The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts following UVA or UVB Irradiation in 2D and 3D Cell Cultures
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture and Treatment
2.2. Proteomic Analysis
2.3. Protein Identification, Grouping, and Label-Free Quantification
2.4. Statistical Analysis
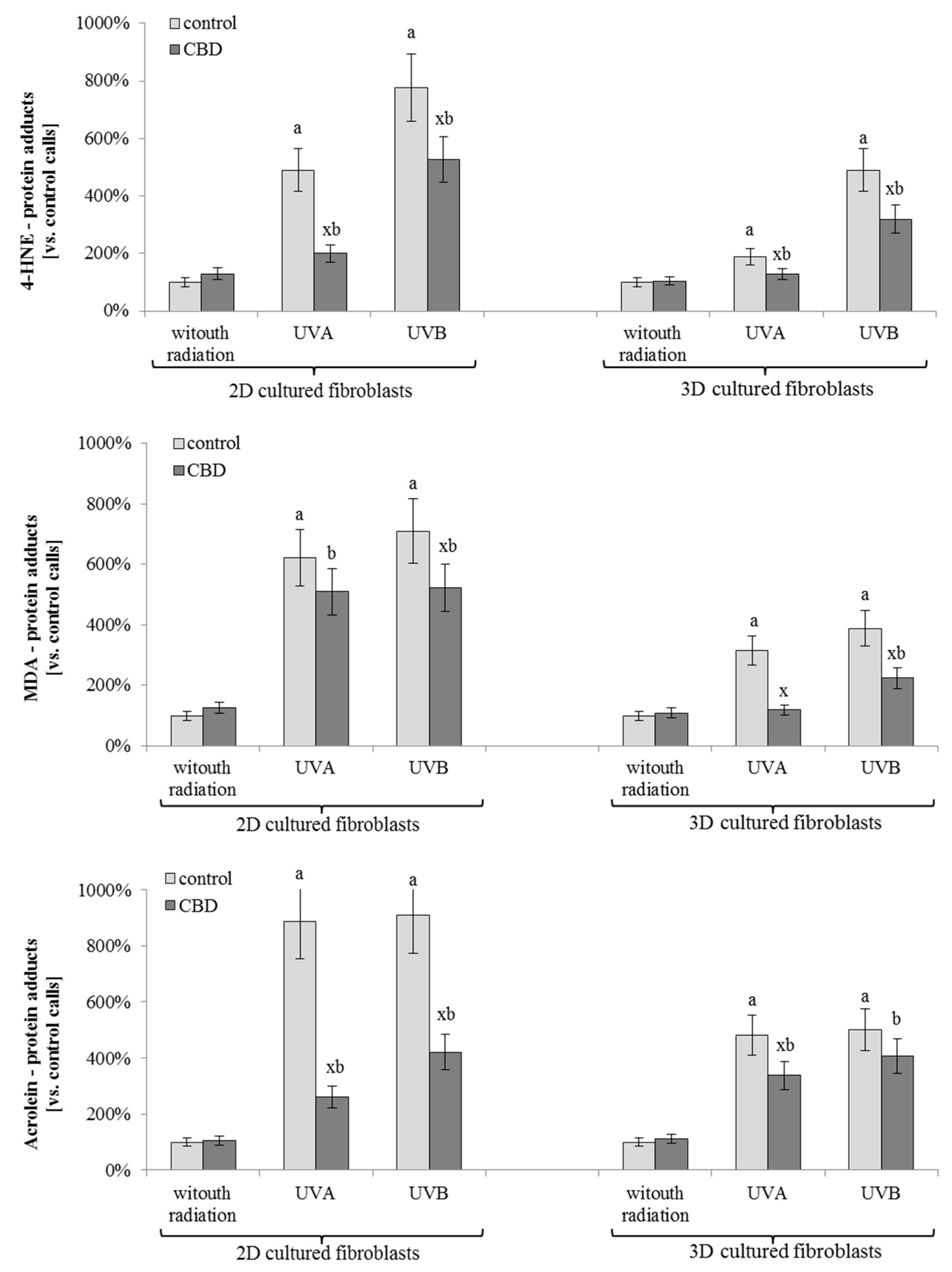
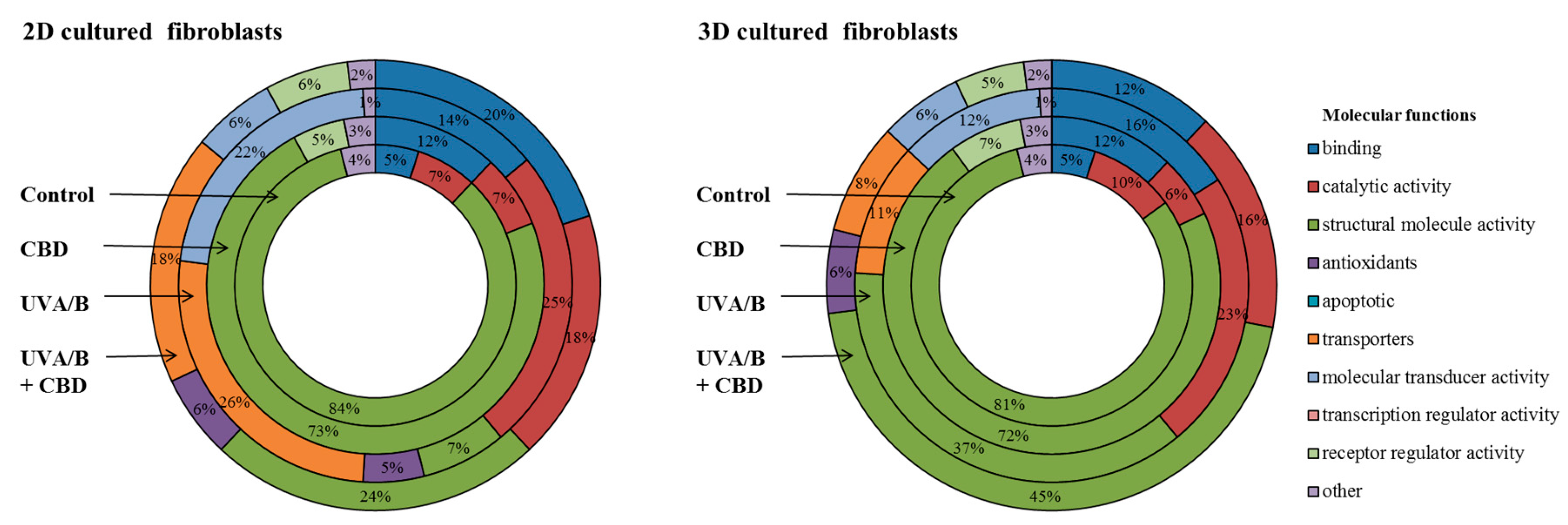
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−) Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Jhawar, N.; Schoenberg, E.; Wang, J.V.; Saedi, N. The growing trend of cannabidiol in skincare products. Clin. Dermatol. 2018, 37, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Briggs, G.H. Cannabidiol, a non-psychoactive marijuana component, exhibits antioxidant and neuroprotective effects in neuronal Alzheimer’s cell model SH-5Y. FASEB J. 2018, 32, 552–556. [Google Scholar]
- Li, H.; Kong, W.; Chambers, C.R.; Yu, D.; Ganea, D.; Tuma, R.F.; Ward, S.J. The non-psychoactive phytocannabinoid cannabidiol (CBD) attenuates pro-inflammatory mediators, T cell infiltration, and thermal sensitivity following spinal cord injury in mice. Cell Immunol. 2018, 329, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar] [CrossRef]
- DaSilva, V.K.; de Freitas, B.S.; Garcia, R.C.L.; Monteiro, R.T.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.S.; Schröder, N. Antiapoptotic effects of cannabidiol in an experimental model of cognitive decline induced by brain iron overload. Transl. Psychiatry 2018, 8, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Chatelain, E.; Gabard, B.; Surber, C. Skin penetration and sun protection factor of five UV filters: Effect of the vehicle. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 28–35. [Google Scholar] [CrossRef]
- Bissell, M.J. Architecture Is the Message: The role of extracellular matrix and 3-D structure in tissue-specific gene expression and breast cancer. Pezcoller Found J. 2007, 16, 2–17. [Google Scholar]
