Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Patients
2.3. Experimental DSS-Induced Colitis
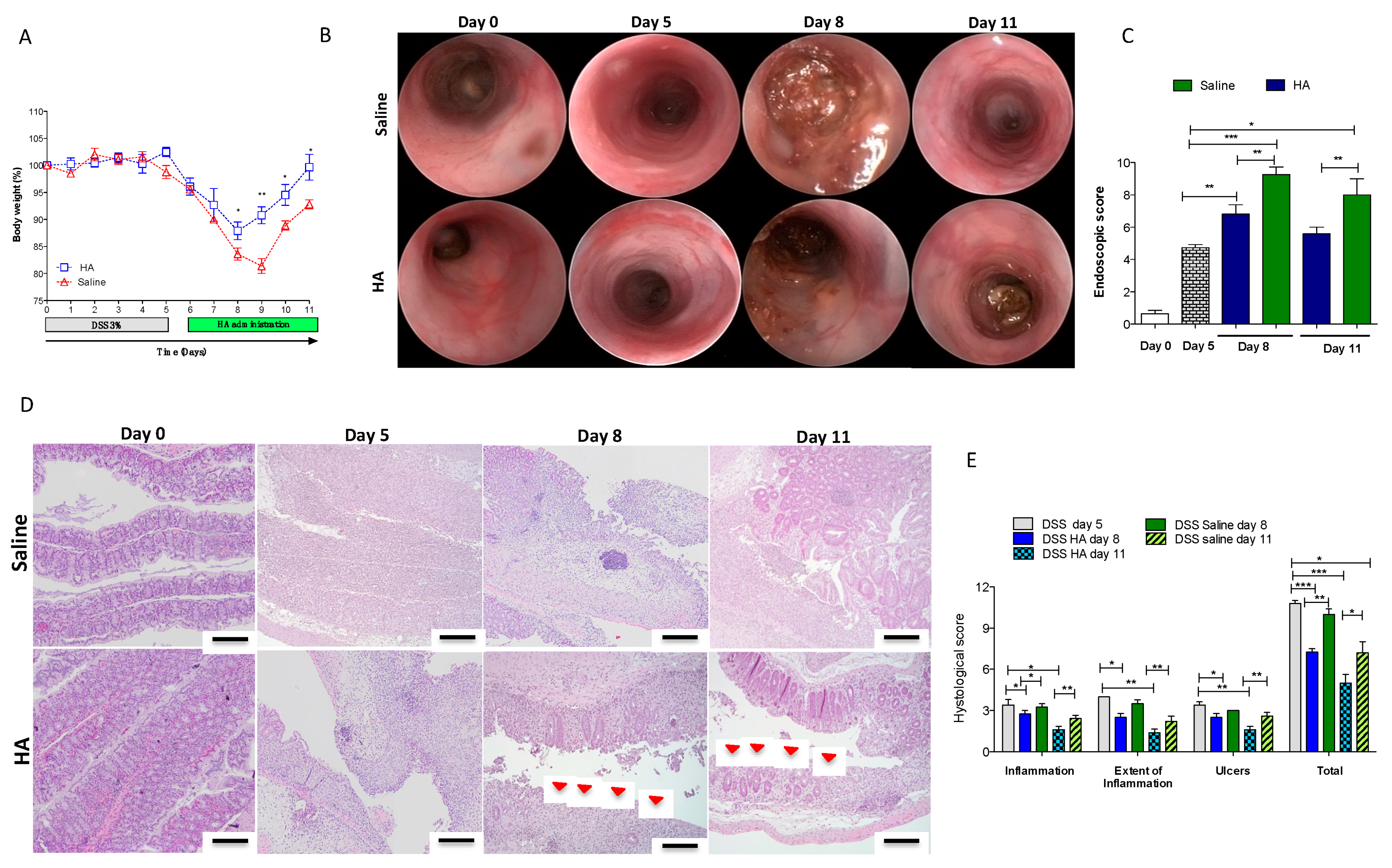
2.4. Endoscopic Assessment
2.5. Histological Analysis
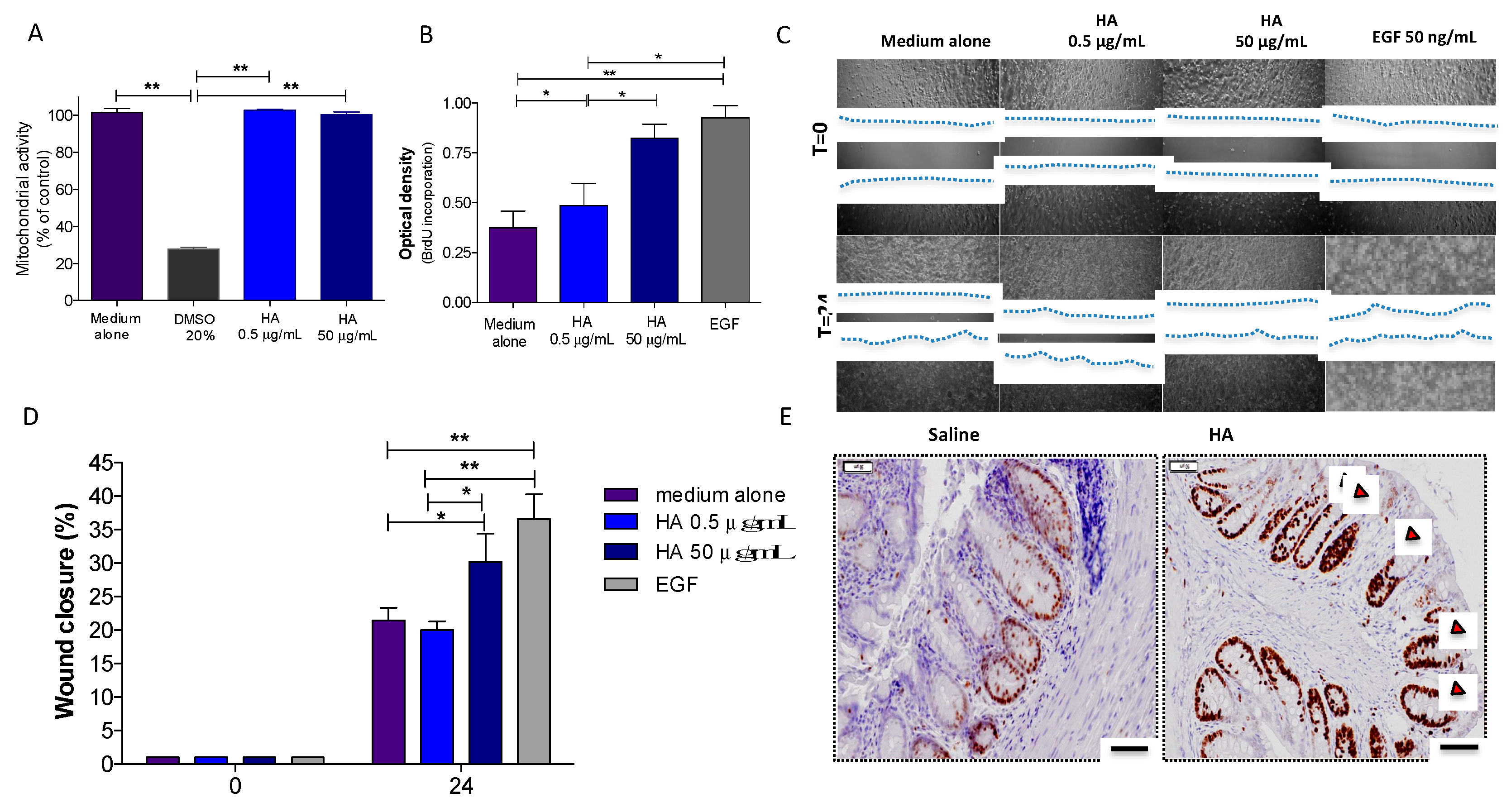
2.6. In Vivo Wound Healing
2.7. Immunohistochemical Staining
2.8. Cell Culture
2.9. In Vitro Cytotoxicity
2.10. Cell Proliferation Assay
2.11. In Vitro Wound Healing
2.12. RNA Extraction
2.13. Statistical Analysis
3. Results
3.1. HA Accelerates the Recovery Phase after Colitis Insult
3.2. HA Treatment Promoted Epithelial Cell Proliferation
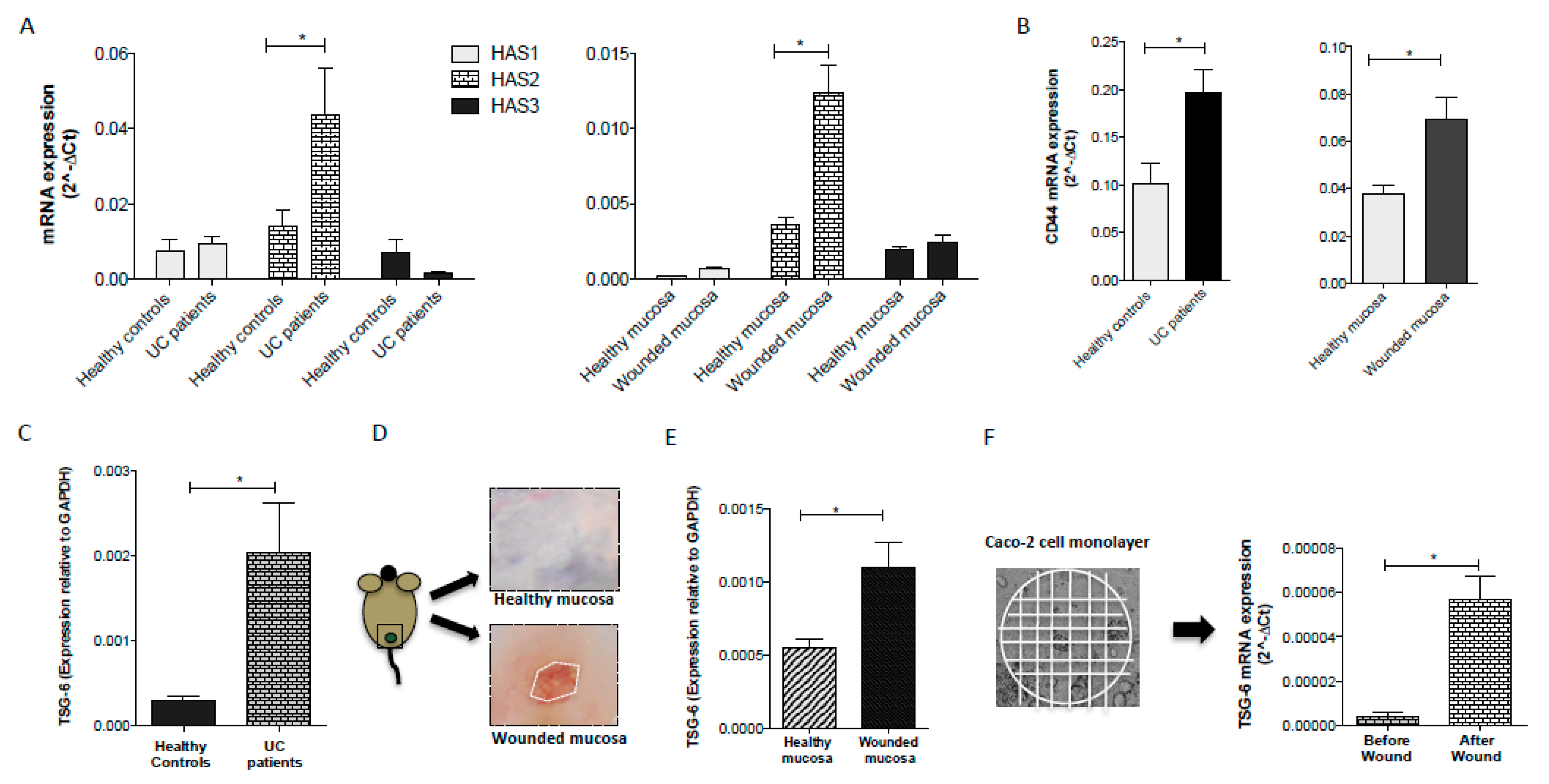
3.3. Inflammatory Conditions Modulated the Synthesis of HA and the Expression of CD44 Receptor
3.4. TSG-6 Expression is Upregulated following Intestinal Injury
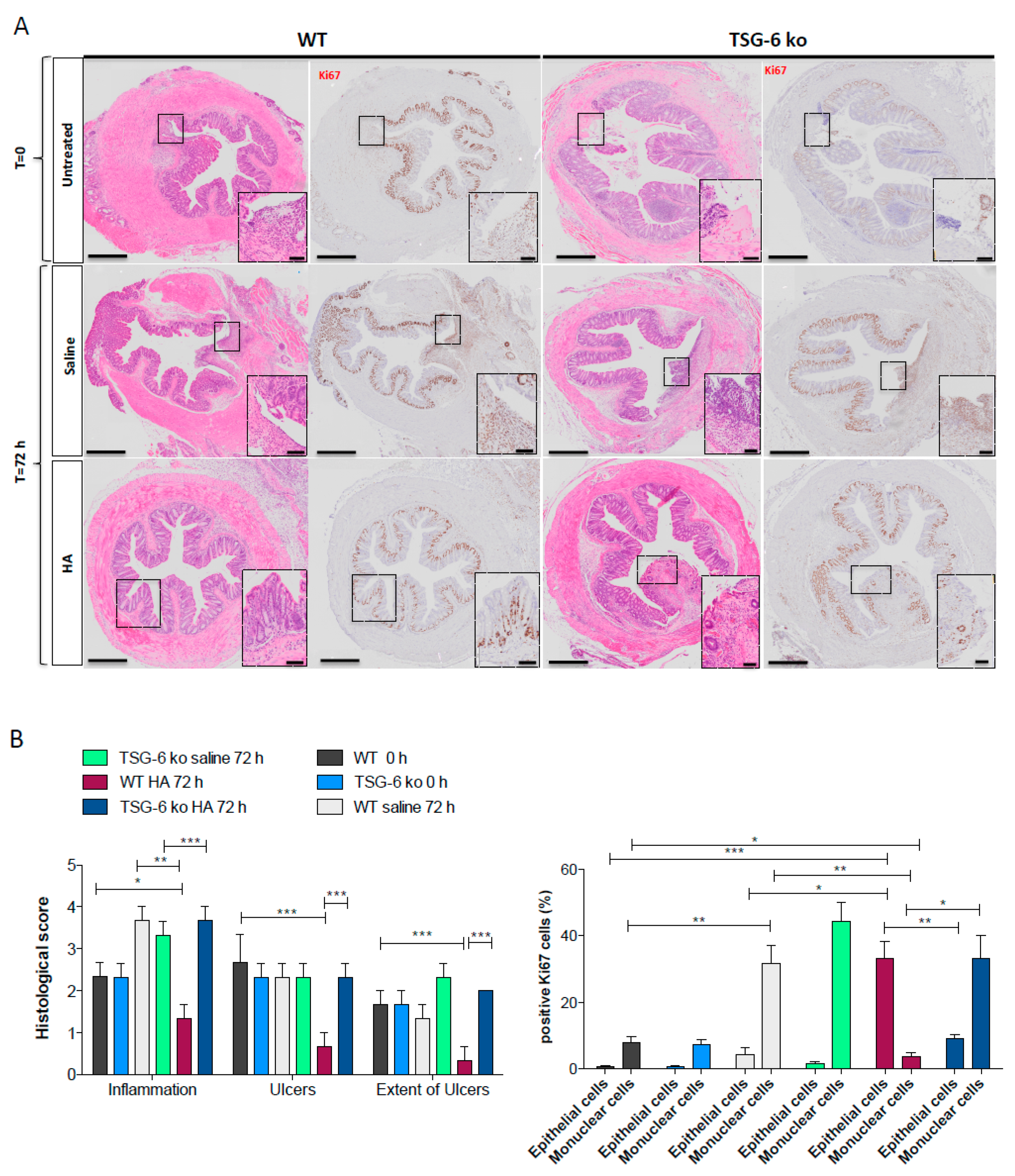
3.5. The Lack of TSG-6 Abolished the Therapeutic Effects of HA Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. The structure and function of hyaluronan: An overview. Immunol. Cell Biol. 1996, 74, A1–A7. [Google Scholar] [CrossRef] [PubMed]
- Weigel, P.H. Hyaluronan Synthase: The Mechanism of Initiation at the Reducing End and a Pendulum Model for Polysaccharide Translocation to the Cell Exterior. Int. J. Cell Biol. 2015, 2015, 367579. [Google Scholar] [CrossRef] [PubMed]
- Tammi, M.I.; Day, A.J.; Turley, E.A. Hyaluronan and homeostasis: A balancing act. J. Biol. Chem. 2002, 277, 4581–4584. [Google Scholar] [CrossRef] [PubMed]
- Garantziotis, S.; Savani, R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan in inflammatory bowel disease: Cross-linking inflammation and coagulation. Matrix Biol. 2019, 78–79, 314–323. [Google Scholar] [CrossRef]
- Wang, A.; de la Motte, C.; Lauer, M.; Hascall, V. Hyaluronan matrices in pathobiological processes. FEBS J. 2011, 278, 1412–1418. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Richter, R.P.; Baranova, N.S.; Day, A.J.; Kwok, J.C. Glycosaminoglycans in extracellular matrix organisation: Are concepts from soft matter physics key to understanding the formation of perineuronal nets? Curr. Opin. Struct. Biol. 2018, 50, 65–74. