De-Glycyrrhizinated Licorice Extract Attenuates High Glucose-Stimulated Renal Tubular Epithelial–Mesenchymal Transition via Suppressing the Notch2 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Reagents and Transfections
2.2. Preparation and Characterization of Licorice Extract and De-Glycyrrhizinated (or GC-Knockout) Licorice Extract
2.3. Western Blot Analysis
2.4. Knockdown of MAML-1 and Overexpression of NICD2
2.5. Measurement of γ-Secretase Activity by Reporter Assay
2.6. Reporter Assay for Monitoring Notch Signaling Activation
2.7. Quantitative Reverse Transcription (RT)-PCR
2.8. Statistical Analysis
3. Results
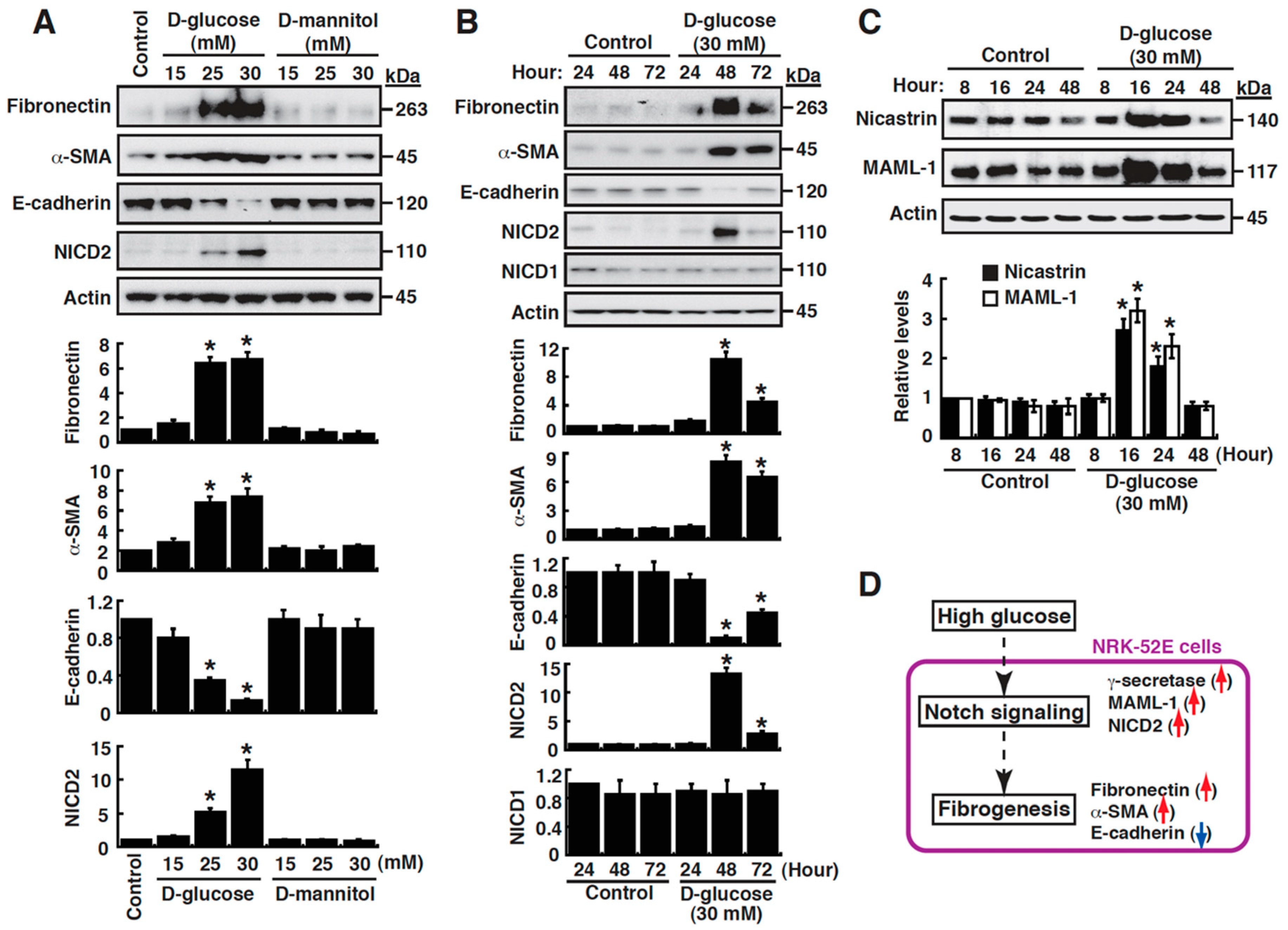
3.1. High Glucose Promotes EMT and Notch2 Activation in Renal Tubular NRK-52E Cells
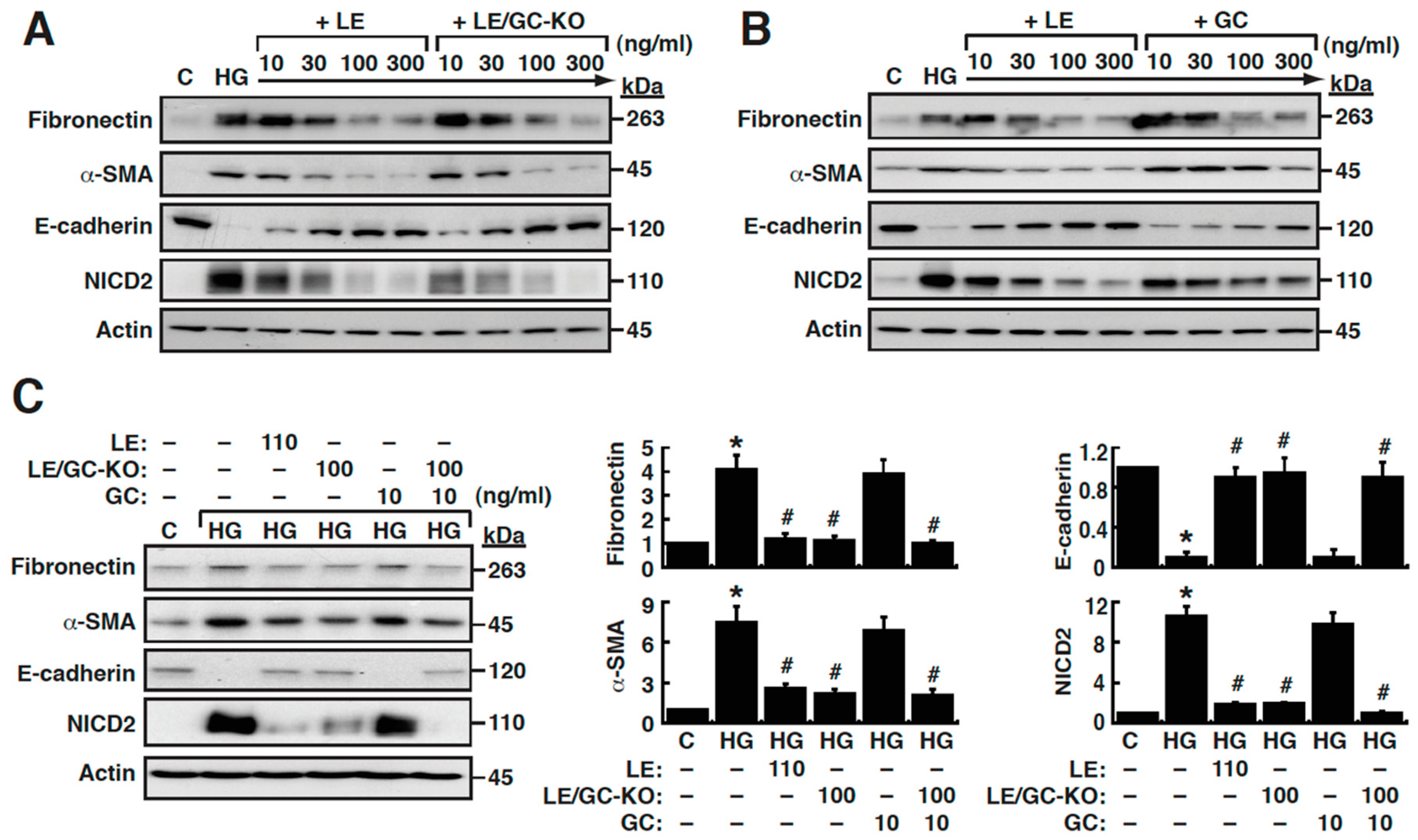
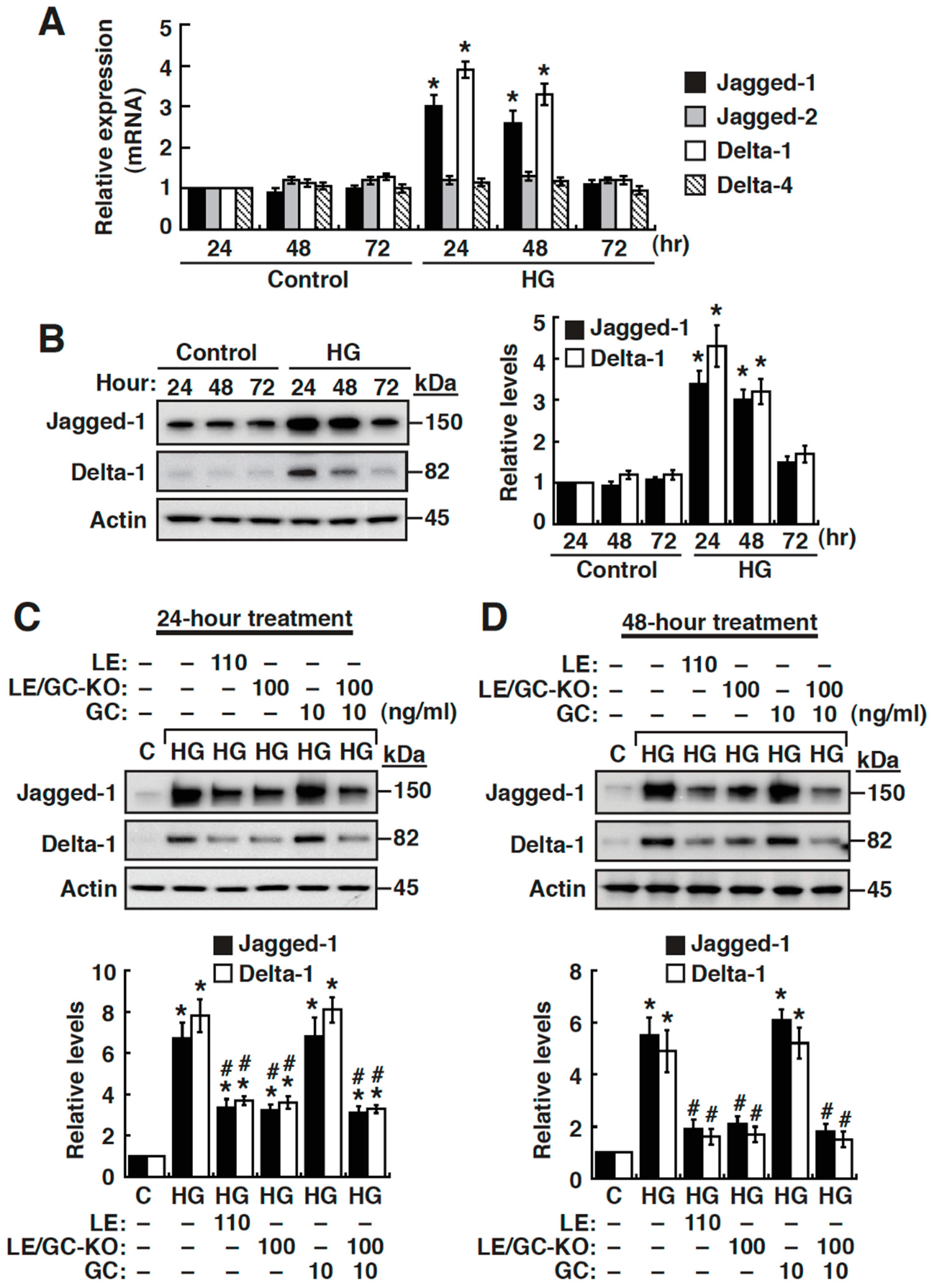
3.2. Effects of Licorice Extract (LE), Glycyrrhizin (GC), and GC-Knockout LE on HG-Induced EMT in NRK-52E Cells
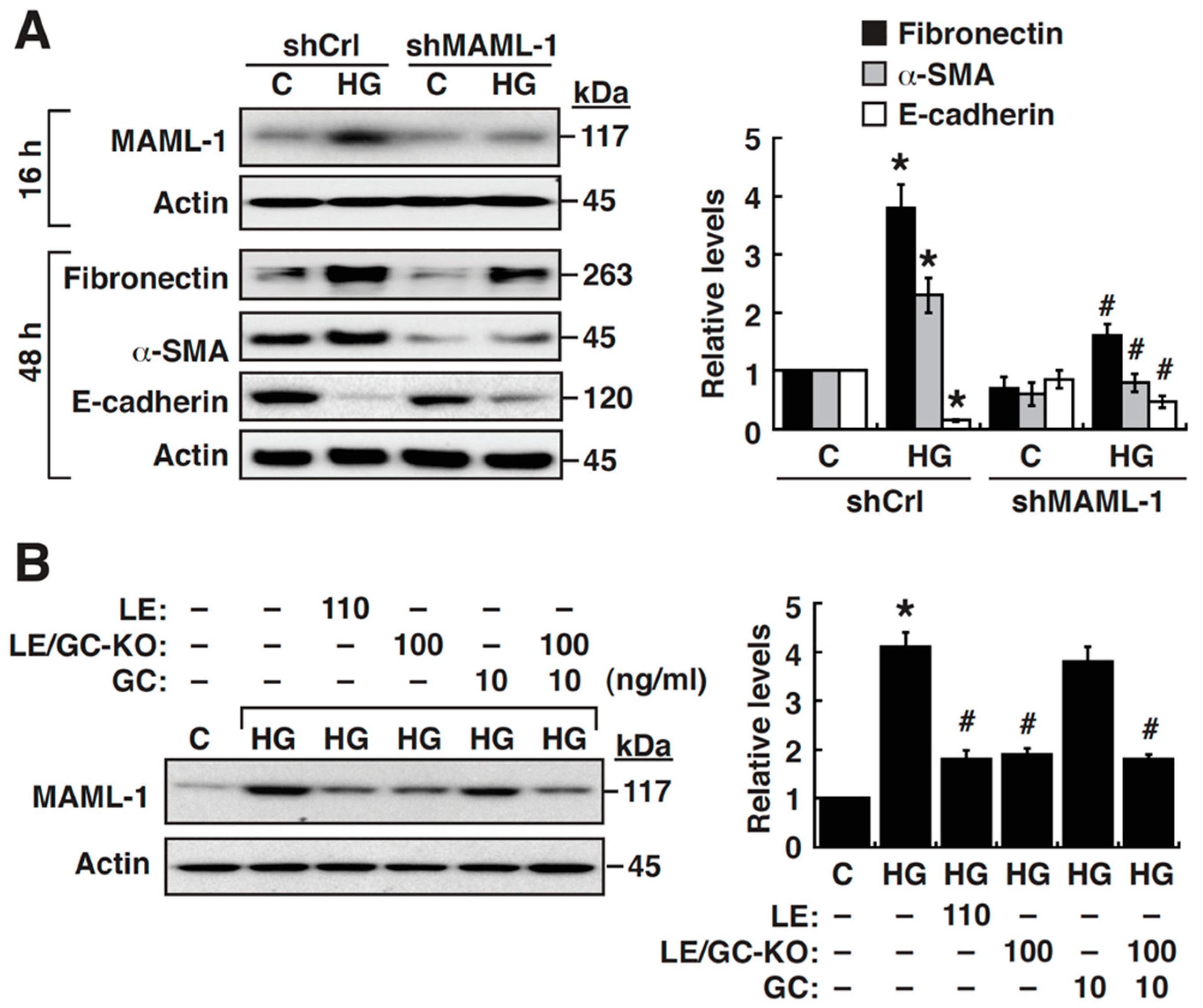
3.3. Upregulation of MAML-1 is Critically Required for HG-Mediated EMT in NRK-52E Cells
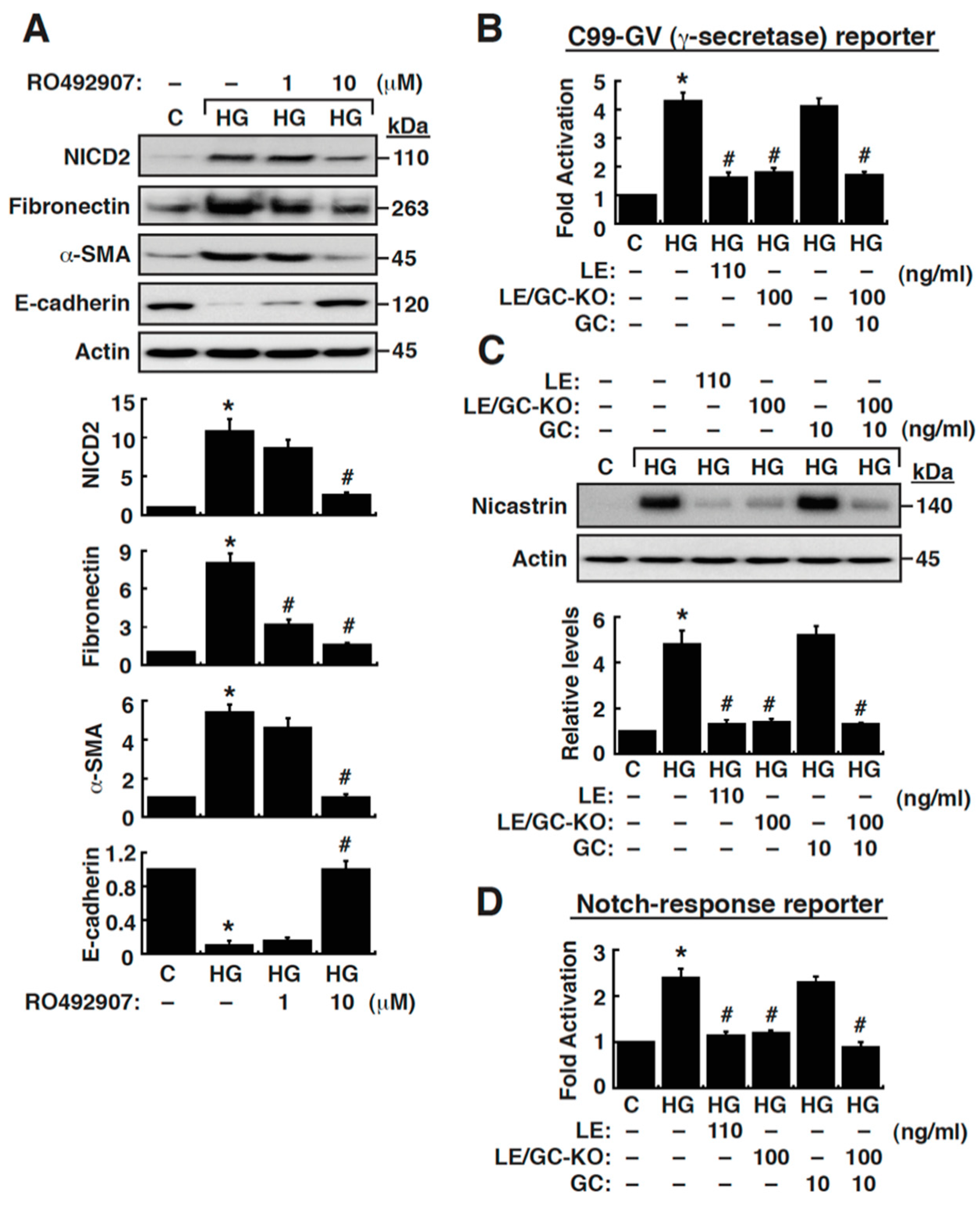
3.4. Suppression of γ-Secretase Activity Prevents HG-Triggered EMT in NRK-52E Cells
3.5. Ectopic Expression of NICD2 in NRK-52E Cells Sufficiently Stimulates EMT