- Sun, T.; Jackson, S.; Haycock, J.W.; MacNeil, S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J. Biotechnol. 2006, 122, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Sommariva, M.; Limonta, P.; Gagliano, N. Epithelial-To-Mesenchymal Transition Markers and CD44 Isoforms Are Differently Expressed in 2D and 3D Cell Cultures of Prostate Cancer Cells. Cells 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Wójcik, P.; Skrzydlewska, E. Proteomic plasma profile of psoriatic patients. J. Pharm. Biomed. Anal. 2018, 155, 185–193. [Google Scholar] [CrossRef]
- Carini, M.; Aldini, G.; Facino, R.M. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom. Rev. 2004, 23, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Miller, I.; Hummel, K.; née Ondrovics, M.N.; Schlosser, S.; Walter, I. Comparative proteome analysis of monolayer and spheroid culture of canine osteosarcoma cells. J. Proteom. 2018, 177, 124–136. [Google Scholar] [CrossRef]
- Meinhardt, M.; Krebs, R.; Anders, A.; Heinrich, U.; Tronnier, H. Wavelength-dependent penetration depths of ultraviolet radiation in human skin. J. Biomed. Opt. 2008, 13, 0440301–0440305. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Kogan, N.M.; Melamed, E.; Wasserman, E.; Raphael, B.; Breuer, A.; Stok, K.S.R.; Escudero, A.V.; Baraghithy, S.; Attar-Namdar, M.; Friedlander-Barenboim, S. Cannabidiol, a Major Non-Psychotropic Cannabis Constituent Enhances Fracture Healing and Stimulates Lysyl Hydroxylase Activity in Osteoblasts. J. Bone Miner. Res. 2015, 30, 1905–1913. [Google Scholar] [CrossRef]
- Meshel, A.S.; Wei, Q.; Adelstein, R.S.; Sheetz, M.P. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat. Cell Biol. 2005, 7, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Serebriiskii, I.; Castelló-Cros, R.; Lamb, A.; Golemis, E.A.; Cukierman, E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol. 2008, 27, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Ghibaudo, M.; Trichet, L.; Le Digabel, J.; Richert, A.; Hersen, P.; Ladoux, B. Substrate topography induces a crossover from 2D to 3D behavior in fibroblast migration. Biophys. J. 2009, 97, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.; Golan, H.; Schiby, G.; PriChen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. 2016, 23, S15–S22. [Google Scholar] [PubMed]
