The Rac3 GTPase in Neuronal Development, Neurodevelopmental Disorders, and Cancer
Abstract
1. Introduction
2. Rac3 in Neuronal Development
2.1. Expression of Rac3
2.2. Rac3 Specifically Influences the Maturation of Neurons in Culture
2.3. Contribution of Rac3 to Mammalian Brain Function
3. Rac3 and Intellectual Disability
4. Rac3 in Cancer
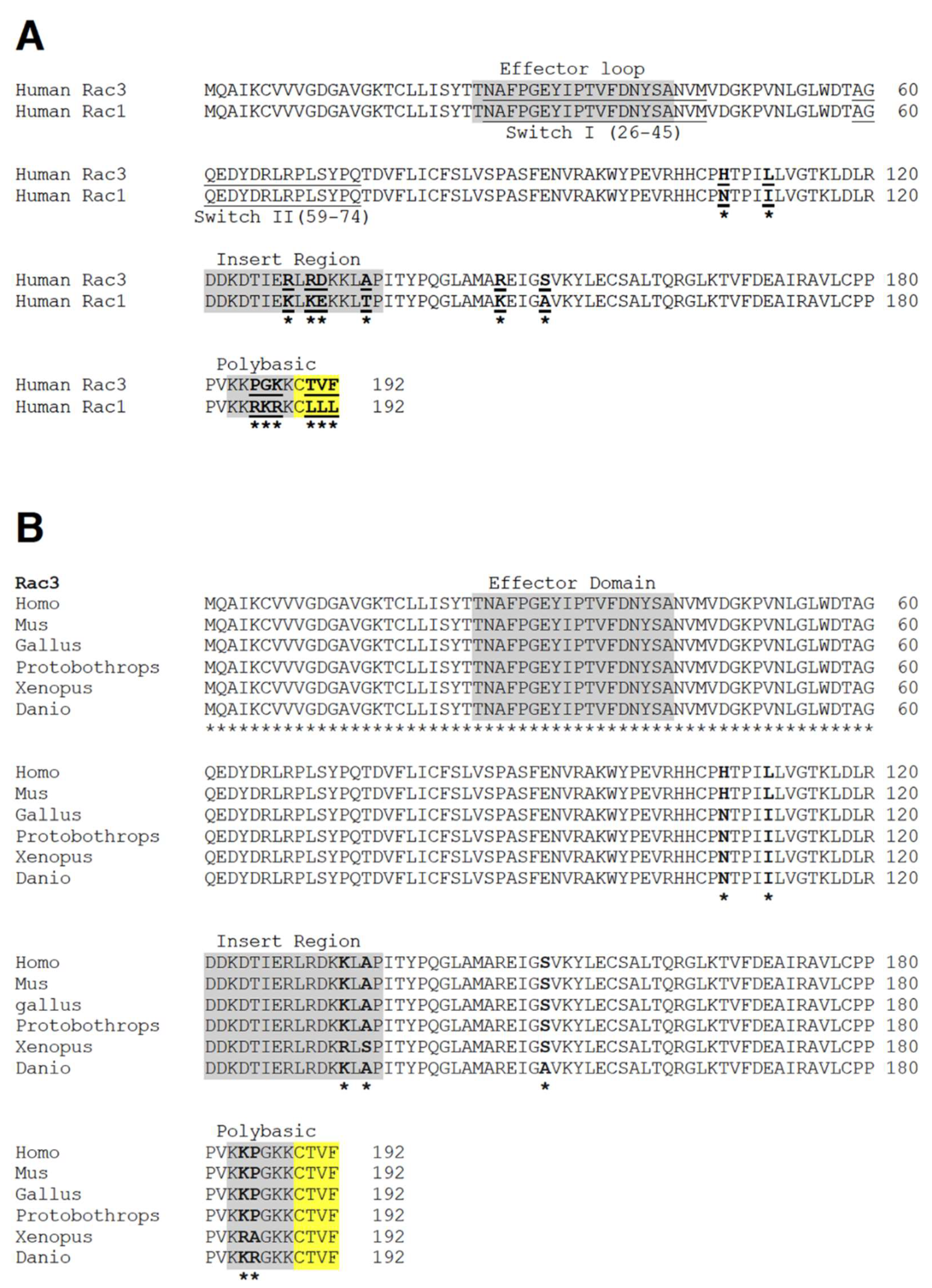
5. Mechanisms Underlying the Specificity of Rac3 Function
6. Conclusions
Funding
Conflicts of Interest
References
- Moll, J.; Sansig, G.; Fattori, E.; van der Putten, H. The murine rac1 gene: cDNA cloning, tissue distribution and regulated expression of rac1 mRNA by disassembly of actin microfilaments. Oncogene 1991, 6, 863–866. [Google Scholar] [PubMed]
- Jordan, P.; Brazåo, R.; Boavida, M.G.; Gespach, C.; Chastre, E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene 1999, 18, 6835–6839. [Google Scholar] [CrossRef]
- Didsbury, J.; Weber, R.F.; Bokoch, G.M.; Evans, T.; Snyderman, R. Rac, a novel ras-related family of proteins that are botulinum toxin substrates. J. Biol. Chem. 1989, 264, 16378–16382. [Google Scholar] [PubMed]
- Cho, Y.J.; Zhang, B.; Kaartinen, V.; Haataja, L.; de Curtis, I.; Groffen, J.; Heisterkamp, N. Generation of Rac3 null mutant mice: Role of Rac3 in Bcr/Abl-caused lymphoblastic leukemia. Mol. Cell Biol. 2005, 25, 5777–5785. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, S.; Gualdoni, S.; Albertinazzi, C.; Paris, S.; Croci, L.; Consalez, G.G.; de Curtis, I. Generation and characterization of Rac3 knockout mice. Mol. Cell Biol. 2005, 25, 5763–5776. [Google Scholar] [CrossRef]
- Li, H.; Gomes, P.J.; Chen, J.D. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc. Natl. Acad. Sci. USA 1997, 94, 8479–8484. [Google Scholar] [CrossRef]
- Haataja, L.; Groffen, J.; Heisterkamp, N. Characterization of RAC3, a novel member of the Rho family. J. Biol. Chem. 1997, 272, 20384–20388. [Google Scholar] [CrossRef]
- Malosio, M.L.; Gilardelli, D.; Paris, S.; Albertinazzi, C.; de Curtis, I. Differential expression of distinct members of the Rho family of GTP-binding proteins during neuronal development: Identification of Rac1B, a new neural-specific member of the family. J. Neurosci. 1997, 17, 6717–6728. [Google Scholar] [CrossRef]
- Kinsella, B.T.; Erdman, R.A.; Maltese, W.A. Carboxyl-terminal isoprenylation of ras-related GTP-binding proteins encoded by rac1, rac2, and ralA. J. Biol. Chem. 1991, 266, 9786–9794. [Google Scholar]
- Ando, S.; Kaibuchi, K.; Sasaki, T.; Hiraoka, K.; Nishiyama, T.; Mizuno, T.; Asada, M.; Nunoi, H.; Matsuda, I.; Matsuura, Y.; et al. Post-translational processing of Rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. J. Biol. Chem. 1992, 267, 25709–25713. [Google Scholar]
