Hypoxic Adaptation of Mitochondrial Metabolism in Rat Cerebellum Decreases in Pregnancy
Abstract
1. Introduction
2. Materials and Method
2.1. Animal Experiments
2.2. Acute Hypobaric Hypoxia
2.3. Enzyme Assays
2.4. Ninhydrine Quantification of Amino Acids
2.5. Data Acquisition and Statistics
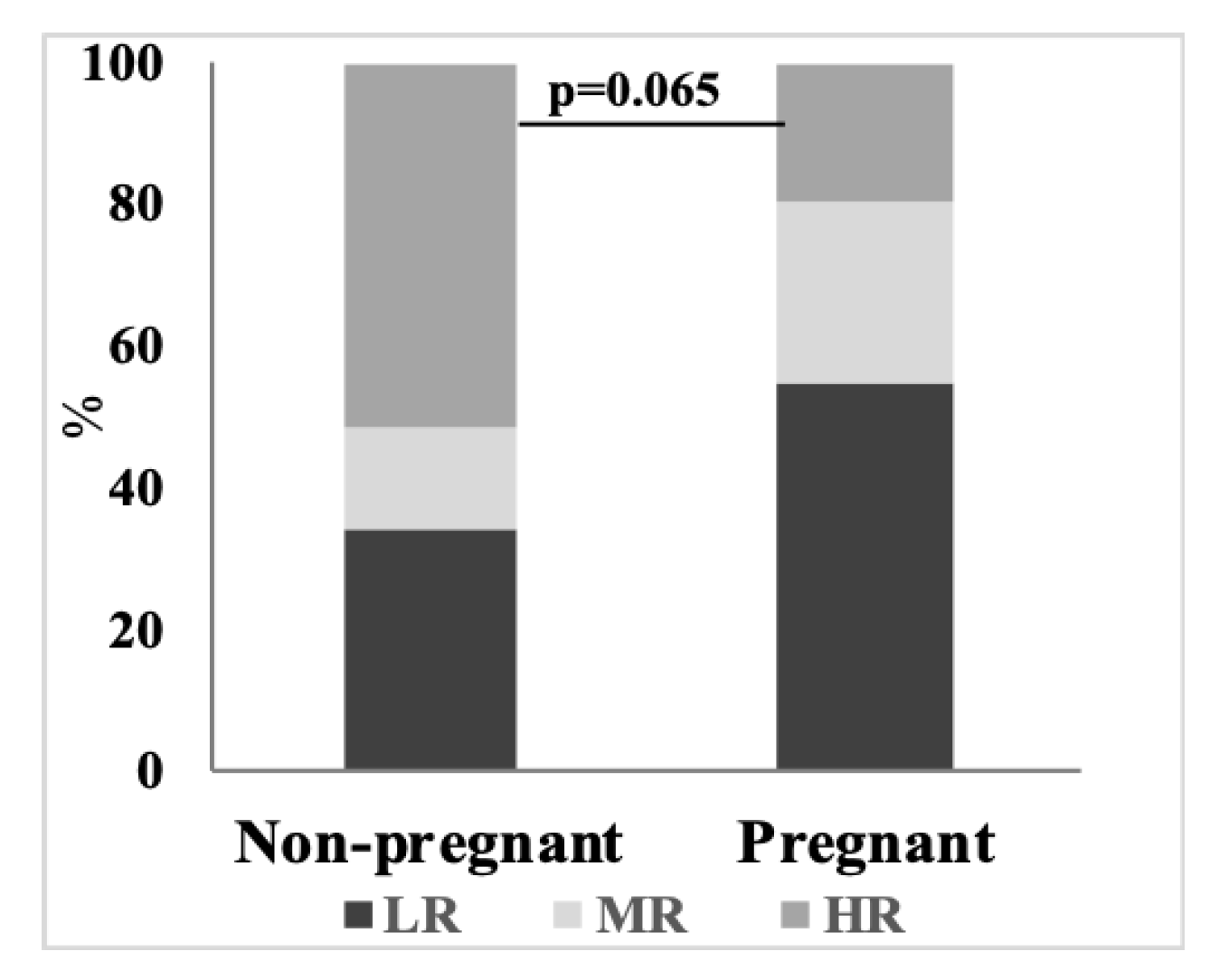
3. Results
3.1. Pregnancy-Induced Changes in Cerebellar Levels of Amino Acids and OGDHC Activity
3.2. Hypoxia-Induced Changes in Cerebellar Pool of Amino Acids Depend on Physiological State
3.3. Interdependence of Cerebellar Levels of OGDHC Activity and/or Amino Acids Varies under Different Physiological Settings
3.4. Concerted Hypoxia-Induced Shift to the Negative Correlations between the Levels of Amino Acids and OGDHC Activity Is not Observed in Pregnancy
3.5. Physiological Consequences of the Hypoxia-Induced Changes in the Interdependent Levels of OGDHC Activity and/or Amino Acids in Cerebellum
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kondoh, T.; Kameishi, M.; Mallick, H.N.; Ono, T.; Torii, K. Lysine and Arginine Reduce the Effects of Cerebral Ischemic Insults and Inhibit Glutamate-Induced Neuronal Activity in Rats. Front. Integr. Neurosci. 2010, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khandare, A.L.; Ankulu, M.; Aparna, N. Role of Glutamate and Nitric Oxide in Onset of Motor Neuron Degeneration in Neurolathyrism. Neurotoxicology 2013, 34, 269–274. [Google Scholar] [CrossRef] [PubMed]
- März, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Böhm, B.O.; Ritz, E.; et al. Homoarginine, Cardiovascular Risk, and Mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bunik, V.I.; Fernie, A.R. Metabolic Control Exerted by the 2-Oxoglutarate Dehydrogenase Reaction: A Cross-Kingdom Comparison of the Crossroad between Energy Production and Nitrogen Assimilation. Biochem. J. 2009, 422, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Bunik, V.I.; Raddatz, G.; Strumilo, S. Translating Enzymology into Metabolic Regulation: The Case of the 2-Oxoglutarate Dehydrogenase Multienzyme Complex. Curr. Chem. Biol. 2013, 7, 74–93. [Google Scholar] [CrossRef]
- Bunik, V.I.; Tylicki, A.; Lukashev, N.V. Thiamin Diphosphate-Dependent Enzymes: From Enzymology to Metabolic Regulation, Drug Design and Disease Models. FEBS J. 2013, 280, 6412–6442. [Google Scholar] [CrossRef]
- Mkrtchyan, G.V.; Graf, A.; Trofimova, L.; Ksenofontov, A.; Baratova, L.; Bunik, V. Positive Correlation between Rat Brain Glutamate Concentrations and Mitochondrial 2-Oxoglutarate Dehydrogenase Activity. Anal. Biochem. 2018, 552, 100–109. [Google Scholar] [CrossRef]
- Araújo, W.L.; Trofimova, L.; Mkrtchyan, G.; Steinhauser, D.; Krall, L.; Graf, A.; Fernie, A.R.; Bunik, V.I. On the Role of the Mitochondrial 2-Oxoglutarate Dehydrogenase Complex in Amino Acid Metabolism. Amino Acids 2013, 44, 683–700. [Google Scholar] [CrossRef]
- Trofimova, L.; Araujo, W.; Strokina, A.; Fernie, A.; Bettendorff, L.; Bunik, V. Consequences of the α-Ketoglutarate Dehydrogenase Inhibition for Neuronal Metabolism and Survival: Implications for Neurodegenerative Diseases. Curr. Med. Chem. 2012, 19, 5895–5906. [Google Scholar] [CrossRef]
- Santos, S.S.; Gibson, G.E.; Cooper, A.J.L.; Denton, T.T.; Thompson, C.M.; Bunik, V.I.; Alves, P.M.; Sonnewald, U. Inhibitors of the α-Ketoglutarate Dehydrogenase Complex Alter [1-13C] Glucose and [U-13C] Glutamate Metabolism in Cerebellar Granule Neurons. J. Neurosci. Res. 2006, 83, 450–458. [Google Scholar] [CrossRef]