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Day, A.J.; de la Motte, C.A. Hyaluronan cross-linking: A protective mechanism in inflammation? Trends Immunol. 2005, 26, 637–643. [Google Scholar] [CrossRef]
- Richette, P.; Chevalier, X.; Ea, H.K.; Eymard, F.; Henrotin, Y.; Ornetti, P.; Sellam, J.; Cucherat, M.; Marty, M. Hyaluronan for knee osteoarthritis: An updated meta-analysis of trials with low risk of bias. RMD Open 2015, 1, e000071. [Google Scholar] [CrossRef]
- Goa, K.L.; Benfield, P. Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs 1994, 47, 536–566. [Google Scholar] [CrossRef]
- Williams, D.L.; Mann, B.K. Efficacy of a crosslinked hyaluronic acid-based hydrogel as a tear film supplement: A masked controlled study. PLoS ONE 2014, 9, e99766. [Google Scholar] [CrossRef]
- Williams, D.L.; Wirostko, B.M.; Gum, G.; Mann, B.K. Topical Cross-Linked HA-Based Hydrogel Accelerates Closure of Corneal Epithelial Defects and Repair of Stromal Ulceration in Companion Animals. Invest. Ophthalmol. Vis. Sci. 2017, 58, 4616–4622. [Google Scholar] [CrossRef]
- Voigt, J.; Driver, V.R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2012, 20, 317–331. [Google Scholar] [CrossRef]
- Fiorino, G.; Gilardi, D.; Naccarato, P.; Sociale, O.R.; Danese, S. Safety and efficacy of sodium hyaluronate (IBD98E) in the induction of clinical and endoscopic remission in subjects with distal ulcerative colitis. Dig. Liver Dis 2014, 46, 330–334. [Google Scholar] [CrossRef]
- Danese, S. Ulcerative colitis: A cinderella story. Curr Drug Targets 2011, 12, 1372. [Google Scholar] [CrossRef]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef]
- Danese, S.; Allez, M.; van Bodegraven, A.A.; Dotan, I.; Gisbert, J.P.; Hart, A.; Lakatos, P.L.; Magro, F.; Peyrin-Biroulet, L.; Schreiber, S.; et al. Unmet Medical Needs in Ulcerative Colitis: An Expert Group Consensus. Dig. Dis. 2019, 37, 266–283. [Google Scholar] [CrossRef]
