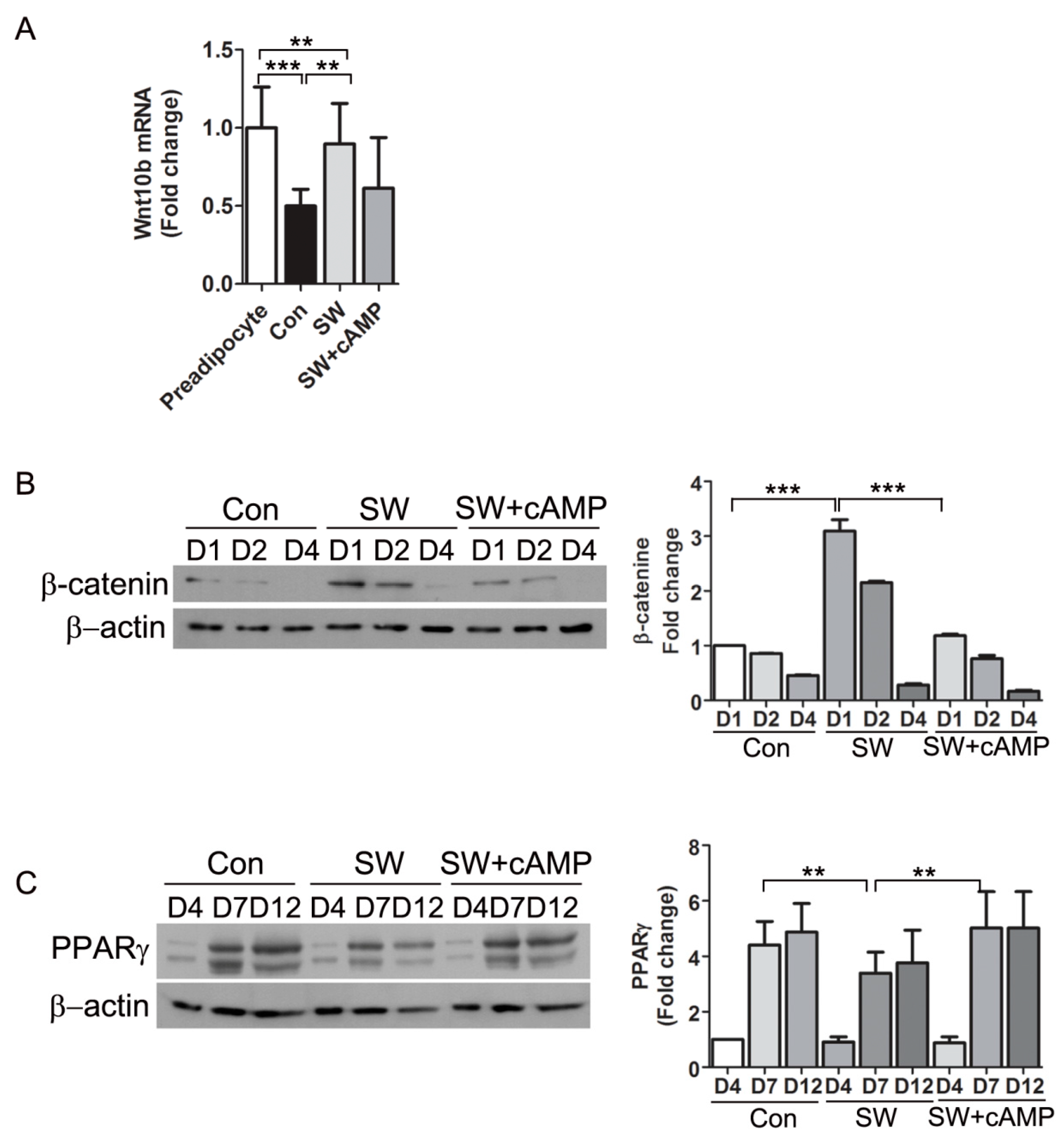
3.6. Shockwaves Activated Wnt Signaling through β-Catenin by Decreasing Intracellular cAMP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The developmental origins of adipose tissue. Development 2013, 140, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Sarraf, P.; Troy, A.E.; Bradwin, G.; Moore, K.; Milstone, D.S.; Spiegelman, B.M.; Mortensen, R.M. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 1999, 4, 611–617. [Google Scholar] [CrossRef]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.T.; Lane, M.D. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc. Natl. Acad. Sci. USA 1994, 91, 8757–8761. [Google Scholar] [CrossRef] [PubMed]
- Chapko, K.M.; Weaver, M.R.; Chapko, M.K.; Pasta, D.; Adamson, G.D. Stability of in vitro fertilization-embryo transfer success rates from the 1989, 1990 and 1991 Clinic-Specific Outcome Assessments. Fertil. Steril. 1995, 64, 757–763. [Google Scholar] [CrossRef]
- Elberg, G.; Gimble, J.M.; Tsai, S.Y. Modulation of the murine peroxisome proliferator-activated receptor gamma 2 promoter activity by CCAAT/enhancer-binding proteins. J. Biol. Chem. 2000, 275, 27815–27822. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef]
- Bhanot, P.; Brink, M.; Samos, C.H.; Hsieh, J.C.; Wang, Y.; Macke, J.P.; Andrew, D.; Nathans, J.; Nusse, R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996, 382, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bejsovec, A. Wnt signaling: An embarrassment of receptors. Curr. Biol. 2000, 10, R919–R922. [Google Scholar] [CrossRef]
- Yost, C.; Torres, M.; Miller, J.R.; Huang, E.; Kimelman, D.; Moon, R.T. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996, 10, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.T.; Strack, P.; Beer-Romero, P.; Chu, C.Y.; Elledge, S.J.; Harper, J.W. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999, 13, 270–283. [Google Scholar] [CrossRef]
- Rubinfeld, B.; Robbins, P.; El-Gamil, M.; Albert, I.; Porfiri, E.; Polakis, P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 1997, 275, 1790–1792. [Google Scholar] [CrossRef]
- Frairia, R.; Berta, L. Biological effects of extracorporeal shock waves on fibroblasts: A review. Muscles Ligaments Tendons J. 2011, 1, 138–147. [Google Scholar]
- Notarnicola, A.; Moretti, B. The biological effects of extracorporeal shock wave therapy (eswt) on tendon tissue. Muscles Ligaments Tendons J. 2012, 2, 33–37. [Google Scholar]
- Seil, R.; Wilmes, R.; Nuhrenborger, C. Extracorporeal shock wave therapy for tendinopathies. Expert Rev. Med. Devices 2006, 3, 463–470. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, C.J.; Yang, K.D.; Kuo, Y.R.; Huang, H.C.; Huang, Y.T.; Sun, Y.C.; Wang, F.S. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-beta1 and IGF-I expression. J. Orthop. Res. 2004, 22, 854–861. [Google Scholar] [CrossRef]
- Chao, Y.H.; Tsuang, Y.H.; Sun, J.S.; Chen, L.T.; Chiang, Y.F.; Wang, C.C.; Chen, M.H. Effects of shock waves on tenocyte proliferation and extracellular matrix metabolism. Ultrasound Med. Biol. 2008, 34, 841–852. [Google Scholar] [CrossRef]