- Stavrum, A.-K.; Heiland, I.; Schuster, S.; Puntervoll, P.; Ziegler, M. Model of Tryptophan Metabolism, Readily Scalable Using Tissue-Specific Gene Expression Data. J. Biol. Chem. 2013, 288, 34555–34566. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Trofimova, L.; Loshinskaja, A.; Mkrtchyan, G.; Strokina, A.; Lovat, M.; Tylicky, A.; Strumilo, S.; Bettendorff, L.; Bunik, V.I. Up-Regulation of 2-Oxoglutarate Dehydrogenase as a Stress Response. Int. J. Biochem. Cell Biol. 2013, 45, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, P.; Laitinen, T.; Lyyra-Laitinen, T.; Raitakari, O.T.; Juonala, M.; Viikari, J.S.A.; Heiskanen, N.; Vanninen, E.; Punnonen, K.; Heinonen, S. Serum L-Homoarginine Concentration Is Elevated during Normal Pregnancy and Is Related to Flow-Mediated Vasodilatation. Circ. J. 2008, 72, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, L.; Lovat, M.; Groznaya, A.; Efimova, E.; Dunaeva, T.; Maslova, M.; Graf, A.; Bunik, V. Behavioral Impact of the Regulation of the Brain 2-Oxoglutarate Dehydrogenase Complex by Synthetic Phosphonate Analog of 2-Oxoglutarate: Implications into the Role of the Complex in Neurodegenerative Diseases. Int. J. Alzheimer’s Dis. 2010, 3, 1–8. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M. Reply: The Cerebellum in Parkinson’s Disease and Parkinsonism in Cerebellar Disorders. Brain 2013, 136, e249. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Hornberger, M.; Balsters, J.H.; Halliday, G.M.; Lewis, S.J.G.; Shine, J.M. Cerebellar Atrophy in Parkinson’s Disease and Its Implication for Network Connectivity. Brain 2016, 139, 845–855. [Google Scholar] [CrossRef]
- Mirdamadi, J.L. Cerebellar Role in Parkinson’s Disease. J. Neurophysiol. 2016, 116, 917–919. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Mourad, I.M.; Mohammed, H.S.; Noor, N.A.; Aboul, H.S. Cerebellar Neurochemical and Histopathological Changes in Rat Model of Parkinson’s Disease Induced by Intrastriatal Injection of Rotenone. Gen. Physiol. Biophys. 2017, 36, 99–108. [Google Scholar] [CrossRef]
- Kishore, A.; Meunier, S.; Popa, T. Cerebellar Influence on Motor Cortex Plasticity: Behavioral Implications for Parkinson’s Disease. Front. Neurol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Roessner, U.; Luedemann, A.; Brust, D.; Fiehn, O.; Linke, T.; Willmitzer, L.; Fernie, A.R. Metabolic Profiling Allows Comprehensive Phenotyping of Genetically or Environmentally Modified Plant Systems. Plant Cell 2001, 13, 11–29. [Google Scholar] [CrossRef]
- Szymanski, J.; Jozefczuk, S.; Nikoloski, Z.; Selbig, J.; Nikiforova, V.; Catchpole, G.; Willmitzer, L. Stability of Metabolic Correlations under Changing Environmental Conditions in Escherichia Coli—A Systems Approach. PLoS ONE 2009, 4, e7441. [Google Scholar] [CrossRef] [PubMed]
- Endrőczi, E. Neuropeptides and Psychosomatic Processes; Taylor & Francis: London, UK, 1983. [Google Scholar]
- Tsepkova, P.M.; Artiukhov, A.V.; Boyko, A.I.; Aleshin, V.A.; Mkrtchyan, G.V.; Zvyagintseva, M.A.; Ryabov, S.I.; Ksenofontov, A.L.; Baratova, L.A.; Graf, A.V.; et al. Thiamine Induces Long-Term Changes in Amino Acid Profiles and Activities of 2-Oxoglutarate and 2-Oxoadipate Dehydrogenases in Rat Brain. Biochemistry (Moscow) 2017, 82, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, H. Effects of Cytotoxic Agents on the Fetus. JAMA 1960, 173, 1616. [Google Scholar] [CrossRef]
