Dynamic Interactions between Autophagosomes and Lipid Droplets in Chlamydomonas reinhardtii
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Cultivation
2.2. Vector Construction
2.3. Generation of mCherry-ATG8 Transgenic Lines
2.4. Protein Isolation and Western Blot Analysis
2.5. Expression Analysis by Real-Time RT-PCR
2.6. Confocal Microscopic Analysis
2.7. Transmission Electron Microscopy (TEM) Analysis
2.8. Flow Cytometry Analysis
2.9. Quantification of Autophagic Structures
2.10. Statistical Analyses
3. Results
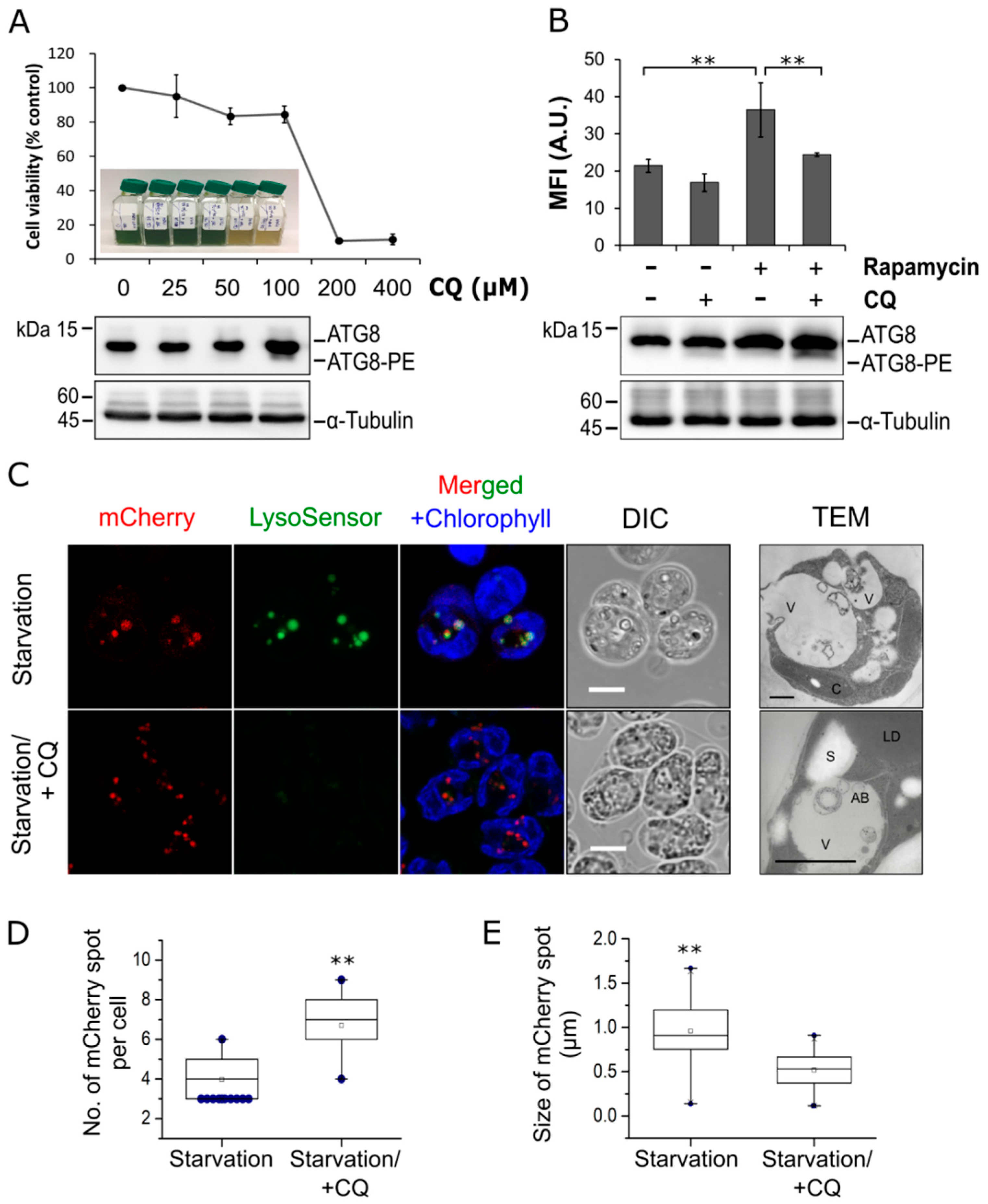
3.1. Autophagic Responses in C. reinhardtii Transgenic Lines Expressing mCherry-ATG8
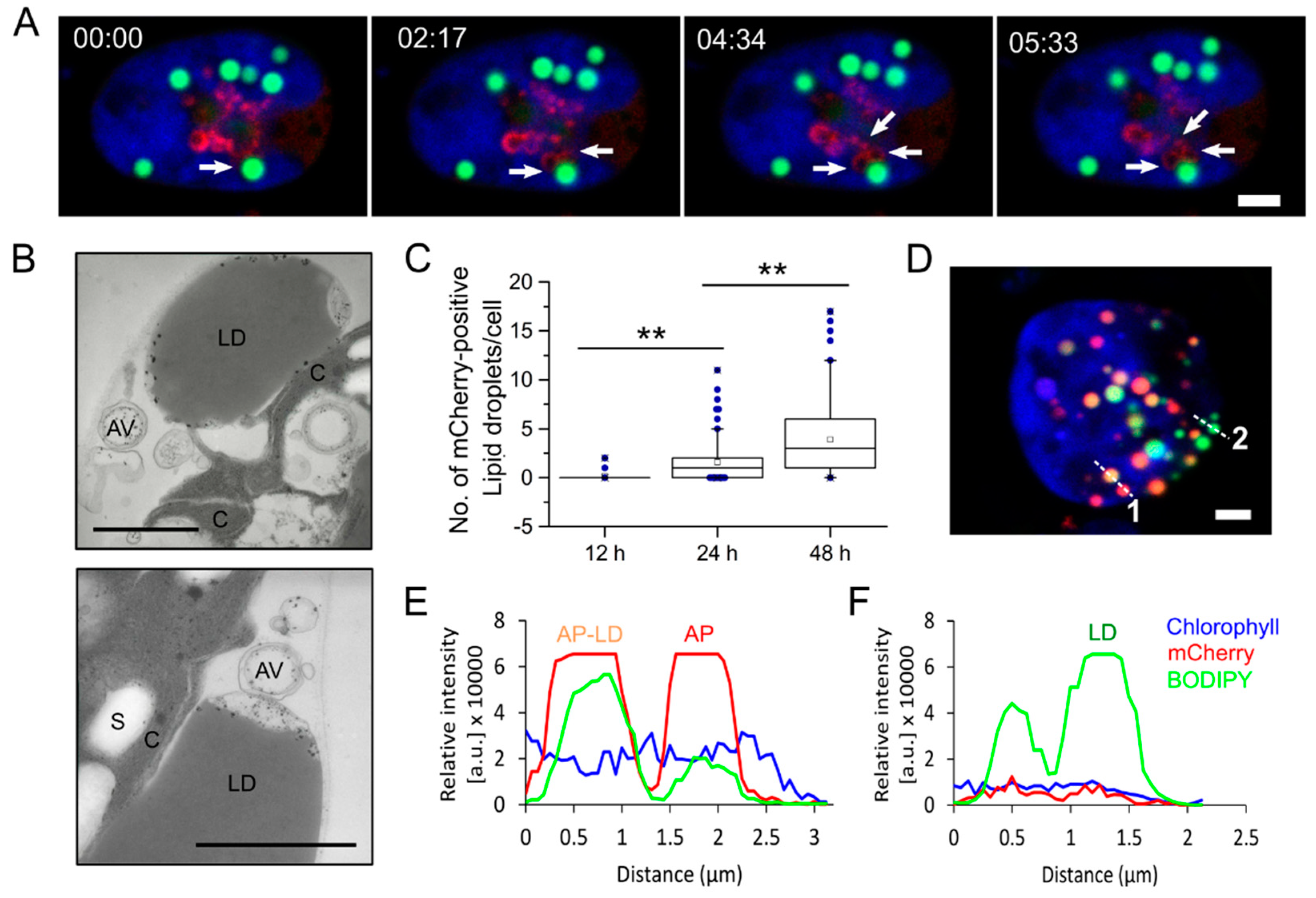
3.2. Live Cell Imaging Revealed Interactions between Autophagosomes and Lipid Droplets in C. reinhardtii
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A Review on the Assessment of Stress Conditions for Simultaneous Production of Microalgal Lipids and Carotenoids. Front. Microbiol. 2016, 7, 546–565. [Google Scholar] [CrossRef]
- Cho, D.-H.; Ramanan, R.; Heo, J.; Shin, D.-S.; Oh, H.-M.; Kim, H.-S. Influence of limiting factors on biomass and lipid productivities of axenic Chlorella vulgaris in photobioreactor under chemostat cultivation. Bioresour. Technol. 2016, 211, 367–373. [Google Scholar] [CrossRef]
- Kakarla, R.; Choi, J.-W.; Yun, J.-H.; Kim, B.-H.; Heo, J.; Lee, S.; Cho, D.-H.; Ramanan, R.; Kim, H.-S. Application of high-salinity stress for enhancing the lipid productivity of Chlorella sorokiniana HS1 in a two-phase process. J. Microbiol. 2018, 56, 56–64. [Google Scholar] [CrossRef]
- Cho, K.; Cho, D.-H.; Heo, J.; Kim, U.; Lee, Y.J.; Choi, D.-Y.; Kim, H.-S. Nitrogen modulation under chemostat cultivation mode induces biomass and lipid production by Chlorella vulgaris and reduces antenna pigment accumulation. Bioresour. Technol. 2019, 281, 118–125. [Google Scholar] [CrossRef]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Bassham, D.C.; Laporte, M.; Marty, F.; Moriyasu, Y.; Ohsumi, Y.; Olsen, L.J.; Yoshimoto, K. Autophagy in development and stress responses of plants. Autophagy 2006, 2, 2–11. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Michaeli, S.; Galili, G.; Genschik, P.; Fernie, A.R.; Avin-Wittenberg, T. Autophagy in Plants—What’s New on the Menu? Trends Plant Sci. 2015, 21, 134–144. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.; Couso, I.; Heredia-Martínez, L.; Crespo, J. Monitoring Autophagy in the Model Green Microalga Chlamydomonas reinhardtii. Cells 2017, 6, 36. [Google Scholar] [CrossRef]
- Crespo, J.L.; Díaz-Troya, S.; Florencio, F.J. Inhibition of Target of Rapamycin Signaling by Rapamycin in the Unicellular Green Alga Chlamydomonas reinhardtii. Plant Physiol. 2005, 139, 1736–1749. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Florencio, F.J.; Crespo, J.L. Inhibition of Target of Rapamycin Signaling and Stress Activate Autophagy in Chlamydomonas reinhardtii. Plant Physiol. 2010, 152, 1874–1888. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Couso, I.; Crespo, J.L. Carotenoid deficiency triggers autophagy in the model green alga Chlamydomonas reinhardtii. Autophagy 2012, 8, 376–388. [Google Scholar] [CrossRef]
- Pérez-Martín, M.; Pérez-Pérez, M.E.; Lemaire, S.D.; Crespo, J.L. Oxidative Stress Contributes to Autophagy Induction in Response to Endoplasmic Reticulum Stress in Chlamydomonas reinhardtii. Plant Physiol. 2014, 166, 997–1008. [Google Scholar] [CrossRef]
- Pérez-Martín, M.; Blaby-Haas, C.E.; Pérez-Pérez, M.E.; Andrés-Garrido, A.; Blaby, I.K.; Merchant, S.S.; Crespo, J.L. Activation of Autophagy by Metals in Chlamydomonas reinhardtii. Eukaryot. Cell 2015, 14, 964–973. [Google Scholar] [CrossRef]
- Couso, I.; Pérez-Pérez, M.E.; Martínez-Force, E.; Kim, H.-S.; He, Y.; Umen, J.G.; Crespo, J.L.; Bozhkov, P. Autophagic flux is required for the synthesis of triacylglycerols and ribosomal protein turnover in Chlamydomonas. J. Exp. Bot. 2017, 69, 1355–1367. [Google Scholar] [CrossRef]