- Sun, D.; Junger, W.G.; Yuan, C.; Zhang, W.; Bao, Y.; Qin, D.; Wang, C.; Tan, L.; Qi, B.; Zhu, D.; et al. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells 2013, 31, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhao, B.; Ge, F.T.; Sun, D.H.; Yu, T. Shockwaves Inhibit Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Association with Adenosine and A2B Receptors. Sci. Rep. 2017, 7, 14377. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.R.; Hirsch, J. Cellularity of adipose depots in six strains of genetically obese mice. J. Lipid Res. 1972, 13, 2–11. [Google Scholar]
- Cleary, M.P.; Brasel, J.A.; Greenwood, M.R. Developmental changes in thymidine kinase, DNA, and fat cellularity in Zucker rats. Am. J. Physiol. 1979, 236, E508–E513. [Google Scholar] [CrossRef]
- Hauner, H.; Entenmann, G.; Wabitsch, M.; Gaillard, D.; Ailhaud, G.; Negrel, R.; Pfeiffer, E.F. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J. Clin. Investig. 1989, 84, 1663–1670. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Yu, T.; Junger, W.G.; Yuan, C.; Jin, A.; Zhao, Y.; Zheng, X.; Zeng, Y.; Liu, J. Shockwaves increase T-cell proliferation and IL-2 expression through ATP release, P2X7 receptors, and FAK activation. Am. J. Physiol. Cell Physiol. 2010, 298, C457–C464. [Google Scholar] [CrossRef]
- Kim, S.P.; Ha, J.M.; Yun, S.J.; Kim, E.K.; Chung, S.W.; Hong, K.W.; Kim, C.D.; Bae, S.S. Transcriptional activation of peroxisome proliferator-activated receptor-gamma requires activation of both protein kinase A and Akt during adipocyte differentiation. Biochem. Biophys. Res. Commun. 2010, 399, 55–59. [Google Scholar] [CrossRef]
- Burnstock, G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 1997, 36, 1127–1139. [Google Scholar] [CrossRef]
- Omatsu-Kanbe, M.; Inoue, K.; Fujii, Y.; Yamamoto, T.; Isono, T.; Fujita, N.; Matsuura, H. Effect of ATP on preadipocyte migration and adipocyte differentiation by activating P2Y receptors in 3T3-L1 cells. Biochem. J. 2006, 393, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Chaussy, C.; Brendel, W.; Schmiedt, E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet 1980, 2, 1265–1268. [Google Scholar] [CrossRef]
- Sauerbruch, T.; Delius, M.; Paumgartner, G.; Holl, J.; Wess, O.; Weber, W.; Hepp, W.; Brendel, W. Fragmentation of gallstones by extracorporeal shock waves. N. Engl. J. Med. 1986, 314, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.E.; Crawford, F.; Murray, G.D. The effectiveness of extra corporeal shock wave therapy for plantar heel pain: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2005, 6, 19. [Google Scholar] [CrossRef]
- Hayashi, D.; Kawakami, K.; Ito, K.; Ishii, K.; Tanno, H.; Imai, Y.; Kanno, E.; Maruyama, R.; Shimokawa, H.; Tachi, M. Low-energy extracorporeal shock wave therapy enhances skin wound healing in diabetic mice: A critical role of endothelial nitric oxide synthase. Wound Repair Regen. 2012, 20, 887–895. [Google Scholar] [CrossRef]
- Sen, B.; Xie, Z.; Case, N.; Ma, M.; Rubin, C.; Rubin, J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 2008, 149, 6065–6075. [Google Scholar] [CrossRef]
- Robinson, J.A.; Chatterjee-Kishore, M.; Yaworsky, P.J.; Cullen, D.M.; Zhao, W.; Li, C.; Kharode, Y.; Sauter, L.; Babij, P.; Brown, E.L.; et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 2006, 281, 31720–31728. [Google Scholar] [CrossRef]