- El-Remessy, A.B.; Tang, Y.; Zhu, G.; Matragoon, S.; Khalifa, Y.; Liu, E.K.; Liu, J.Y.; Hanson, E.; Mian, S.; Fatteh, N.; et al. Neuroprotective effects of cannabidiol in endotoxin-induced uveitis: Critical role of p38 MAPK activation. Mol. Vis. 2008, 14, 2190–2203. [Google Scholar] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Mittal, R.; Chaudhry, N.; Mukherjee, T.K. Targeting breast cancer cell signaling molecules PI3K and Akt by phytochemicals Cannabidiol, Nimbin and Acetogenin: An in silico approach. J. Biomed. 2018, 3, 60–63. [Google Scholar] [CrossRef][Green Version]
- Giacoppo, S.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia 2017, 116, 77–84. [Google Scholar] [CrossRef]
- Lanza Cariccio, V.; Scionti, D.; Raffa, A.; Iori, R.; Pollastro, F.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Treatment of Periodontal Ligament Stem Cells with MOR and CBD Promotes Cell Survival and Neuronal Differentiation via the PI3K/Akt/mTOR Pathway. Int. J. Mol. Sci. 2018, 19, 2341. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Sandalcioglu, E.; Karsak, M. Cannabinoids in glioblastoma therapy: New applications for old drugs. Front. Mol. Neurosci. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs. 3D culture reveals differences in AKT–mTOR–S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Bauvy, C.; Tonelli, G.; Yue, W.; Delomenie, C.; Nicolas, V.; Zhu, Y.; Domergue, V.; Marin-Esteban, V.; Tharinger, H.; et al. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene 2013, 32, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. WIREs Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef] [PubMed]
- Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front. Physiol. 2016, 7, 1–17. [Google Scholar]