3.6. High Glucose Upregulates the Expression of Jagged-1 and Delta-like 1 in NRK-52E Cells
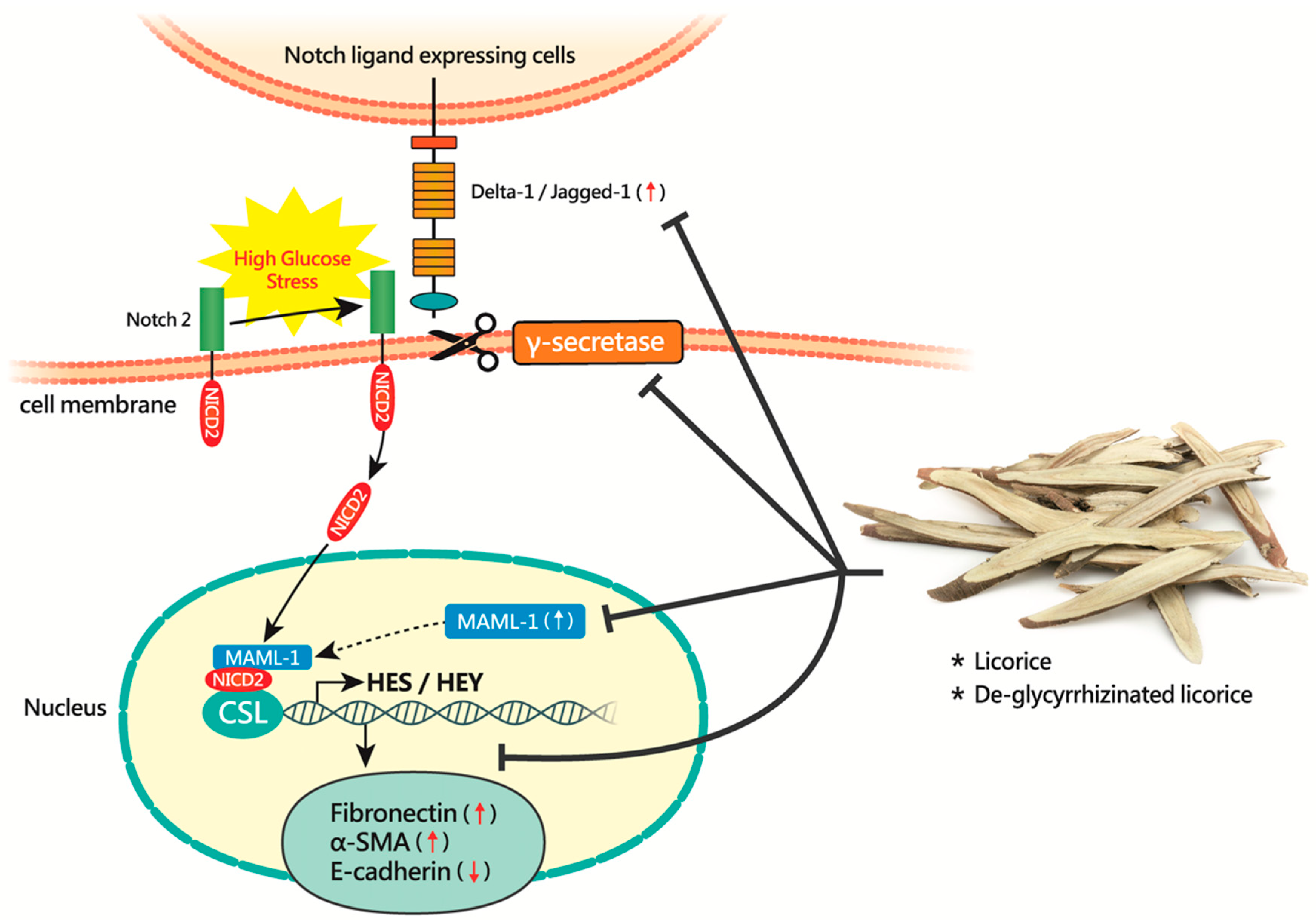
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Badal, S.S.; Danesh, F.R. New insights into molecular mechanisms of diabetic kidney disease. Am. J. Kidney Dis. 2014, 63, S63–S83. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Zeng, X.X.; Rychlik, I. Clinical manifestation and natural history of diabetic nephropathy. Contrib. Nephrol. 2011, 170, 19–27. [Google Scholar] [PubMed]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.; Qu, X.; Caruana, G.; Li, J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 2016, 92, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Yang, C. Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 2001, 159, 1465–1475. [Google Scholar] [CrossRef]
- Fan, J.M.; Ng, Y.Y.; Hill, P.A.; Nikolic-Paterson, D.J.; Mu, W.; Atkins, R.C.; Lan, H.Y. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999, 56, 1455–1467. [Google Scholar] [CrossRef]
- Veerasamy, M.; Nguyen, T.Q.; Motazed, R.; Pearson, A.L.; Goldschmeding, R.; Dockrell, M.E. Differential regulation of E-cadherin and alpha-smooth muscle actin by BMP 7 in human renal proximal tubule epithelial cells and its implication in renal fibrosis. Am. J. Physiol. Renal Physiol. 2009, 297, F1238–F1248. [Google Scholar] [CrossRef]