- Lau, K.H.; Kapur, S.; Kesavan, C.; Baylink, D.J. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J. Biol. Chem. 2006, 281, 9576–9588. [Google Scholar] [CrossRef]
- Rubin, C.T.; Capilla, E.; Luu, Y.K.; Busa, B.; Crawford, H.; Nolan, D.J.; Mittal, V.; Rosen, C.J.; Pessin, J.E.; Judex, S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl. Acad. Sci. USA 2007, 104, 17879–17884. [Google Scholar] [CrossRef]
- Hu, J.; Liao, H.; Ma, Z.; Chen, H.; Huang, Z.; Zhang, Y.; Yu, M.; Chen, Y.; Xu, J. Focal Adhesion Kinase Signaling Mediated the Enhancement of Osteogenesis of Human Mesenchymal Stem Cells Induced by Extracorporeal Shockwave. Sci. Rep. 2016, 6, 20875. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug. Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schon, M.R.; Abumrad, N.A.; Bluher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Takahashi, S.; Matsubara, T.; Jiang, C.; Sakamoto, W.; Chanturiya, T.; Teng, R.; Gavrilova, O.; Gonzalez, F.J. Adipocyte-specific disruption of fat-specific protein 27 causes hepatosteatosis and insulin resistance in high-fat diet-fed mice. J. Biol. Chem. 2015, 290, 3092–3105. [Google Scholar] [CrossRef]
- Stephens, J.M. The fat controller: Adipocyte development. PLoS Biol. 2012, 10, e1001436. [Google Scholar] [CrossRef]
- Scherer, P.E. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef]
- Hirsch, J.; Han, P.W. Cellularity of rat adipose tissue: Effects of growth, starvation, and obesity. J. Lipid Res. 1969, 10, 77–82. [Google Scholar]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Lemonnier, D. Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J. Clin. Investig. 1972, 51, 2907–2915. [Google Scholar] [CrossRef]
- Faust, I.M.; Johnson, P.R.; Stern, J.S.; Hirsch, J. Diet-induced adipocyte number increase in adult rats: A new model of obesity. Am. J. Physiol. 1978, 235, E279–E286. [Google Scholar] [CrossRef]
- Tchoukalova, Y.D.; Votruba, S.B.; Tchkonia, T.; Giorgadze, N.; Kirkland, J.L.; Jensen, M.D. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl. Acad. Sci. USA 2010, 107, 18226–18231. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, I. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schon, M.R.; Kern, M.; Stumvoll, M.; Bluher, M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E506–E515. [Google Scholar] [CrossRef]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [PubMed]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Koutnikova, H.; Cock, T.A.; Watanabe, M.; Houten, S.M.; Champy, M.F.; Dierich, A.; Auwerx, J. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc. Natl. Acad. Sci. USA 2003, 100, 14457–14462. [Google Scholar] [CrossRef]
- Wang, N.D.; Finegold, M.J.; Bradley, A.; Ou, C.N.; Abdelsayed, S.V.; Wilde, M.D.; Taylor, L.R.; Wilson, D.R.; Darlington, G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef]
- Tzameli, I.; Fang, H.; Ollero, M.; Shi, H.; Hamm, J.K.; Kievit, P.; Hollenberg, A.N.; Flier, J.S. Regulated production of a peroxisome proliferator-activated receptor-gamma ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J. Biol. Chem. 2004, 279, 36093–36102. [Google Scholar] [CrossRef]
- Hansen, J.B.; Zhang, H.; Rasmussen, T.H.; Petersen, R.K.; Flindt, E.N.; Kristiansen, K. Peroxisome proliferator-activated receptor delta PPARδ-mediated regulation of preadipocyte proliferation and gene expression is dependent on cAMP signaling. J. Biol. Chem. 2001, 276, 3175–3182. [Google Scholar] [CrossRef]