- Sánchez-Mendoza, M.E.; López-Lorenzo, Y.; Cruz-Antonio, L.; Matus-Meza, A.S.; Sánchez-Mendoza, Y.; Arrieta, J. Gastroprotection of Calein D against Ethanol-Induced Gastric Lesions in Mice: Role of Prostaglandins, Nitric Oxide and Sulfhydryls. Molecules 2019, 24, 622. [Google Scholar]
- Lehrke, M.; Lazar, M.A. The many faces of PPARγ. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef]
- Jouzeau, J.Y.; Moulin, D.; Koufany, M.; Sebillaud, S.; Bianchi, A.; Netter, P. Pathophysiological relevance of peroxisome proliferators activated receptors (PPAR) to joint diseases-the pro and con of agonists. J. Soc. Biol. 2008, 202, 289–312. [Google Scholar] [CrossRef]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br. J. Pharmacol. 2016, 173, 815–825. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhao, Q.; Liu, H.; Guo, Y.; Xu, H. PPAR-α agonist WY-14643 inhibits LPS-induced inflammation in synovial fibroblasts via NF-kB pathway. J. Mol. Neurosci. 2016, 59, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A. Effects of antiepileptic drugs on mitochondrial functions, morphology, kinetics, biogenesis, and survival. Epilepsy Res. 2017, 136, 5–11. [Google Scholar] [CrossRef] [PubMed]
- DeMorrow, S.; Francis, H.; Gaudio, E.; Ueno, Y.; Venter, J.; Onori, P.; Franchitto, A.; Vaculin, B.; Vaculin, S.; Alpini, G. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G506–G519. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Rudd, L.P.; Kabler, S.L.; Morrow, C.S.; Townsend, A.J. Enhanced glutathione depletion, protein adduct formation, and cytotoxicity following exposure to 4-hydroxy-2-nonenal (HNE) in cells expressing human multidrug resistance protein-1 (MRP1) together with human glutathione S-transferase-M1 (GSTM1). Chem. Biol. Interact. 