- Maslova, M.V.; Graf, A.V.; Maklakova, A.S.; Krushinskaya, Y.V.; Sokolova, N.A.; Koshelev, V.B. Acute Hypoxia during Organogenesis Affects Cardiac Autonomic Balance in Pregnant Rats. Bull. Exp. Biol. Med. 2005, 139, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Lukyanova, L.D.; Germanova, E.L.; Kopaladze, R.A. Development of Resistance of an Organism under Various Conditions of Hypoxic Preconditioning: Role of the Hypoxic Period and Reoxygenation. Bull. Exp. Biol. Med. 2009, 147, 400. [Google Scholar] [CrossRef]
- Malyshev, A.Y.; Luk’yanova, L.D.; Krapivin, S.V. Effect of Hypoxia of Increasing Severity on the Dynamics of Cerebral Cortex EEGs in Rats with Different Resistance to Acute Oxygen Deficiency. Bull. Exp. Biol. Med. 1996, 122, 879–884. [Google Scholar] [CrossRef]
- Bondarenko, N.A.; Germanova, E.L.; Luk’yanova, L.D. Behavioral Peculiarities and Reactions of Brain Dopaminergic Systems in Rats with Different Resistance to Acute Hypobaric Hypoxia. Bull. Exp. Biol. Med. 2000, 130, 849–851. [Google Scholar] [CrossRef]
- Luk’yanova, L.D.; Romanova, V.E.; Chernobaeva, G.N. Oxidative Phosphorylation in Brain Mitochondria of Rats Differing in Their Sensitivity to Hypoxia. Bull. Exp. Biol. Med. 1991, 112, 962–965. [Google Scholar] [CrossRef]
- Graf, A.; Kabysheva, M.; Klimuk, E.; Trofimova, L.; Dunaeva, T.; Zündorf, G.; Kahlert, S.; Reiser, G.; Storozhevykh, T.; Pinelis, V.; et al. Role of 2-Oxoglutarate Dehydrogenase in Brain Pathologies Involving Glutamate Neurotoxicity. J. Mol. Catal. B Enzym. 2009, 61, 80–87. [Google Scholar] [CrossRef]
- Ksenofontov, A.L.; Boyko, A.I.; Mkrtchyan, G.V.; Tashlitsky, V.N.; Timofeeva, A.V.; Graf, A.V.; Bunik, V.I.; Baratova, L.A. Analysis of Free Amino Acids in Mammalian Brain Extracts. Biochemistry (Moscow) 2017, 82, 1183–1192. [Google Scholar] [CrossRef]
- Smiley, K.O.; Ladyman, S.R.; Gustafson, P.; Grattan, D.R.; Brown, R.S.E. Neuroendocrinology and Adaptive Physiology of Maternal Care. Curr. Top Behav. Neurosci. 2019. [Google Scholar] [CrossRef]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Storey-Workley, M.; Usip, S.; Hafemeister, J.; Miller, K.E.; Papka, R.E. Glutamate and Metabotropic Glutamate Receptors Associated with Innervation of the Uterine Cervix during Pregnancy: Receptor Antagonism Inhibits c-Fos Expression in Rat Lumbosacral Spinal Cord at Parturition. J. Neurosci. Res. 2007, 85, 1318–1335. [Google Scholar] [CrossRef] [PubMed]
- Bhandage, A.K.; Jin, Z.; Hellgren, C.; Korol, S.V.; Nowak, K.; Williamsson, L.; Sundström-Poromaa, I.; Birnir, B. AMPA, NMDA and Kainate Glutamate Receptor Subunits Are Expressed in Human Peripheral Blood Mononuclear Cells (PBMCs) Where the Expression of GluK4 Is Altered by Pregnancy and GluN2D by Depression in Pregnant Women. J. Neuroimmunol. 2017, 305, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, H.; Dagdeviren, H.; Caypinar, S.S.; Kanawati, A.; Yildiz, S.; Ekin, M. Plasma Serotonin Levels Are Elevated in Pregnant Women with Hyperemesis Gravidarum. Arch. Gynecol. Obstet. 2015, 291, 1271–1276. [Google Scholar] [CrossRef]
- Thibeault, A.-A.H.; Sanderson, J.T.; Vaillancourt, C. Serotonin-Estrogen Interactions: What Can We Learn from Pregnancy? Biochimie 2019, 161, 88–108. [Google Scholar] [CrossRef]
- Luan, H.; Meng, N.; Liu, P.; Feng, Q.; Lin, S.; Fu, J.; Davidson, R.; Chen, X.; Rao, W.; Chen, F.; et al. Pregnancy-Induced Metabolic Phenotype Variations in Maternal Plasma. J. Proteome Res. 2014, 13, 1527–1536. [Google Scholar] [CrossRef]
- Di Giulio, A.M.; Carelli, S.; Castoldi, R.E.; Gorio, A.; Taricco, E.; Cetin, I. Plasma Amino Acid Concentrations throughout Normal Pregnancy and Early Stages of Intrauterine Growth Restricted Pregnancy. J. Matern. Neonatal Med. 2004, 15, 356–362. [Google Scholar] [CrossRef]