- Fulop, C.; Szanto, S.; Mukhopadhyay, D.; Bardos, T.; Kamath, R.V.; Rugg, M.S.; Day, A.J.; Salustri, A.; Hascall, V.C.; Glant, T.T.; et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 2003, 130, 2253–2261. [Google Scholar] [CrossRef]
- Rugg, M.S.; Willis, A.C.; Mukhopadhyay, D.; Hascall, V.C.; Fries, E.; Fulop, C.; Milner, C.M.; Day, A.J. Characterization of complexes formed between TSG-6 and inter-alpha-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J. Biol. Chem. 2005, 280, 25674–25686. [Google Scholar] [CrossRef]
- Briggs, D.C.; Birchenough, H.L.; Ali, T.; Rugg, M.S.; Waltho, J.P.; Ievoli, E.; Jowitt, T.A.; Enghild, J.J.; Richter, R.P.; Salustri, A.; et al. Metal Ion-dependent Heavy Chain Transfer Activity of TSG-6 Mediates Assembly of the Cumulus-Oocyte Matrix. J. Biol. Chem. 2015, 290, 28708–28723. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78-79, 60–83. [Google Scholar] [CrossRef]
- Zhuo, L.; Kanamori, A.; Kannagi, R.; Itano, N.; Wu, J.; Hamaguchi, M.; Ishiguro, N.; Kimata, K. SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem. 2006, 281, 20303–20314. [Google Scholar] [CrossRef]
- Tseng, S.C. HC-HA/PTX3 Purified From Amniotic Membrane as Novel Regenerative Matrix: Insight Into Relationship Between Inflammation and Regeneration. Invest. Ophthalmol. Vis. Sci. 2016, 57, ORSFh1–ORSFh8. [Google Scholar] [CrossRef]
- Milner, C.M.; Day, A.J. TSG-6: A multifunctional protein associated with inflammation. J. Cell Sci. 2003, 116, 1863–1873. [Google Scholar] [CrossRef]
- Sivakumar, A.; Mahadevan, A.; Lauer, M.E.; Narvaez, R.J.; Ramesh, S.; Demler, C.M.; Souchet, N.R.; Hascall, V.C.; Midura, R.J.; Garantziotis, S.; et al. Midgut Laterality Is Driven by Hyaluronan on the Right. Dev. Cell 2018, 46, 533–551.e5. [Google Scholar] [CrossRef]
- Bell, T.J.; Brand, O.J.; Morgan, D.J.; Salek-Ardakani, S.; Jagger, C.; Fujimori, T.; Cholewa, L.; Tilakaratna, V.; Ostling, J.; Thomas, M.; et al. Defective lung function following influenza virus is due to prolonged, reversible hyaluronan synthesis. Matrix Biol. 2019, 80, 14–28. [Google Scholar] [CrossRef]