- Phanish, M.K.; Wahab, N.A.; Colville-Nash, P.; Hendry, B.M.; Dockrell, M.E. The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem. J. 2006, 393, 601–607. [Google Scholar] [CrossRef]
- Wei, M.G.; Sun, W.; He, W.M.; Ni, L.; Yang, Y.Y. Ferulic Acid Attenuates TGF-beta1-Induced Renal Cellular Fibrosis in NRK-52E Cells by Inhibiting Smad/ILK/Snail Pathway. Evid. Based Complement. Altern. Med. 2015, 2015, 619720. [Google Scholar] [CrossRef]
- He, W.; Dai, C. Key Fibrogenic Signaling. Curr. Pathobiol. Rep. 2015, 3, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Edeling, M.; Ragi, G.; Huang, S.; Pavenstadt, H.; Susztak, K. Developmental signalling pathways in renal fibrosis: The roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 2016, 12, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Sirin, Y.; Susztak, K. Notch in the kidney: Development and disease. J. Pathol. 2012, 226, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Murea, M.; Park, J.K.; Sharma, S.; Kato, H.; Gruenwald, A.; Niranjan, T.; Si, H.; Thomas, D.B.; Pullman, J.M.; Melamed, M.L.; et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int. 2010, 78, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Bielesz, B.; Sirin, Y.; Si, H.; Niranjan, T.; Gruenwald, A.; Ahn, S.; Kato, H.; Pullman, J.; Gessler, M.; Haase, V.H.; et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Investig. 2010, 120, 4040–4054. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Nixon, D.W. Licorice and cancer. Nutr. Cancer 2001, 39, 1–11. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.Q.; Yuan, B.C.; Liu, Y. The Pharmacological Activities of Licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.R.; Komarova, I.; El-Ghonemi, M.; Fathy, A.; Rashad, R.; Abdelmalak, H.D.; Yerramadha, M.R.; Ali, Y.; Helal, E.; Camporesi, E.M. Licorice abuse: Time to send a warning message. Ther. Adv. Endocrinol. Metab. 2012, 3, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Morinaga, O.; Tanaka, H.; Shoyama, Y. Analysis of the synergistic effect of glycyrrhizin and other constituents in licorice extract on lipopolysaccharide-induced nitric oxide production using knock-out extract. Biochem. Biophys. Res. Commun. 2012, 417, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Morinaga, O.; Uto, T.; Fuji, S.; Aboagye, F.A.; Tung, N.H.; Li, X.W.; Putalun, W.; Shoyama, Y. Application of Monoclonal Antibodies against Bioactive Natural Products: Eastern Blotting and Preparation of Knockout Extract. Int. J. Anal. Chem. 2012, 2012, 260425. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Hsu, Y.C.; Huang, Y.T.; Shih, Y.H.; Wang, C.J.; Chiang, W.C.; Chang, P.J. A KDM6A-KLF10 reinforcing feedback mechanism aggravates diabetic podocyte dysfunction. EMBO Mol. Med. 2019, 11, e9828. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.F.; Wang, B.J.; Cheng, H.T.; Kuo, L.H.; Wolfe, M.S. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J. Biol. Chem. 2004, 279, 49523–49532. [Google Scholar] [CrossRef]
- Chang, P.J.; Yang, Y.H.; Chen, P.C.; Chen, L.W.; Wang, S.S.; Shih, Y.J.; Chen, L.Y.; Chen, C.J.; Hung, C.H.; Lin, C.L. Diabetes and risk of Kaposi’s sarcoma: Effects of high glucose on reactivation and infection of Kaposi’s sarcoma-associated herpesvirus. Oncotarget 2017, 8, 80595–80611. [Google Scholar] [CrossRef]
- Oikawa, N.; Walter, J. Presenilins and gamma-Secretase in Membrane Proteostasis. Cells 2019, 8, 209. [Google Scholar] [CrossRef]
- Sharma, S.; Sirin, Y.; Susztak, K. The story of Notch and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 56–61. [Google Scholar] [CrossRef]