2011, 194, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, E.; D’Angelo, B.; Cristiano, L.; Di Giacomo, E.; Fanelli, F.; Moreno, S.; Cecconi, F.; Fidoamore, A.; Antonosante, A.; Falcone, R.; et al. Involvement of peroxisome proliferator-activated receptor β/δ (PPAR β/δ) in BDNF signaling during aging and in Alzheimer disease: Possible role of 4-hydroxynonenal (4-HNE). Cell Cycle 2014, 13, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Vindis, C.; Escargueil-Blanc, I.; Elbaz, M.; Marcheix, B.; Grazide, M.H.; Uchida, K.; Salvayre, R.; Nègre-Salvayre, A. Desensitization of platelet-derived growth factor receptor-β by oxidized lipids in vascular cells and atherosclerotic lesions: Prevention by aldehyde scavengers. Circ. Res. 2006, 98, 785–792. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Hirota, S.; Ohashi, A.; Nishida, T.; Isozaki, K.; Kinoshita, K.; Shinomura, Y.; Kitamura, Y. Gain-of-function mutations of platelet-derived growth factor receptor α gene in gastrointestinal stromal tumors. Gastroenterology 2003, 125, 660–667. [Google Scholar] [CrossRef]
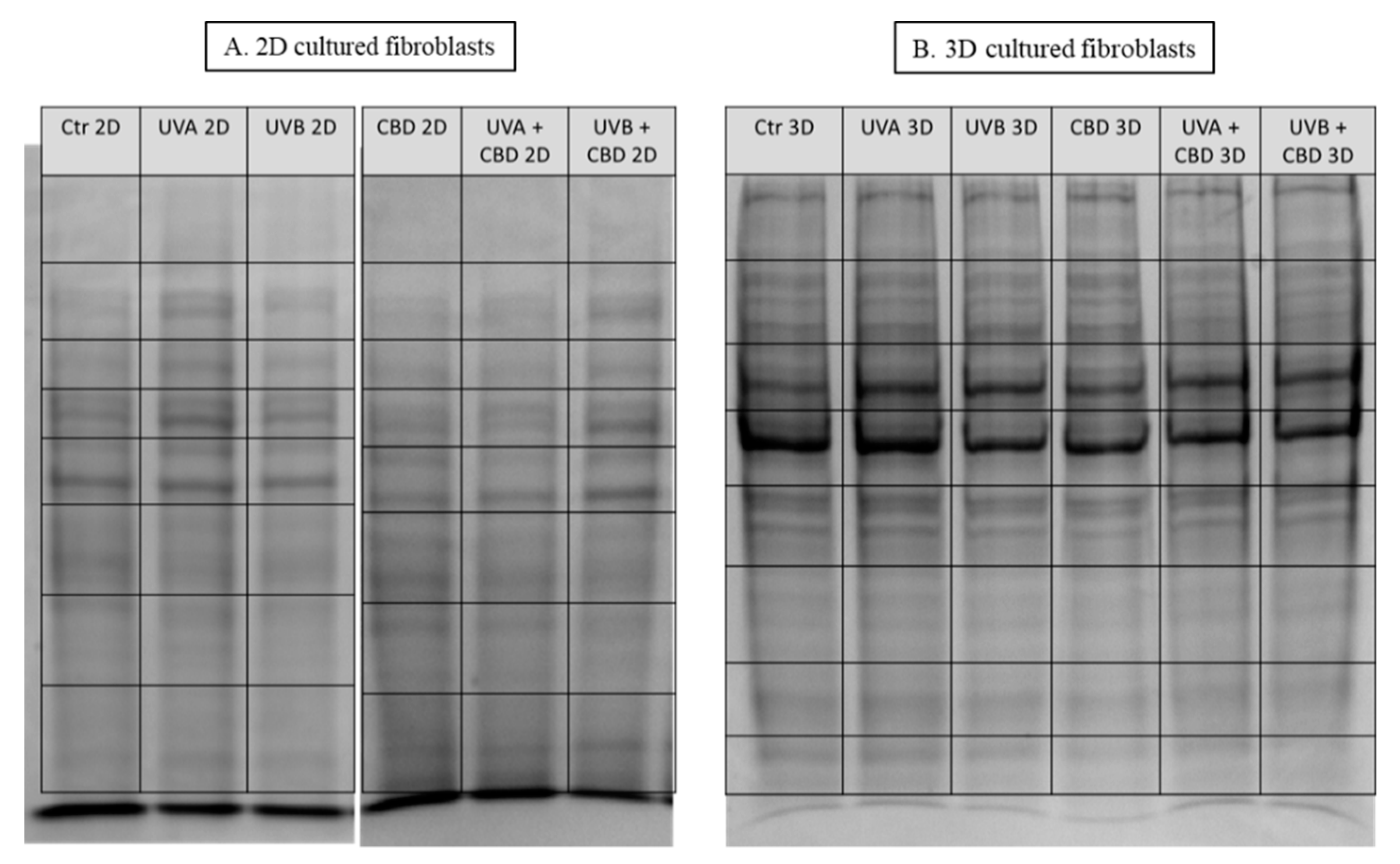
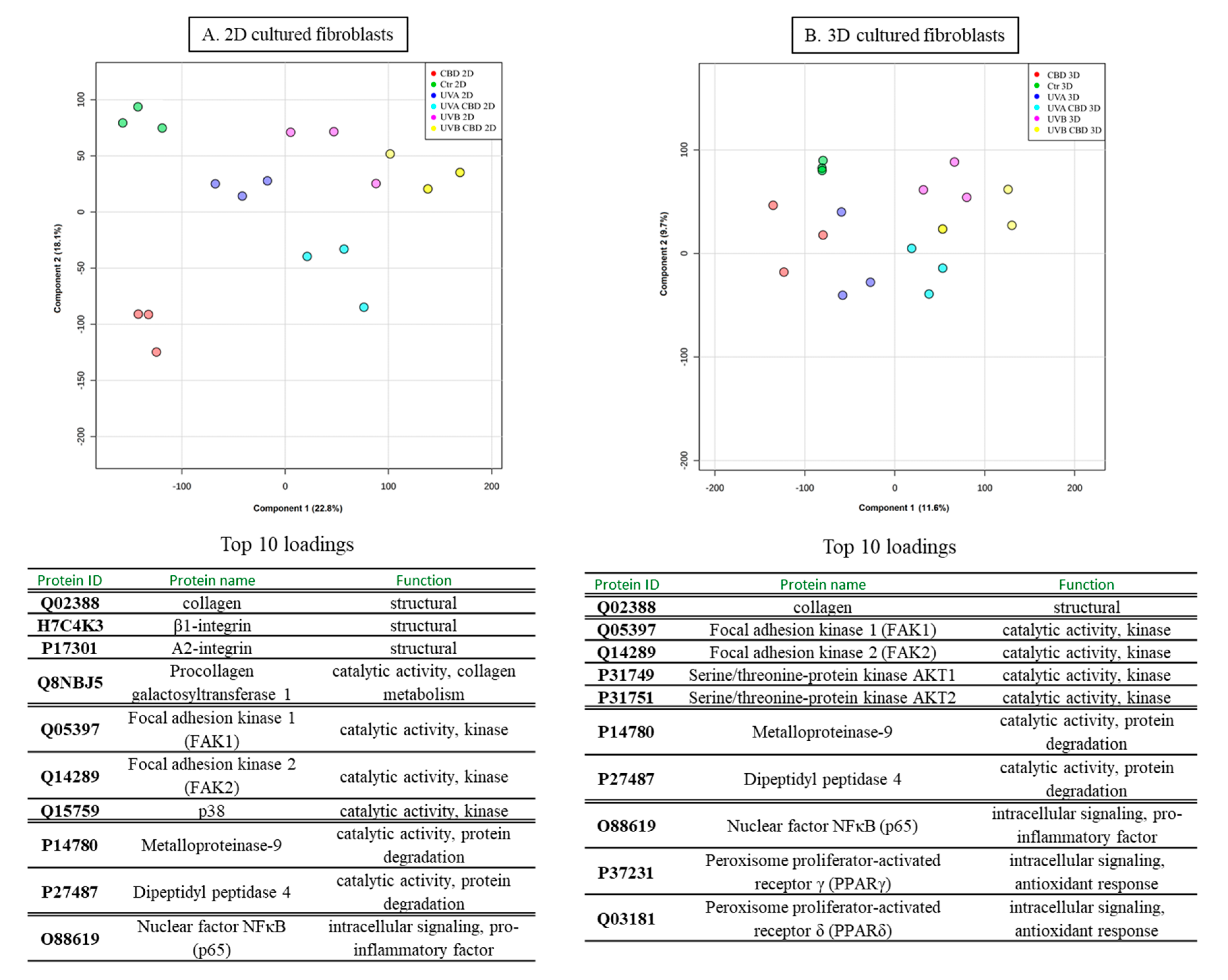
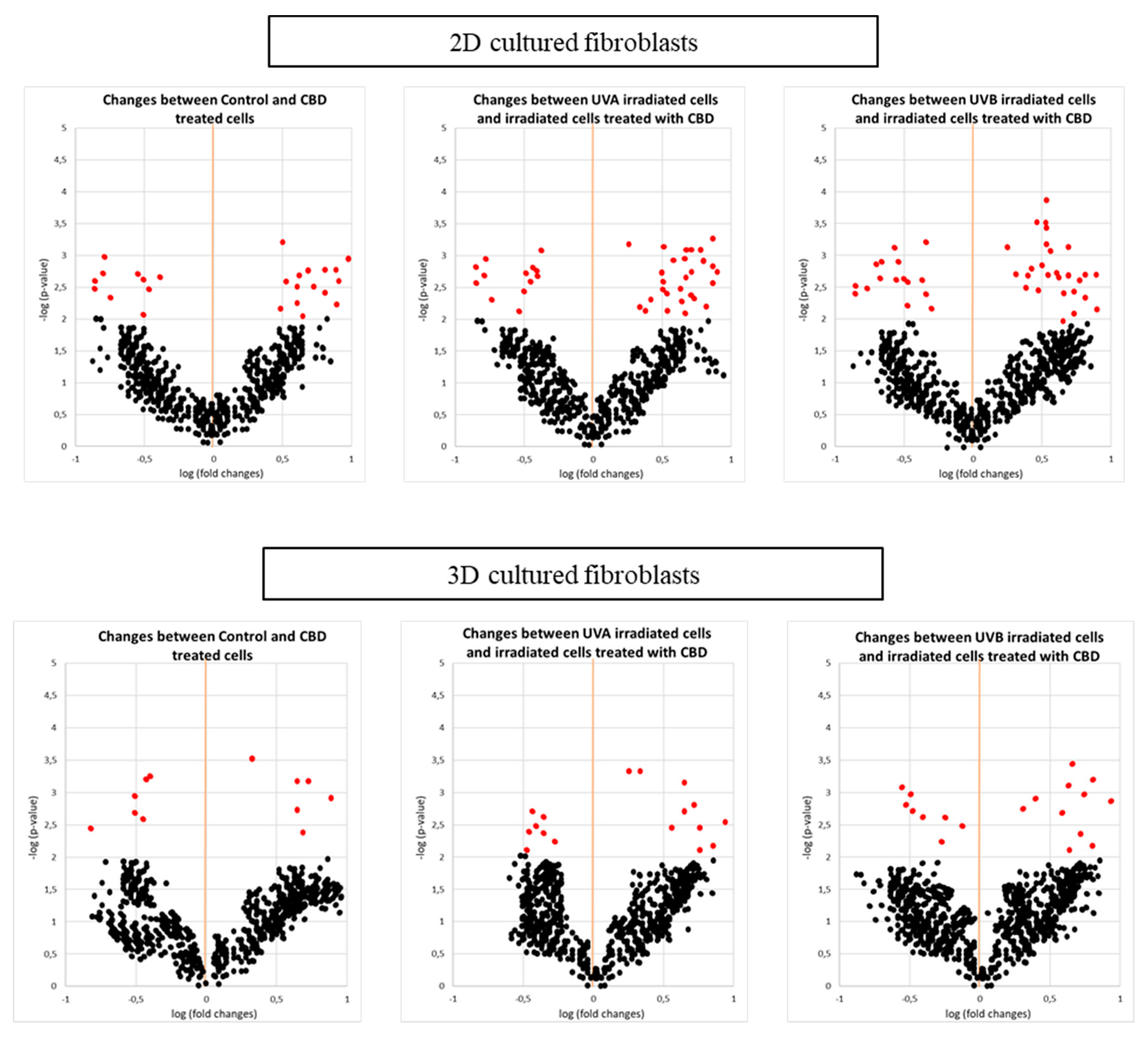
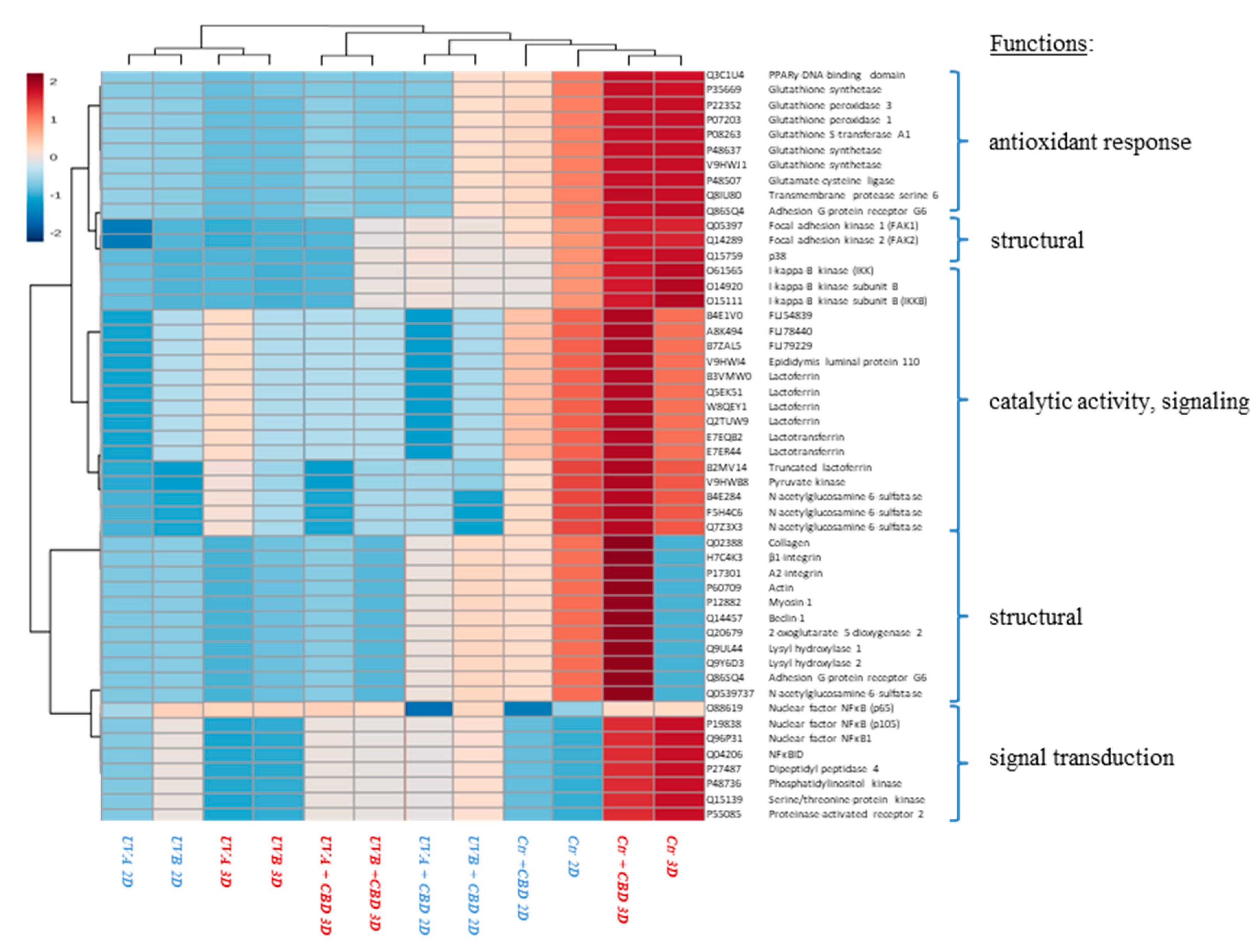
| Changes | ID | Protein Name | Biological/Molecular Functions | Fold Change between Control and CBD Treated Cells | Fold Change between UVA Irradiated Cells and Irradiated Cells Treated with CBD | Fold Change between UVB Irradiated Cells and Irradiated Cells Treated with CBD | |||
|---|---|---|---|---|---|---|---|---|---|
| 2D Cultures | 3D Cultures | 2D Cultures | 3D Cultures | 2D Cultures | 3D Cultures | ||||
| INCREASE | Q02388 | collagen | structural | 3.7 | 2.1 | 4.1 | 2.3 | 4.0 | 2.7 |
| H7C4K3 | β1-integrin | structural | 6.1 | 4.5 | 4.0 | ||||
| P17301 | A2-integrin | structural | 4.9 | 6.1 | 5.4 | ||||
| P60709 | Actin | structural | 3.3 | 4.3 | 4.0 | ||||
| P12882 | Myosin-1 | structural | 3.6 | 3.9 | 3.5 | ||||
| Q14457 | Beclin-1 | structural | 6.2 | 6.1 | 6.7 | ||||
| Q20679 | 2-oxoglutarate 5-dioxygenase 2 | structural, collagen synthesis | 5.1 | ||||||
| Q9UL44 | Lysyl hydroxylase 1 | structural, collagen synthesis | 3.8 | 2.7 | |||||