- Diaz, S.O.; Barros, A.S.; Goodfellow, B.J.; Duarte, I.F.; Carreira, I.M.; Galhano, E.; Pita, C.; do Céu Almeida, M.; Gil, A.M. Following Healthy Pregnancy by Nuclear Magnetic Resonance (NMR) Metabolic Profiling of Human Urine. J. Proteome Res. 2012, 12, 969–979. [Google Scholar] [CrossRef]
- Pinto, J.; Barros, A.S.; Domingues, M.R.M.; Goodfellow, B.J.; Galhano, E.; Pita, C.; Almeida, M.D.C.; Carreira, I.M.; Gil, A.M. Following Healthy Pregnancy by NMR Metabolomics of Plasma and Correlation to Urine. J. Proteome Res. 2015, 14, 1263–1274. [Google Scholar] [CrossRef]
- Pinto, J.; Almeida, L.M.; Martins, A.S.; Duarte, D.; Barros, A.S.; Galhano, E.; Pita, C.; Almeida, M.d.C.; Carreira, I.M.; Gil, A.M. Prediction of Gestational Diabetes through NMR Metabolomics of Maternal Blood. J. Proteome Res. 2015, 14, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhang, X.; Xie, Y.; Jiang, S.; Liu, Y.; Liu, X.; Xie, W.; Jia, X.; Bade, R.; Shi, R.; et al. 5-Aza-2′-Deoxycytidine Increases Hypoxia Tolerance-Dependent Autophagy in Mouse Neuronal Cells by Initiating the TSC1/MTOR Pathway. Biomed. Pharmacother. 2019, 118, 109219. [Google Scholar] [CrossRef]
- Huang, Q.; Zhan, L.; Cao, H.; Li, J.; Lyu, Y.; Guo, X.; Zhang, J.; Ji, L.; Ren, T.; An, J.; et al. Increased Mitochondrial Fission Promotes Autophagy and Hepatocellular Carcinoma Cell Survival through the ROS-Modulated Coordinated Regulation of the NFKB and TP53 Pathways. Autophagy 2016, 12, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-E.; Kang, G.M.; Min, S.H.; Jo, D.S.; Jung, Y.-K.; Kim, K.; Kim, M.-S.; Cho, D.-H. Primary Cilia Mediate Mitochondrial Stress Responses to Promote Dopamine Neuron Survival in a Parkinson’s Disease Model. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, G. Autophagy in Mitochondrial Quality Control. In Autophagy: Biology and Diseases; Springer: Berlin, Germany, 2019; pp. 421–434. [Google Scholar]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-Activated Protein Kinase Mediates Mitochondrial Fission in Response to Energy Stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Zhao, Y.; Fan, R.; Bai, Y.; Guo, X.; Li, J.; Chen, C. Role of the PI3K-MTOR Autophagy Pathway in Nerve Damage in Rats with Intermittent Hypoxia-Aggravated Whole Brain Ischemia. Mol. Med. Rep. 2019, 20, 1411–1417. [Google Scholar] [CrossRef]
- Tang, T.; Gao, D.; Yang, X.; Hua, X.; Li, S.; Sun, H. Exogenous Netrin-1 Inhibits Autophagy of Ischemic Brain Tissues and Hypoxic Neurons via PI3K/MTOR Pathway in Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1338–1345. [Google Scholar] [CrossRef]
- Sun, B.; Ou, H.; Ren, F.; Huan, Y.; Zhong, T.; Gao, M.; Cai, H. Propofol Inhibited Autophagy through Ca 2+/CaMKKβ/AMPK/MTOR Pathway in OGD/R-Induced Neuron Injury. Mol. Med. 2018, 24, 58. [Google Scholar] [CrossRef]
- Lübke, K.T.; Busch, A.; Hoenen, M.; Schaal, B.; Pause, B.M. Pregnancy Reduces the Perception of Anxiety. Sci. Rep. 2017, 7, 9213. [Google Scholar] [CrossRef]
- Entringer, S.; Buss, C.; Shirtcliff, E.A.; Cammack, A.L.; Yim, I.S.; Chicz-DeMet, A.; Sandman, C.A.; Wadhwa, P.D. Attenuation of Maternal Psychophysiological Stress Responses and the Maternal Cortisol Awakening Response over the Course of Human Pregnancy. Stress 2010, 13, 258–268. [Google Scholar] [CrossRef]