- Tighe, R.M.; Garantziotis, S. Hyaluronan interactions with innate immunity in lung biology. Matrix Biol. 2019, 78-79, 84–99. [Google Scholar] [CrossRef]
- Baranova, N.S.; Nileback, E.; Haller, F.M.; Briggs, D.C.; Svedhem, S.; Day, A.J.; Richter, R.P. The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J. Biol. Chem. 2011, 286, 25675–25686. [Google Scholar] [CrossRef]
- Lesley, J.; Gal, I.; Mahoney, D.J.; Cordell, M.R.; Rugg, M.S.; Hyman, R.; Day, A.J.; Mikecz, K. TSG-6 modulates the interaction between hyaluronan and cell surface CD44. J. Biol. Chem. 2004, 279, 25745–25754. [Google Scholar] [CrossRef]
- Baranova, N.S.; Foulcer, S.J.; Briggs, D.C.; Tilakaratna, V.; Enghild, J.J.; Milner, C.M.; Day, A.J.; Richter, R.P. Inter-alpha-inhibitor impairs TSG-6-induced hyaluronan cross-linking. J. Biol. Chem. 2013, 288, 29642–29653. [Google Scholar] [CrossRef]
- Kota, D.J.; Wiggins, L.L.; Yoon, N.; Lee, R.H. TSG-6 produced by hMSCs delays the onset of autoimmune diabetes by suppressing Th1 development and enhancing tolerogenicity. Diabetes 2013, 62, 2048–2058. [Google Scholar] [CrossRef]
- Dyer, D.P.; Salanga, C.L.; Johns, S.C.; Valdambrini, E.; Fuster, M.M.; Milner, C.M.; Day, A.J.; Handel, T.M. The Anti-inflammatory Protein TSG-6 Regulates Chemokine Function by Inhibiting Chemokine/Glycosaminoglycan Interactions. J. Biol. Chem. 2016, 291, 12627–12640. [Google Scholar] [CrossRef]
- Romano, B.; Elangovan, S.; Erreni, M.; Sala, E.; Petti, L.; Kunderfranco, P.; Massimino, L.; Restelli, S.; Sinha, S.; Lucchetti, D.; et al. TNF-Stimulated Gene-6 Is a Key Regulator in Switching Stemness and Biological Properties of Mesenchymal Stem Cells. Stem Cells 2019, 37, 973–987. [Google Scholar] [CrossRef]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology 2015, 149, 163–176.e20. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Sans, M.; Vetrano, S.; Graziani, C.; De Cristofaro, R.; Gerlitz, B.; Repici, A.; Arena, V.; Malesci, A.; Panes, J.; et al. Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease. J. Clin. Invest. 2007, 117, 1951–1960. [Google Scholar] [CrossRef]