- Huang, S.; Park, J.; Qiu, C.; Chung, K.W.; Li, S.Y.; Sirin, Y.; Han, S.H.; Taylor, V.; Zimber-Strobl, U.; Susztak, K. Jagged1/Notch2 controls kidney fibrosis via Tfam-mediated metabolic reprogramming. PLoS Biol. 2018, 16, e2005233. [Google Scholar] [CrossRef]
- Kobayashi, T.; Terada, Y.; Kuwana, H.; Tanaka, H.; Okado, T.; Kuwahara, M.; Tohda, S.; Sakano, S.; Sasaki, S. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int. 2008, 73, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.R.; Lendahl, U. Therapeutic modulation of Notch signalling--are we there yet? Nat. Rev. Drug Discov. 2014, 13, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, A.S.; Raman, R.; Siemers, E.R.; Becerra, L.; Clark, C.M.; Dean, R.A.; Farlow, M.R.; Galvin, J.E.; Peskind, E.R.; Quinn, J.F.; et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch. Neurol. 2008, 65, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Siemers, E.R.; Quinn, J.F.; Kaye, J.; Farlow, M.R.; Porsteinsson, A.; Tariot, P.; Zoulnouni, P.; Galvin, J.E.; Holtzman, D.M.; Knopman, D.S.; et al. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology 2006, 66, 602–604. [Google Scholar] [CrossRef]
- Zecchini, V.; Domaschenz, R.; Winton, D.; Jones, P. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 2005, 19, 1686–1691. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, F.; Li, Y.; Gao, L.; Zhang, J. The protective effect of glycyrrhizic acid on renal tubular epithelial cell injury induced by high glucose. Int. J. Mol. Sci. 2014, 15, 15026–15043. [Google Scholar] [CrossRef]