- Klinkenberg, A.V.; Nater, U.M.; Nierop, A.; Bratsikas, A.; Zimmermann, R.; Ehlert, U. Heart Rate Variability Changes in Pregnant and Non-Pregnant Women during Standardized Psychosocial Stress 1. Acta Obstet. Gynecol. Scand. 2009, 88, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, J.Y.; Lee, J.H.; Kim, S.-H. Plasma Levels of Lysine, Tyrosine, and Valine during Pregnancy Are Independent Risk Factors of Insulin Resistance and Gestational Diabetes. Metab. Syndr. Relat. Disord. 2015, 13, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Che, D.; Qin, G.; Farouk, M.H.; Hailong, J.; Rui, H. Novel Biosynthesis, Metabolism and Physiological Functions of L-Homoarginine. Curr. Protein Pept. Sci. 2019, 20, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K. Hypoxia in the Pathogenesis of Preeclampsia. Hypertens. Res. Pregnancy 2018, 5, 46–51. [Google Scholar] [CrossRef]
- Logue, O.C.; George, E.M.; Bidwell, G.L., III. Preeclampsia and the Brain: Neural Control of Cardiovascular Changes during Pregnancy and Neurological Outcomes of Preeclampsia. Clin. Sci. 2016, 130, 1417–1434. [Google Scholar] [CrossRef]
- Siepmann, T.; Boardman, H.; Bilderbeck, A.; Griffanti, L.; Kenworthy, Y.; Zwager, C.; McKean, D.; Francis, J.; Neubauer, S.; Grace, Z.Y.; et al. Long-Term Cerebral White and Gray Matter Changes after Preeclampsia. Neurology 2017, 88, 1256–1264. [Google Scholar] [CrossRef]
- Junewar, V.; Verma, R.; Sankhwar, P.L.; Garg, R.K.; Singh, M.K.; Malhotra, H.S.; Sharma, P.K.; Parihar, A. Neuroimaging Features and Predictors of Outcome in Eclamptic Encephalopathy: A Prospective Observational Study. Am. J. Neuroradiol. 2014, 35, 1728–1734. [Google Scholar] [CrossRef]
- Smriga, M.; Torii, K. L-Lysine Acts like a Partial Serotonin Receptor 4 Antagonist and Inhibits Serotonin-Mediated Intestinal Pathologies and Anxiety in Rats. Proc. Natl. Acad. Sci. USA 2003, 100, 15370–15375. [Google Scholar] [CrossRef]
- Hiramatsu, M. A Role for Guanidino Compounds in the Brain. Mol. Cell. Biochem. 2003, 244, 57–62. [Google Scholar] [CrossRef]
| Parameter | Rats | ||
|---|---|---|---|
| Non-Pregnant | Pregnant | p Values | |
| OGDHC | 1.13 ± 0.13 | 1.21 ± 0.15 | 0.61 |
| ALA | 0.59 ± 0.05 | 0.62 ± 0.04 | 0.67 |
| ARG | 0.15 ± 0.03 | 0.15 ± 0.02 | 0.22 |
| ASP | 1.96 ± 0.09 | 2.01 ± 0.11 | 0.32 |
| GABA | 1.72 ± 0.18 | 1.63 ± 0.16 | 0.67 |
| GLU | 9.11 ± 0.36 | 11.00 ± 0.39 | 0.02↑ |
| GLY | 0.82 ± 0.10 | 0.75 ± 0.10 | 0.47 |
| HIS | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.19 |
| ILE | 0.03 ± 0.01 | 0.03 ± 0.01 | 1.00 |
| LEU | 0.08± 0.02 | 0.07 ± 0.01 | 0.71 |
| LYS | 0.35 ± 0.02 | 0.37 ± 0.02 | 0.24 |
| MET | 0.05 ± 0.00 | 0.06 ± 0.01 | 0.59 |
| PHE | 0.06 ± 0.01 | 0.08 ± 0.02 | 0.16 |
| SER | 0.61 ± 0.03 | 0.56 ± 0.03 | 0.24 |
| TRP | 0.07 ± 0.00 | 0.11 ± 0.03 | 0.02↑ |
| TYR | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.89 |
| Groups | Non-Pregnant Rats | Pregnant Rats | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Control | Hypoxia | p Values | Control | Hypoxia | p Values | |
| OGDH | 1.13 ± 0.13 | 1.11 ± 0.18 | 0.561 | 1.21 ± 0.15 | 1.24 ± 0.18 | 0.875 | |
| ALA | 0.59 ± 0.05 | 0.73 ± 0.06 | 0.081 | 0.62 ± 0.04 | 0.60 ± 0.04 | 0.633 | |
| ARG | 0.15 ± 0.03 | 0.23 ± 0.04 | 0.048↑ | 0.15 ± 0.02 | 0.16 ± 0.02 | 0.762 | |
| ASP | 1.96 ± 0.09 | 2.30 ± 0.19 | 0.180 | 2.01 ± 0.11 | 2.08 ± 0.09 | 0.573 | |
| GABA | 1.72 ± 0.18 | 2.22 ± 0.25 | 0.091 | 1.63 ± 0.16 | 1.54 ± 0.11 | 0.460 | |
| Glu | 9.11 ± 0.36 | 10.93 ±0.61 | 0.027↑ | 11.00 ± 0.01 | 10.56 ± 0.42 | 0.274 | |
| GLY | 0.82 ± 0.10 | 1.03 ± 0.14 | 0.180 | 0.75 ± 0.10 | 0.77 ± 0.08 | 1.000 | |
| HIS | 0.06 ± 0.01 | 0.08 ± 0.01 | 0.055 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.696 | |
| ILE | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.162 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.696 | |
| LEU | 0.08± 0.02 | 0.12 ±0.02 | 0.116 | 0.07 ±0.01 | 0.10 ± 0.02 | 0.829 | |