| Q9Y6D3 | Lysyl hydroxylase 2 | structural, collagen synthesis | 4.3 | 3.1 | |||||
| Q86SQ4 | Adhesion G-protein coupled receptor G6 | structural, collagen binding | 4.2 | ||||||
| Q05397 | Focal adhesion kinase 1 (FAK1) | catalytic activity, kinase | 3.8 | 6.1 | 2.2 | 4.0 | 2.1 | 4.1 | |
| Q14289 | Focal adhesion kinase 2 (FAK2) | catalytic activity, kinase | 4.1 | 3.1 | 5.7 | 3.7 | 5.4 | ||
| Q15759 | p38 | catalytic activity, kinase | 6.4 | 8.1 | 6.1 | 9.0 | |||
| P31749 | Serine/threonine-protein kinase AKT1 | catalytic activity, kinase | 3.2 | 4.5 | 3.3 | 6.1 | 3.3 | 5.4 | |
| P31751 | Serine/threonine-protein kinase AKT2 | catalytic activity, kinase | 4.2 | 3.6 | 4.3 | 3.4 | 4.0 | ||
| Q9H8T0 | AKT-interacting protein (AktIP) | catalytic activity, kinase | 2.4 | 2.3 | |||||
| P27986 | Phosphoinositide 3-kinase (PI3K) | catalytic activity, kinase | 4.1 | 4.0 | 3.1 | 5.4 | 3.7 | 5.1 | |
| Q8IW41 | MAP kinase-activated protein kinase 5 (MAPKAPK5) | catalytic activity, kinase | 3.8 | 3.4 | |||||
| Q04206 | NF-kappa-B-repressing factor (NKRF) | intracellular signaling, anti-inflammatory factor | 4.3 | 3.9 | |||||
| P37231 | Peroxisome proliferator-activated receptor γ (PPARγ) | intracellular signaling, antioxidant response | 2.9 | 4.2 | 2.4 | 4.7 | |||
| Q03181 | Peroxisome proliferator-activated receptor δ (PPARδ) | intracellular signaling, antioxidant response | 3.4 | 5.1 | 3.7 | 5.2 | |||
| Q9UBK2 | Peroxisome proliferator-activated receptor gamma coactivator 1A (PPARGC1A) | intracellular signaling, antioxidant response | 2.6 | 4.9 | |||||
| D2KUA6 | NR1C3 (PPARγ subunit) | intracellular signaling, antioxidant response | 4.9 | 3.9 | |||||
| Q3C1U4 | PPARγ-DNA-binding domain-interacting protein 1β (PDIP1β) | intracellular signaling, antioxidant response | 3.3 | 3.1 | |||||
| P35669 | γ-Glutamate cysteine ligase A1 (γ-GCS A1) | catalytic activity, antioxidant response | 3.4 | 3.6 | |||||