- Yao, K.; Chen, H.; Lee, M.H.; Li, H.; Ma, W.; Peng, C.; Song, N.R.; Lee, K.W.; Bode, A.M.; Dong, Z.; et al. Licochalcone A, a natural inhibitor of c-Jun N-terminal kinase 1. Cancer Prev. Res. 2014, 7, 139–149. [Google Scholar] [CrossRef]
- Park, H.G.; Bak, E.J.; Woo, G.H.; Kim, J.M.; Quan, Z.; Kim, J.M.; Yoon, H.K.; Cheon, S.H.; Yoon, G.; Yoo, Y.J.; et al. Licochalcone E has an antidiabetic effect. J. Nutr. Biochem. 2012, 23, 759–767. [Google Scholar] [CrossRef]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar] [CrossRef]
- Wu, F.; Jin, Z.; Jin, J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol. Med. Rep. 2013, 7, 1278–1282. [Google Scholar] [CrossRef]
- Weidner, C.; de Groot, J.C.; Prasad, A.; Freiwald, A.; Quedenau, C.; Kliem, M.; Witzke, A.; Kodelja, V.; Han, C.T.; Giegold, S.; et al. Amorfrutins are potent antidiabetic dietary natural products. Proc. Natl. Acad. Sci. USA 2012, 109, 7257–7262. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Chang, P.-J.; Tung, C.-W.; Shih, Y.-H.; Ni, W.-C.; Li, Y.-C.; Uto, T.; Shoyama, Y.; Ho, C.; Lin, C.