- Becker, C.; Fantini, M.C.; Wirtz, S.; Nikolaev, A.; Kiesslich, R.; Lehr, H.A.; Galle, P.R.; Neurath, M.F. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 2005, 54, 950–954. [Google Scholar] [CrossRef]
- Simsek, H.D.; Basyigit, S.; Aktas, B.; Simsek, G.G.; Vargol, E.; Kucukazman, M.; Nazligul, Y. Assessment of the Correlation between Endoscopic Activity and Histological Activity in Ulcerative Colitis Patients. Med. Princ. Pract. 2016, 25, 378–384. [Google Scholar] [CrossRef]
- Vetrano, S.; Ploplis, V.A.; Sala, E.; Sandoval-Cooper, M.; Donahue, D.L.; Correale, C.; Arena, V.; Spinelli, A.; Repici, A.; Malesci, A.; et al. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc. Natl. Acad. Sci. USA 2011, 108, 19830–19835. [Google Scholar] [CrossRef]
- Kuo, Y.Z.; Fang, W.Y.; Huang, C.C.; Tsai, S.T.; Wang, Y.C.; Yang, C.L.; Wu, L.W. Hyaluronan synthase 3 mediated oncogenic action through forming inter-regulation loop with tumor necrosis factor alpha in oral cancer. Oncotarget 2017, 8, 15563–15583. [Google Scholar] [CrossRef] [PubMed]
- Siiskonen, H.; Oikari, S.; Pasonen-Seppanen, S.; Rilla, K. Hyaluronan synthase 1: A mysterious enzyme with unexpected functions. Front. Immunol. 2015, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Heldin, P.; Lin, C.Y.; Kolliopoulos, C.; Chen, Y.H.; Skandalis, S.S. Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol. 2019, 78-79, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Invest. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Beltran, S.R.; Svoboda, K.K.; Kerns, D.G.; Sheth, A.; Prockop, D.J. Anti-inflammatory protein tumor necrosis factor-alpha-stimulated protein 6 (TSG-6) promotes early gingival wound healing: An in vivo study. J. Periodontol. 2015, 86, 62–71. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Li, X.J.; Ma, L.; Tang, Y.L. Anti-inflammatory cytokine TSG-6 inhibits hypertrophic scar formation in a rabbit ear model. Eur. J. Pharmacol. 2015, 751, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Di, G.; Du, X.; Qi, X.; Zhao, X.; Duan, H.; Li, S.; Xie, L.; Zhou, Q. Mesenchymal Stem Cells Promote Diabetic Corneal Epithelial Wound Healing Through TSG-6-Dependent Stem Cell Activation and Macrophage Switch. Invest. Ophthalmol. Vis. Sci. 2017, 58, 4344–4354. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.; Duan, H.; Chai, J.; Yang, J.; Li, X.; Yu, Y.; Zhang, X.; Hu, X.; Xiao, M.; et al. TSG-6 secreted by human umbilical cord-MSCs attenuates severe burn-induced excessive inflammation via inhibiting activations of P38 and JNK signaling. Sci. Rep. 2016, 6, 30121. [Google Scholar] [CrossRef]
- Janssen, U.; Thomas, G.; Glant, T.; Phillips, A. Expression of inter-alpha-trypsin inhibitor and tumor necrosis factor-stimulated gene 6 in renal proximal tubular epithelial cells. Kidney Int. 2001, 60, 126–136. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, D.H.; Ryu, J.S.; Ko, A.Y.; Ko, J.H.; Kim, M.K.; Wee, W.R.; Khwarg, S.I.; Oh, J.Y. Topical TSG-6 Administration Protects the Ocular Surface in Two Mouse Models of Inflammation-Related Dry Eye. Invest. Ophthalmol. Vis. Sci. 2015, 56, 5175–5181. [Google Scholar] [CrossRef]
- Filpa, V.; Bistoletti, M.; Caon, I.; Moro, E.; Grimaldi, A.; Moretto, P.; Baj, A.; Giron, M.C.; Karousou, E.; Viola, M.; et al. Changes in hyaluronan deposition in the rat myenteric plexus after experimentally-induced colitis. Sci. Rep. 2017, 7, 17644. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Brinck, J.; Briskin, M.J.; Spicer, A.P.; Heldin, P. Expression of human hyaluronan synthases in response to external stimuli. Biochem. J. 2000, 348 Pt 1, 29–35. [Google Scholar] [CrossRef]