| P22352 | Glutathione peroxidase 3 (GSH -Px3) | catalytic activity, antioxidant response | 6.2 | 3.7 | 2.0 | ||||
| P07203 | Glutathione peroxidase 1 (GSH -Px1) | catalytic activity, antioxidant response | 5.1 | 4.8 | 2.2 | ||||
| P08263 | Glutathione S-transferase A1 (GSTA1) | catalytic activity, antioxidant response | 3.2 | 2.1 | |||||
| P48637 | Glutathione synthetase | catalytic activity, antioxidant response | 3.2 | 3.8 | 4.4 | ||||
| V9HWJ1 | Glutathione synthetase, HEL-S-64p | catalytic activity, antioxidant response | 4.1 | 3.7 | |||||
| P48507 | Glutamate-cysteine ligase | catalytic activity, antioxidant response | 8.9 | ||||||
| Q8IU80 | Transmembrane protease serine 6 | catalytic activity, protease | 6.7 | ||||||
| P04083 | Annexin A1 | structural, transport | 4.2 | ||||||
| DECREASE | Q8NBJ5 | Procollagen galactosyltransferase 1 | catalytic activity, collagen metabolism | 0.3 | 0.4 | 0.2 | 0.2 | ||
| Q15582 | Transforming growth factor-beta-induced protein ig-h3 | structural, cell-collagen interactions | 0.2 | ||||||
| Q06828 | Fibromodulin | structural | 0.4 | ||||||
| P16671 | Platelet glycoprotein 4 | structural, cell-collagen interactions | 0.2 | 0.4 | 0.2 | 0.5 | 0.3 | 0.6 | |
| O14495 | Phospholipid phosphatase 3 | catalytic activity, phosphatase | 0.3 | 0.3 | 0.2 | ||||
| P45452 | Metalloproteinase-13 | catalytic activity, protein degradation | 0.3 | 0.4 | |||||
| P14780 | Metalloproteinase-9 | catalytic activity, protein degradation | 0.2 | 0.3 | 0.3 | 0.4 | 0.4 | 0.5 | |
| Q12884 | Prolyl endopeptidase FAP | catalytic activity, protein degradation | 0.3 | 0.4 | 0.4 | ||||
| P25774 | Cathepsin S | catalytic activity, protein degradation | 0.2 | 0.2 | |||||
| O61565 | I-kappa-B kinase (IKK) | catalytic activity, kinase, pro-inflammatory factor | 0.2 | 0.5 | 0.2 | 0.5 | |||
| O14920 | I-kappa-B kinase subunit B (IKKB) | catalytic activity, kinase, pro-inflammatory factor | 0.4 | 0.6 | 0.3 | ||||
| O15111 | I-kappa-B kinase subunit A (IKKA) | catalytic activity, kinase, pro-inflammatory factor | 0.2 | 0.4 | 0.3 | ||||