- Moores, S.L.; Schaber, M.D.; Mosser, S.D.; Rands, E.; O’Hara, M.B.; Garsky, V.M.; Marshall, M.S.; Pompliano, D.L.; Gibbs, J.B. Sequence dependence of protein isoprenylation. J. Biol. Chem. 1991, 266, 14603–14610. [Google Scholar] [PubMed]
- Morris, C.M.; Haataja, L.; McDonald, M.; Gough, S.; Markie, D.; Groffen, J.; Heisterkamp, N. The small GTPase RAC3 gene is located within chromosome band 17q25.3 outside and telomeric of a region commonly deleted in breast and ovarian tumours. Cytogenet. Cell Genet. 2000, 89, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Cropp, C.S.; Lidereau, R.; Campbell, G.; Champene, M.H.; Callahan, R. Loss of heterozygosity on chromosomes 17 and 18 in breast carcinoma: Two additional regions identified. Proc. Natl. Acad. Sci. USA 1990, 87, 7737–7741. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, R.S.; Devilee, P.; van Vliet, M.; Kuipers-Dijkshoorn, N.; Kersenmaeker, A.; Bardoel, A.; Khan, P.M.; Cornelisse, C.J. Allele loss patterns on chromosome 17q in 109 breast carcinomas indicate at least two distinct target regions. Oncogene 1993, 8, 781–785. [Google Scholar] [PubMed]
- Philips, A.; Blein, M.; Robert, A.; Chambon, J.P.; Baghdiguian, S.; Weill, M.; Fort, P. Ascidians as a vertebrate-like model organism for physiological studies of Rho GTPase signaling. Biol. Cell 2003, 95, 295–302. [Google Scholar] [CrossRef]
- Albertinazzi, C.; Gilardelli, D.; Paris, S.; Longhi, R.; de Curtis, I. Overexpression of a neural-specific Rho family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. J. Cell Biol. 1998, 142, 815–825. [Google Scholar] [CrossRef]
- Bolis, A.; Corbetta, S.; Cioce, A.; de Curtis, I. Differential distribution of Rac1 and Rac3 GTPases in the developing mouse brain: Implications for a role of Rac3 in Purkinje cell differentiation. Eur. J. Neurosci. 2003, 18, 2417–2424. [Google Scholar] [CrossRef]
- Cox, A.D.; Der, C.J. Protein prenylation: More than just glue? Curr. Opin. Cell Biol. 1992, 4, 1008–1016. [Google Scholar] [CrossRef]
- Joyce, P.L.; Cox, A.D. Rac1 and Rac3 are targets for geranylgeranyltransferase I inhibitor-mediated inhibition of signaling, transformation, and membrane ruffling. Cancer Res. 2003, 63, 7959–7967. [Google Scholar]
- Corbetta, S.; Gualdoni, S.; Ciceri, G.; Monari, M.; Zuccaro, E.; Tybulewicz, V.L.; de Curtis, I. Essential role of Rac1 and Rac3 GTPases in neuronal development. FASEB J. 2009, 23, 1347–1357. [Google Scholar] [CrossRef]