-L. De-Glycyrrhizinated Licorice Extract Attenuates High Glucose-Stimulated Renal Tubular Epithelial–Mesenchymal Transition via Suppressing the Notch2 Signaling Pathway. Cells 2020, 9, 125. https://doi.org/10.3390/cells9010125
Hsu Y-C, Chang P-J, Tung C-W, Shih Y-H, Ni W-C, Li Y-C, Uto T, Shoyama Y, Ho C, Lin C-L. De-Glycyrrhizinated Licorice Extract Attenuates High Glucose-Stimulated Renal Tubular Epithelial–Mesenchymal Transition via Suppressing the Notch2 Signaling Pathway. Cells. 2020; 9(1):125. https://doi.org/10.3390/cells9010125
Chicago/Turabian StyleHsu, Yung-Chien, Pey-Jium Chang, Chun-Wu Tung, Ya-Hsueh Shih, Wen-Chiu Ni, Yi-Chen Li, Takuhiro Uto, Yukihiro Shoyama, Cheng Ho, and Chun-Liang Lin. 2020. "De-Glycyrrhizinated Licorice Extract Attenuates High Glucose-Stimulated Renal Tubular Epithelial–Mesenchymal Transition via Suppressing the Notch2 Signaling Pathway" Cells 9, no. 1: 125. https://doi.org/10.3390/cells9010125
APA StyleHsu, Y.-C., Chang, P.-J., Tung, C.-W., Shih, Y.-H., Ni, W.-C., Li, Y.-C., Uto, T., Shoyama, Y., Ho, C., & Lin, C.-L. (2020). De-Glycyrrhizinated Licorice Extract Attenuates High Glucose-Stimulated Renal Tubular Epithelial–Mesenchymal Transition via Suppressing the Notch2 Signaling Pathway. Cells, 9(1), 125. https://doi.org/10.3390/cells9010125