| LYS | 0.35 ± 0.02 | 0.42 ± 0.03 | 0.048↑ | 0.37 ± 0.02 | 0.39 ± 0.03 | 0.515 | |
| MET | 0.05 ± 0.00 | 0.09 ± 0.00 | 0.014↑ | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.573 | |
| PHE | 0.06 ± 0.01 | 0.13 ± 0.02 | 0.004↑ | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.829 | |
| SER | 0.51 ± 0.03 | 0.61 ± 0.02 | 0.036↑ | 0.56 ± 0.03 | 0.56 ± 0.05 | 0.274 | |
| TRP | 0.07 ± 0.00 | 0.10 ± 0.01 | 0.048↑ | 0.11 ± 0.03 | 0.09 ± 0.01 | 0.083 | |
| TYR | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.351 | 0.07 ± 0.01 | 0.07 ± 0.01 | 1.000 | |
| (A) Non-Pregnant Rats | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | OGDHC | ALA | ARG | ASP | GABA | GLU | GLY | HIS | ILE | LEU | LYS | MET | PHE | SER | TRP | TYR | |
| C | |||||||||||||||||
| OGDHC | −0.94 0.001 | −0.94 0.001 | −0.90 0.002 | −0.95 0.000 | −0.84 0.009 | −0.95 0.000 | −0.94 0.000 | −0.95 0.000 | −0.95 0.000 | −0.69 0.059 | −0.65 0.084 | −0.95 0.000 | −0.19 0.649 | 0.53 0.181 | −0.89 0.003 | ||
| ALA | −0.42 0.407 | 0.99 0.000 | 0.86 0.006 | 0.97 0.000 | 0.82 0.013 | 0.96 0.000 | 0.94 0.000 | 0.97 0.000 | 0.98 0.000 | 0.81 0.015 | 0.71 0.046 | 0.99 0.000 | 0.31 0.452 | −0.68 0.065 | 0.93 0.001 | ||
| ARG | −0.62 0.187 | 0.87 0.025 | 0.85 0.008 | 0.98 0.000 | 0.83 0.010 | 0.99 0.000 | 0.94 0.000 | 0.98 0.000 | 0.98 0.000 | 0.78 0.022 | 0.62 0.103 | 0.97 0.000 | 0.27 0.518 | −0.66 0.078 | 0.97 0.000 | ||
| ASP | −0.68 0.135 | 0.78 0.068 | 0.97 0.001 | 0.91 0.001 | 0.94 0.001 | 0.87 0.005 | 0.91 0.002 | 0.89 0.003 | 0.88 0.004 | 0.80 0.017 | 0.71 0.050 | 0.86 0.007 | 0.56 0.147 | −0.33 0.422 | 0.86 0.006 | ||
| GABA | −0.63 0.178 | 0.91 0.011 | 0.92 0.009 | 0.93 0.008 | 0.92 0.001 | 0.99 0.000 | 0.94 0.000 | 0.97 0.000 | 0.96 0.000 | 0.80 0.017 | 0.60 0.116 | 0.97 0.000 | 0.35 0.397 | −0.56 0.152 | 0.97 0.000 | ||
| GLU | −0.42 0.402 | 0.76 0.081 | 0.84 0.036 | 0.90 0.014 | 0.90 0.014 | 0.89 0.003 | 0.90 0.003 | 0.85 0.007 | 0.84 0.009 | 0.84 0.009 | 0.46 0.246 | 0.83 0.011 | 0.53 0.176 | −0.39 0.340 | 0.90 0.002 | ||
| GLY | −0.64 0.170 | 0.86 0.027 | 0.98 0.000 | 0.96 0.002 | 0.94 0.005 | 0.83 0.040 | 0.95 0.000 | 0.98 0.000 | 0.97 0.000 | 0.76 0.030 | 0.53 0.172 | 0.97 0.000 | 0.25 0.546 | −0.56 0.148 | 0.96 0.000 | ||
| HIS | 0.03 0.955 | −0.05 0.918 | 0.12 0.814 | 0.06 0.911 | −0.05 0.932 | −0.17 0.750 | 0.22 0.671 | 0.97 0.000 | 0.98 0.000 | 0.85 0.008 | 0.64 0.085 | 0.93 0.001 | 0.39 0.340 | −0.58 0.132 | 0.93 0.001 | ||
| ILE | −0.72 0.106 | 0.93 0.008 | 0.91 0.011 | 0.87 0.025 | 0.95 0.003 | 0.74 0.093 | 0.93 0.007 | 0.02 0.970 | 0.99 0.000 | 0.78 0.024 | 0.64 0.091 | 0.97 0.000 | 0.30 0.468 | −0.56 0.151 | 0.94 0.000 | ||
| LEU | −0.70 0.125 | 0.94 0.005 | 0.90 0.015 | 0.86 0.028 | 0.96 0.002 | 0.76 0.078 | 0.91 0.011 | −0.04 0.940 | 0.99 0.000 | 0.80 0.018 | 0.66 0.073 | 0.97 0.000 | 0.31 0.455 | −0.60 0.117 | 0.93 0.001 | ||
| LYS | −0.56 0.251 | 0.67 0.142 | 0.60 0.206 | 0.69 0.127 | 0.85 0.031 | 0.81 0.049 | 0.65 0.159 | −0.32 0.543 | 0.74 0.096 | 0.77 0.071 | 0.64 0.090 | 0.74 0.034 | 0.72 0.043 | −0.67 0.068 | 0.84 0.009 | ||
| MET | 0.67 0.144 | −0.65 0.158 | −0.48 0.341 | −0.38 0.454 | −0.57 0.240 | −0.19 0.726 | −0.50 0.311 | 0.09 0.859 | −0.77 0.075 | −0.76 0.079 | −0.46 0.356 | 0.65 0.081 | 0.53 0.179 | −0.43 0.284 | 0.54 0.172 | ||
| PHE | −0.64 0.174 | 0.75 0.089 | 0.90 0.014 | 0.96 0.002 | 0.91 0.011 | 0.96 0.003 | 0.88 0.022 | −0.18 0.730 | 0.81 0.050 | 0.82 0.047 | 0.78 0.068 | −0.32 0.541 | 0.23 0.580 | −0.60 0.116 | 0.91 0.002 | ||