- Pugkaew, W.; Meetam, M.; Ponpuak, M.; Yokthongwattana, K.; Pokethitiyook, P. Role of autophagy in triacylglycerol biosynthesis in Chlamydomonas reinhardtii revealed by chemical inducer and inhibitors. J. Appl. Phycol. 2017, 30, 15–22. [Google Scholar] [CrossRef]
- Ramanan, R.; Tran, Q.-G.; Cho, D.-H.; Jung, J.-E.; Kim, B.-H.; Shin, S.-Y.; Choi, S.-H.; Liu, K.-H.; Kim, D.-S.; Lee, S.-J.; et al. The Ancient Phosphatidylinositol 3-Kinase Signaling System Is a Master Regulator of Energy and Carbon Metabolism in Algae. Plant Physiol. 2018, 177, 1050–1065. [Google Scholar] [CrossRef]
- Tran, Q.-G.; Cho, K.; Kim, U.; Yun, J.-H.; Cho, D.-H.; Heo, J.; Park, S.-B.; Kim, J.W.; Lee, Y.J.; Ramanan, R.; et al. Enhancement of β-carotene production by regulating the autophagy-carotenoid biosynthesis seesaw in Chlamydomonas reinhardtii. Bioresour. Technol. 2019, 292, 121937. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, J.; Wu, Q. Autophagy-like processes are involved in lipid droplet degradation in Auxenochlorella protothecoides during the heterotrophy-autotrophy transition. Front. Plant Sci. 2014, 5, 400. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 2007, 130, 165–178. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo-Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Harris, E.H. Chapter 8—Chlamydomonas in the Laboratory. In The Chlamydomonas Sourcebook, 2nd ed.; Harris, E.H., Stern, D.B., Witman, G.B., Eds.; Academic Press: London, UK, 2009; pp. 241–302. [Google Scholar]
- Rasala, B.A.; Barrera, D.J.; Ng, J.; Plucinak, T.M.; Rosenberg, J.N.; Weeks, D.P.; Oyler, G.A.; Peterson, T.C.; Haerizadeh, F.; Mayfield, S.P. Expanding the spectral palette of fluorescent proteins for the green microalga Chlamydomonas reinhardtii. Plant. J. 2013, 74, 545–556. [Google Scholar] [CrossRef]
- Klionsky, D.J. For the last time, it is GFP-Atg8, not Atg8-GFP (and the same goes for LC3). Autophagy 2011, 7, 1093–1094. [Google Scholar] [CrossRef]