- de la Motte, C.A.; Hascall, V.C.; Drazba, J.; Bandyopadhyay, S.K.; Strong, S.A. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: Inter-alpha-trypsin inhibitor is crucial to structure and function. Am. J. Pathol. 2003, 163, 121–133. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Deed, R.; Rooney, P.; Kumar, P.; Norton, J.D.; Smith, J.; Freemont, A.J.; Kumar, S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int. J. Cancer 1997, 71, 251–256. [Google Scholar] [CrossRef]
- Danese, S.; Sans, M.; de la Motte, C.; Graziani, C.; West, G.; Phillips, M.H.; Pola, R.; Rutella, S.; Willis, J.; Gasbarrini, A.; et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 2006, 130, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef]
- Dubacheva, G.V.; Curk, T.; Auzely-Velty, R.; Frenkel, D.; Richter, R.P. Designing multivalent probes for tunable superselective targeting. Proc. Natl. Acad. Sci. USA 2015, 112, 5579–5584. [Google Scholar] [CrossRef]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Pure, E. CD44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrix remodeling and tensile strength during cutaneous wound healing. Matrix Biol. 2019, 75–76, 314–330. [Google Scholar] [CrossRef]
- Higman, V.A.; Briggs, D.C.; Mahoney, D.J.; Blundell, C.D.; Sattelle, B.M.; Dyer, D.P.; Green, D.E.; DeAngelis, P.L.; Almond, A.; Milner, C.M.; et al. A refined model for the TSG-6 link module in complex with hyaluronan: Use of defined oligosaccharides to probe structure and function. J. Biol. Chem. 2014, 289, 5619–5634. [Google Scholar] [CrossRef]
- Tan, K.T.; McGrouther, D.A.; Day, A.J.; Milner, C.M.; Bayat, A. Characterization of hyaluronan and TSG-6 in skin scarring: Differential distribution in keloid scars, normal scars and unscarred skin. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Lilly, C.M.; Tateno, H.; Oguma, T.; Israel, E.; Sonna, L.A. Effects of allergen challenge on airway epithelial cell gene expression. Am. J. Respir. Crit. Care Med. 2005, 171, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Riehl, T.E.; Stenson, W.F. Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology 2009, 137, 2041–2051. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequences |
|---|---|
| mm-HAS 1 h-HAS 1 | Forward: 5′-GCGAGCACTCACGATCATCTT-3′ Reverse: 5′-GTCCATAGCGATCTGAAGCCA-3′ Forward: 5′-GAGCCTCTTCGCGTACCTG-3′ Reverse: 5′-CCTCCTGGTAGGCGGAGAT-3′ |
| mm-HAS 2 h-HAS 2 | Forward: 5′-GTACGGTGCCTTTTTAGCCTC-3′ Reverse: 5′-TAATCGGGGTTTCAAGGGACT-3′ Forward: 5′-TCCTGGATCTCATTCCTCAGC-3′ Reverse: 5′-TGCACTGAACACACCCAAAATA-3′ |
| mm-HAS 3 h-HAS 3 | Forward: 5′-CAATCGCCAGGAAGATACCTAC-3′ Reverse: 5′-GGAAATTGCTACGCCACACAA-3′ Forward: 5′-CAGCCTATGTGACGGGCTAC-3′ Reverse: 5′-CCTCCTGGTATGCGGCAAT-3′ |
| mm-CD44 h-CD44 | Forward: 5′-ACTTTGCCTCTTGCAGTTGAG-3′ Reverse: 5′-TTTCTCCACATGGAATACACCTG-3′ Forward: 5′-CTGCCGCTTTGCAGGTGTA-3′ Reverse: 5′-CATTGTGGGCAAGGTGCTATT-3′ |
| mm-GAPDH h-GAPDH | Forward: 5′-CCATGTTCGTCATGGGTGTG-3′ Reverse: 5′-CAGGGGTGCTAAGCAGTTGG-3′ Forward: 5′-TGTGTCCGTCGTGGATCTGA-3′ Reverse: 5′-CCTGCTTCACCACCTTCTTGA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammarco, G.; Shalaby, M.; Elangovan, S.; Petti, L.; Roda, G.; Restelli, S.; Arena, V.; Ungaro, F.; Fiorino, G.; Day, A.J.; et al. Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6. Cells 2019, 8, 1074. https://doi.org/10.3390/cells8091074
Sammarco G, Shalaby M, Elangovan S, Petti L, Roda G, Restelli S, Arena V, Ungaro F, Fiorino G, Day AJ, et al. Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6. Cells. 2019; 8(9):1074. https://doi.org/10.3390/cells8091074
Chicago/Turabian StyleSammarco, Giusy, Mohammad Shalaby, Sudharshan Elangovan, Luciana Petti, Giulia Roda, Silvia Restelli, Vincenzo Arena, Federica Ungaro, Gionata Fiorino, Anthony J. Day, and et al. 2019. "Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6" Cells 8, no. 9: 1074. https://doi.org/10.3390/cells8091074
APA StyleSammarco, G., Shalaby, M., Elangovan, S., Petti, L., Roda, G., Restelli, S., Arena, V., Ungaro, F., Fiorino, G., Day, A. J., D’Alessio, S., & Vetrano, S. (2019). Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6. Cells, 8(9), 1074. https://doi.org/10.3390/cells8091074