| SER | 0.32 0.531 | −0.63 0.181 | −0.72 0.104 | −0.79 0.059 | −0.75 0.086 | −0.93 0.006 | −0.66 0.158 | 0.42 0.403 | −0.57 0.234 | −0.61 0.203 | −0.68 0.134 | 0.05 0.924 | −0.91 0.011 | −0.11 0.789 | 0.40 0.329 | ||
| TRP | 0.79 0.061 | −0.17 0.745 | −0.51 0.305 | −0.54 0.272 | −0.38 0.458 | −0.17 0.744 | −0.57 0.239 | −0.56 0.246 | −0.49 0.327 | −0.43 0.400 | −0.17 0.753 | 0.39 0.446 | −0.36 0.483 | −0.02 0.965 | −0.63 0.097 | ||
| TYR | −0.06 0.906 | 0.87 0.025 | 0.79 0.062 | 0.699 0.126 | 0.76 0.078 | 0.74 0.095 | 0.79 0.059 | 0.23 0.666 | 0.70 0.120 | 0.71 0.115 | 0.46 0.357 | −0.25 0.629 | 0.63 0.182 | −0.59 0.221 | −0.06 0.912 | ||
| (B) Pregnant Rats | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | OGDHC | ALA | ARG | ASP | GABA | GLU | GLY | HIS | ILE | LEU | LYS | MET | PHE | SER | TRP | TYR | |
| C | |||||||||||||||||
| OGDHC | −0.17 0.629 | −0.03 0.931 | −0.06 0.864 | −0.12 0.748 | −0.22 0.532 | −0.15 0.670 | 0.03 0.944 | 0.14 0.708 | 0.19 0.602 | 0.24 0.506 | 0.63 0.050 | 0.08 0.832 | 0.44 0.201 | 0.05 0.891 | 0.13 0.725 | ||
| ALA | −0.35 0.569 | 0.76 0.010 | 0.28 0.439 | 0.61 0.062 | 0.10 0.791 | 0.77 0.009 | 0.60 0.069 | 0.83 0.003 | 0.79 0.006 | 0.40 0.255 | 0.40 0.248 | 0.60 0.067 | 0.67 0.034 | −0.02 0.949 | 0.73 0.016 | ||
| ARG | −0.33 0.582 | 0.86 0.063 | 0.03 0.926 | 0.84 0.002 | −0.42 0.226 | 0.94 0.000 | 0.30 0.406 | 0.77 0.009 | 0.64 0.048 | −0.07 0.838 | 0.46 0.180 | 0.91 0.000 | 0.36 0.313 | −0.52 0.122 | 0.37 0.298 | ||
| ASP | −0.04 0.953 | 0.85 0.066 | 0.85 0.067 | 0.35 0.323 | 0.75 0.012 | 0.22 0.548 | 0.65 0.042 | −0.11 0.769 | −0.10 0.777 | 0.16 0.653 | −0.22 0.544 | −0.05 0.885 | 0.10 0.787 | 0.58 0.081 | 0.25 0.478 | ||
| GABA | −0.55 0.340 | 0.88 0.052 | 0.96 0.010 | 0.82 0.087 | −0.17 0.631 | 0.96 0.000 | 0.44 0.204 | 0.53 0.118 | 0.37 0.291 | −0.24 0.510 | 0.16 0.650 | 0.80 0.005 | 0.14 0.704 | −0.45 0.189 | 0.22 0.548 | ||
| GLU | 0.33 0.588 | 0.49 0.398 | 0.21 0.732 | 0.68 0.205 | 0.23 0.710 | −0.25 0.480 | 0.45 0.192 | −0.36 0.306 | −0.28 0.432 | 0.23 0.522 | −0.49 0.152 | −0.55 0.100 | 0.02 0.947 | 0.89 0.000 | 0.11 0.769 | ||
| GLY | −0.51 0.380 | 0.78 0.118 | 0.96 0.010 | 0.80 0.104 | 0.98 0.003 | 0.15 0.808 | 0.44 0.203 | 0.72 0.020 | 0.58 0.081 | −0.08 0.818 | 0.32 0.375 | 0.87 0.001 | 0.31 0.390 | −0.49 0.153 | 0.38 0.274 | ||
| HIS | 0.76 0.136 | 0.24 0.700 | 0.27 0.660 | 0.39 0.519 | 0.01 0.990 | 0.34 0.576 | 0.01 0.989 | 0.30 0.393 | 0.34 0.341 | 0.49 0.150 | 0.27 0.452 | 0.38 0.279 | 0.54 0.104 | 0.30 0.405 | 0.59 0.075 | ||
| ILE | −0.44 0.456 | 0.93 0.022 | 0.98 0.003 | 0.85 0.071 | 0.98 0.003 | 0.26 0.672 | 0.95 0.015 | 0.17 0.780 | 0.98 0.000 | 0.46 0.184 | 0.74 0.015 | 0.69 0.028 | 0.77 0.010 | −0.39 0.268 | 0.77 0.009 | ||
| LEU | −0.42 0.479 | 0.90 0.036 | 0.99 0.001 | 0.84 0.076 | 0.98 0.004 | 0.22 0.724 | 0.96 0.011 | 0.20 0.752 | 0.99 0.000 | 0.62 0.054 | 0.78 0.008 | 0.57 0.084 | 0.86 0.001 | −0.27 0.450 | 0.86 0.001 | ||
| LYS | 0.07 0.910 | 0.80 0.106 | 0.81 0.095 | 0.71 0.177 | 0.66 0.230 | 0.26 0.670 | 0.61 0.275 | 0.70 0.191 | 0.79 0.112 | 0.80 0.106 | 0.60 0.068 | −0.05 0.898 | 0.81 0.005 | 0.33 0.353 | 0.87 0.001 | ||
| MET | −0.15 0.812 | 0.00 0.994 | 0.39 0.518 | −0.08 0.893 | 0.24 0.698 | −0.74 0.156 | 0.33 0.585 | 0.21 0.740 | 0.28 0.651 | 0.34 0.578 | 0.39 0.512 | 0.54 0.111 | 0.78 0.007 | −0.31 0.377 | 0.66 0.039 | ||
| PHE | −0.04 0.951 | 0.50 0.396 | 0.84 0.077 | 0.58 0.310 | 0.67 0.213 | −0.14 0.827 | 0.74 0.149 | 0.49 0.402 | 0.72 0.166 | 0.77 0.125 | 0.78 0.123 | 0.76 0.137 | 0.36 0.307 | −0.64 0.046 | 0.34 0.340 | ||