- Molnar, A.; Bassett, A.; Thuenemann, E.; Schwach, F.; Karkare, S.; Ossowski, S.; Weigel, D.; Baulcombe, D. Highly specific gene silencing by artificial microRNAs in the unicellular alga Chlamydomonas reinhardtii. Plant. J. 2009, 58, 165–174. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Couso, I.; Crespo, J.L. The TOR Signaling Network in the Model Unicellular Green Alga Chlamydomonas reinhardtii. Biomolecules 2017, 7, 54. [Google Scholar] [CrossRef]
- Crespo, J.L.; Hall, M.N. Elucidating TOR Signaling and Rapamycin Action: Lessons from Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2002, 66, 579–591. [Google Scholar] [CrossRef]
- Kuma, A.; Matsui, M.; Mizushima, N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: Caution in the interpretation of LC3 localization. Autophagy 2007, 3, 323–328. [Google Scholar] [CrossRef]
- Galindo, F.; Burguete, M.I.; Vigara, L.; Luis, S.V.; Kabir, N.; Gavrilovic, J.; Russell, D.A. Synthetic macrocyclic peptidomimetics as tunable pH probes for the fluorescence imaging of acidic organelles in live cells. Angew. Chem. Int. Ed. 2005, 117, 6662–6666. [Google Scholar] [CrossRef]
- Gao, Q.; Goodman, J.M. The lipid droplet—A well-connected organelle. Front. Cell Dev. Biol. 2015, 3, 49. [Google Scholar] [CrossRef]
- Tran, Q.-G.; Cho, K.; Park, S.-B.; Kim, U.; Lee, Y.J.; Kim, H.-S. Impairment of starch biosynthesis results in elevated oxidative stress and autophagy activity in Chlamydomonas reinhardtii. Sci. Rep. 2019, 9, 9856. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131. [Google Scholar] [CrossRef] [PubMed]
- Bozhkov, P.V. Plant autophagy: Mechanisms and functions. J. Exp. Bot. 2018, 69, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas Genome Reveals the Evolution of Key Animal and Plant Functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gutman, B.L.; Niyogi, K.K. Chlamydomonas and Arabidopsis. A Dynamic Duo. Plant Physiol. 2004, 135, 607–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagy, P.; Varga, Á.; Kovács, A.L.; Takáts, S.; Juhász, G. How and why to study autophagy in Drosophila: It’s more than just a garbage chute. Methods 2015, 75, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef]