| O88619 | Nuclear factor NFκB (p65) | intracellular signaling, pro-inflammatory factor | 0.3 | 0.5 | 0.2 | 0.4 | |||
| P19838 | Nuclear factor NFκB (p105) | intracellular signaling, pro-inflammatory factor | 0.3 | 0.3 | 0.4 | ||||
| Q96P31 | Nuclear factor NFκB1 | intracellular signaling, pro-inflammatory factor | 0.2 | 0.2 | |||||
| Q04206 | NFκBID | intracellular signaling, pro-inflammatory factor | 0.3 | 0.3 | |||||
| P27487 | Dipeptidyl peptidase 4 | catalytic activity, protein degradation | 0.2 | 0.5 | 0.4 | 0.5 | 0.3 | 0.4 | |
| P48736 | Phosphatidylinositol 4,5-bisphosphate 3-kinase | catalytic activity, kinase | 0.2 | 0.8 | |||||
| Q15139 | Serine/threonine-protein kinase | catalytic activity, kinase | 0.3 | 0.6 | |||||
| P55085 | Proteinase-activated receptor 2 | catalytic activity, protein degradation | 0.4 | ||||||
| P35354 | Prostaglandin G/H synthase 2 | catalytic activity, lipid metabolism | 0.5 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gęgotek, A.; Atalay, S.; Domingues, P.; Skrzydlewska, E. The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts following UVA or UVB Irradiation in 2D and 3D Cell Cultures. Cells 2019, 8, 995. https://doi.org/10.3390/cells8090995
Gęgotek A, Atalay S, Domingues P, Skrzydlewska E. The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts following UVA or UVB Irradiation in 2D and 3D Cell Cultures. Cells. 2019; 8(9):995. https://doi.org/10.3390/cells8090995
Chicago/Turabian StyleGęgotek, Agnieszka, Sinemyiz Atalay, Pedro Domingues, and Elżbieta Skrzydlewska. 2019. "The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts following UVA or UVB Irradiation in 2D and 3D Cell Cultures" Cells 8, no. 9: 995. https://doi.org/10.3390/cells8090995
APA StyleGęgotek, A., Atalay, S., Domingues, P., & Skrzydlewska, E. (2019). The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts following UVA or UVB Irradiation in 2D and 3D Cell Cultures. Cells, 8(9), 995. https://doi.org/10.3390/cells8090995