| SER | 0.36 0.549 | 0.43 0.466 | 0.60 0.288 | 0.45 0.446 | 0.35 0.565 | 0.02 0.978 | 0.36 0.546 | 0.86 0.065 | 0.50 0.393 | 0.53 0.353 | 0.89 0.045 | 0.61 0.272 | 0.82 0.086 | 0.12 0.739 | 0.90 0.000 | ||
| TRP | 0.85 0.070 | −0.21 0.740 | −0.45 0.444 | 0.01 0.988 | −0.55 0.339 | 0.64 0.242 | −0.59 0.295 | 0.53 0.358 | −0.46 0.433 | −0.48 0.409 | −0.08 0.903 | −0.63 0.255 | −0.43 0.470 | 0.02 0.979 | 0.09 0.815 | ||
| TYR | −0.08 0.900 | 0.81 0.097 | 0.96 0.008 | 0.86 0.061 | 0.85 0.068 | 0.27 0.663 | 0.86 0.064 | 0.52 0.373 | 0.91 0.031 | 0.93 0.022 | 0.90 0.035 | 0.42 0.482 | 0.89 0.041 | 0.77 0.129 | −0.26 0.669 | ||
| Groups | Non-Pregnant Rats | Pregnant Rats | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∑ | + | − | ∑ | + | − | ||||||||||||
| Parameter | Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | |
| OGDHC | 7.90 | 12.26 | 0.53 | 0.82 | 0 | 0 | 0 | 11 | 5.28 | 2.68 | 0.35 | 0.18 | 0 | 1 | 0 | 0 | |
| ALA | 10.26 | 12.86 | 0.68 | 0.86 | 5 | 12 | 0 | 1 | 9.03 | 7.73 | 0.60 | 0.52 | 3 | 6 | 0 | 0 | |
| ARG | 11.13 | 12.75 | 0.74 | 0.85 | 8 | 11 | 0 | 1 | 10.46 | 7.42 | 0.70 | 0.49 | 5 | 7 | 0 | 0 | |
| ASP | 11.06 | 12.13 | 0.74 | 0.81 | 7 | 12 | 0 | 1 | 8.81 | 3.91 | 0.59 | 0.26 | 0 | 2 | 0 | 0 | |
| GABA | 11.41 | 12.84 | 0.76 | 0.86 | 8 | 11 | 0 | 1 | 9.71 | 6.4 | 0.65 | 0.43 | 5 | 3 | 0 | 0 | |
| GLU | 10.12 | 11.78 | 0.67 | 0.79 | 6 | 11 | 1 | 1 | 4.98 | 5.29 | 0.33 | 0.35 | 0 | 2 | 0 | 0 | |
| GLY | 11.32 | 12.58 | 0.75 | 0.84 | 8 | 11 | 0 | 1 | 9.59 | 7.48 | 0.64 | 0.50 | 4 | 5 | 0 | 0 | |
| HIS | 2.56 | 12.79 | 0.17 | 0.85 | 0 | 11 | 0 | 1 | 5.7 | 6.12 | 0.38 | 0.41 | 0 | 1 | 0 | 0 | |
| ILE | 11.14 | 12.74 | 0.74 | 0.85 | 6 | 11 | 0 | 1 | 10.21 | 8.56 | 0.68 | 0.57 | 6 | 8 | 0 | 0 | |
| LEU | 11.16 | 12.8 | 0.74 | 0.85 | 6 | 11 | 0 | 1 | 10.35 | 8.23 | 0.69 | 0.55 | 6 | 6 | 0 | 0 | |
| LYS | 9.21 | 11.52 | 0.61 | 0.77 | 0 | 12 | 0 | 0 | 9.25 | 5.65 | 0.62 | 0.38 | 2 | 2 | 0 | 0 | |
| MET | 6.53 | 9.01 | 0.44 | 0.60 | 0 | 0 | 0 | 0 | 5.57 | 7.36 | 0.37 | 0.49 | 0 | 3 | 0 | 0 | |
| PHE | 10.81 | 12.54 | 0.72 | 0.84 | 7 | 11 | 1 | 1 | 9.17 | 7.43 | 0.61 | 0.50 | 1 | 4 | 0 | 1 | |
| SER | 8.65 | 5.45 | 0.58 | 0.36 | 0 | 1 | 2 | 0 | 7.57 | 7.18 | 0.50 | 0.48 | 1 | 6 | 0 | 0 | |
| TRP | 5.61 | 7.89 | 0.37 | 0.53 | 0 | 0 | 0 | 0 | 6.19 | 5.45 | 0.41 | 0.36 | 0 | 1 | 0 | 1 | |
| TYR | 8.33 | 12.6 | 0.56 | 0.84 | 1 | 11 | 0 | 1 | 10.29 | 7.27 | 0.69 | 0.48 | 5 | 6 | 0 | 0 | |
| Sum or Average | 147.20 | 184.54 | 0.61 | 0.77 | 62 | 136 | 4 | 22 | 132.16 # | 104.16# | 0.55 # | 0.43 # | 38 | 63 # | 0 | 2 # | |
| p value of the difference | 0.004 | 0.004 | 0.002 | 0.04 | 0.004 | 0.004 | 0.005 | 0.18 | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, A.; Trofimova, L.; Ksenofontov, A.; Baratova, L.; Bunik, V. Hypoxic Adaptation of Mitochondrial Metabolism in Rat Cerebellum Decreases in Pregnancy. Cells 2020, 9, 139. https://doi.org/10.3390/cells9010139
Graf A, Trofimova L, Ksenofontov A, Baratova L, Bunik V. Hypoxic Adaptation of Mitochondrial Metabolism in Rat Cerebellum Decreases in Pregnancy. Cells. 2020; 9(1):139. https://doi.org/10.3390/cells9010139
Chicago/Turabian StyleGraf, Anastasia, Lidia Trofimova, Alexander Ksenofontov, Lyudmila Baratova, and Victoria Bunik. 2020. "Hypoxic Adaptation of Mitochondrial Metabolism in Rat Cerebellum Decreases in Pregnancy" Cells 9, no. 1: 139. https://doi.org/10.3390/cells9010139
APA StyleGraf, A., Trofimova, L., Ksenofontov, A., Baratova, L., & Bunik, V. (2020). Hypoxic Adaptation of Mitochondrial Metabolism in Rat Cerebellum Decreases in Pregnancy. Cells, 9(1), 139. https://doi.org/10.3390/cells9010139