- Singh, R.; Cuervo, A.M. Lipophagy: Connecting Autophagy and Lipid Metabolism. Int. J. Cell Biol. 2012, 2012, 282041. [Google Scholar] [CrossRef]
- Kurusu, T.; Koyano, T.; Hanamata, S.; Kubo, T.; Noguchi, Y.; Yagi, C.; Nagata, N.; Yamamoto, T.; Ohnishi, T.; Okazaki, Y. OsATG7 is required for autophagy-dependent lipid metabolism in rice postmeiotic anther development. Autophagy 2014, 10, 878–888. [Google Scholar] [CrossRef]
- Van Zutphen, T.; Todde, V.; de Boer, R.; Kreim, M.; Hofbauer, H.F.; Wolinski, H.; Veenhuis, M.; van der Klei, I.J.; Kohlwein, S.D. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 2014, 25, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Czaja, M.J. Regulation of lipid droplets by autophagy. Trends Endocrinol. Metab. 2011, 22, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Czaja, M.J. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2012, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yu, L.; Xu, C. Dual Role for Autophagy in Lipid Metabolism in Arabidopsis. Plant Cell 2019, 31, 1598–1613. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, Q.-G.; Yoon, H.R.; Cho, K.; Lee, S.-J.; Crespo, J.L.; Ramanan, R.; Kim, H.-S. Dynamic Interactions between Autophagosomes and Lipid Droplets in Chlamydomonas reinhardtii. Cells 2019, 8, 992. https://doi.org/10.3390/cells8090992
Tran Q-G, Yoon HR, Cho K, Lee S-J, Crespo JL, Ramanan R, Kim H-S. Dynamic Interactions between Autophagosomes and Lipid Droplets in Chlamydomonas reinhardtii. Cells. 2019; 8(9):992. https://doi.org/10.3390/cells8090992
Chicago/Turabian StyleTran, Quynh-Giao, Hyang Ran Yoon, Kichul Cho, Seon-Jin Lee, José L. Crespo, Rishiram Ramanan, and Hee-Sik Kim. 2019. "Dynamic Interactions between Autophagosomes and Lipid Droplets in Chlamydomonas reinhardtii" Cells 8, no. 9: 992. https://doi.org/10.3390/cells8090992
APA StyleTran, Q.-G., Yoon, H. R., Cho, K., Lee, S.-J., Crespo, J. L., Ramanan, R., & Kim, H.-S. (2019). Dynamic Interactions between Autophagosomes and Lipid Droplets in Chlamydomonas reinhardtii. Cells, 8(9), 992. https://doi.org/10.3390/cells8090992