- Christy, R.J.; Kaestner, K.H.; Geiman, D.E.; Lane, M.D. CCAAT/enhancer binding protein gene promoter: Binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Klemm, D.J.; Vinson, C.; Lane, M.D. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J. Biol. Chem. 2004, 279, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Regulation of PPARgamma activity during adipogenesis. Int. J. Obes. 2005, 29, S13–S16. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Jiang, M.S.; Lane, M.D. Repressive effect of Sp1 on the C/EBPalpha gene promoter: Role in adipocyte differentiation. Mol. Cell Biol. 1999, 19, 4855–4865. [Google Scholar] [CrossRef]
- Choi, O.M.; Cho, Y.H.; Choi, S.; Lee, S.H.; Seo, S.H.; Kim, H.Y.; Han, G.; Min, D.S.; Park, T.; Choi, K.Y. The small molecule indirubin-3’-oxime activates Wnt/beta-catenin signaling and inhibits adipocyte differentiation and obesity. Int. J. Obes. (Lond.) 2014, 38, 1044–1052. [Google Scholar] [CrossRef]
- Shiomi, Y.; Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Nakayama, R.; Orikawa, Y.; Yoshioka, Y.; Tanaka, K.; Ueki, K.; Kadowaki, T. A Novel Peroxisome Proliferator-activated Receptor (PPAR) α Agonist and PPARγ Antagonist, Z-551, Ameliorates High-fat Diet-induced Obesity and Metabolic Disorders in Mice. J. Biol. Chem. 2015, 290, 14567–14581. [Google Scholar] [CrossRef]
- Duan, S.Z.; Ivashchenko, C.Y.; Whitesall, S.E.; D’Alecy, L.G.; Duquaine, D.C.; Brosius, F.C.; Gonzalez, F.J.; Vinson, C.; Pierre, M.A.; Milstone, D.S.; et al. Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J. Clin. Investig. 2007, 117, 812–822. [Google Scholar] [CrossRef]
| Forward | Reverse | |
|---|---|---|
| PPARγ1 | CAA GAA TAC CAA AGT GCG ATC AA | GAG CTG GGT CTT TTC AGA ATA ATA AG |
| PPARγ2 | CCA GAG CAT GGT GCC TTC GCT | CAG CAA CCA TTG GGT CAG CTC |
| C/EBPα | AGC, AAC GAG TAC CGG GTA CG | TGT TTG GCT TTA TCT CGG CTC |
| perilipin | TGC TGG ATG GAG ACC TC | ACC GGC TCC ATG CTC CA |
| Fsp27 | CTG GAG GAA GAT GGC ACA ATC GTG | CAG CCA ATA AAG TCC TGA GGG TTC A |
| FAS | GCT GCT GTT GGA AGT CAG C | AGT GTT CGT TCC TCG GAG TG |
| PREF1 | CGG GAA ATT CTG CGA AAT AG | TGT GCA GGA GCA TTC GAT CT |
| ACC | GGA CAG ACT GAT CGC AGA GAA AG | TGG AGA GCC CCA CAC AC |
| Wnt10b | ACGACATGGACTTCGGAGAGAAGT | CATTCTCGCCTGGATGTCCC |
| GAPDH | AAC TTT GGC ATT GTG GAA GG | GGA TGC AGG GAT GAT GTT CT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, W.; Kim, S.; Jeong, M.; Park, Y.M. Shockwaves Suppress Adipocyte Differentiation via Decrease in PPARγ. Cells 2020, 9, 166. https://doi.org/10.3390/cells9010166
Cho W, Kim S, Jeong M, Park YM. Shockwaves Suppress Adipocyte Differentiation via Decrease in PPARγ. Cells. 2020; 9(1):166. https://doi.org/10.3390/cells9010166
Chicago/Turabian StyleCho, Wonkyoung, SeoYeon Kim, Myeongsook Jeong, and Young Mi Park. 2020. "Shockwaves Suppress Adipocyte Differentiation via Decrease in PPARγ" Cells 9, no. 1: 166. https://doi.org/10.3390/cells9010166
APA StyleCho, W., Kim, S., Jeong, M., & Park, Y. M. (2020). Shockwaves Suppress Adipocyte Differentiation via Decrease in PPARγ. Cells, 9(1), 166. https://doi.org/10.3390/cells9010166