- Pennucci, R.; Gucciardi, I.; de Curtis, I. Rac1 and Rac3 GTPases differently influence the morphological maturation of dendritic spines in hippocampal neurons. PLoS ONE 2019, 14, e0220496. [Google Scholar] [CrossRef] [PubMed]
- Honkura, N.; Matsuzaki, M.; Noguchi, J.; Ellis-Davies, G.C.; Kasai, H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron 2008, 57, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.W.; Cai, X.; Stone, M.R.; Bloch, R.J.; Thompson, S.M. The actin binding domain of βI-spectrin regulates the morphological and functional dynamics of dendritic spines. PLoS ONE 2011, 6, e16197. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, I. Functions of Rac GTPases during neuronal development. Dev. Neurosci. 2008, 30, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Nakatsuji, N.; Nakamura, K.; Nakao, K.; Hashimoto, R.; Otani, H.; Sakagami, H.; Kondo, H.; Nozawa, S.; Aiba, A.; et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene 1998, 17, 3427–3433. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liao, G.; Waclaw, R.R.; Burns, K.A.; Linquist, D.; Campbell, K.; Zheng, Y.; Kuan, C.Y. Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons. J. Neurosci. 2007, 27, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Kassai, H.; Terashima, T.; Fukaya, M.; Nakao, K.; Sakahara, M.; Watanabe, M.; Aiba, A. Rac1 in cortical projection neurons is selectively required for midline crossing of commissural axonal formation. Eur. J. Neurosci. 2008, 28, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Grimsley-Myers, C.M.; Sipe, C.W.; Géléoc, G.S.; Lu, X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J. Neurosci. 2009, 29, 15859–15869. [Google Scholar] [CrossRef] [PubMed]
- Haditsch, U.; Leone, D.P.; Farinelli, M.; Chrostek-Grashoff, A.; Brakebusch, C.; Mansuy, I.M.; McConnell, S.K.; Palmer, T.D. A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Mol. Cell Neurosci. 2009, 41, 409–419. [Google Scholar] [CrossRef]
- Haruta, M.; Bush, R.A.; Kjellstrom, S.; Vijayasarathy, C.; Zeng, Y.; Le, Y.Z.; Sieving, P.A. Depleting Rac1 in mouse rod photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc. Natl. Acad. Sci. USA 2009, 106, 9397–9402. [Google Scholar] [CrossRef]
- Leone, D.P.; Srinivasan, K.; Brakebusch, C.; McConnell, S.K. The rho GTPase Rac1 is required for proliferation and survival of progenitors in the developing forebrain. Dev. Neurobiol. 2010, 70, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Tahirovic, S.; Hellal, F.; Neukirchen, D.; Hindges, R.; Garvalov, B.K.; Flynn, K.C.; Stradal, T.E.; Chrostek-Grashoff, A.; Brakebusch, C.; Bradke, F. Rac1 regulates neuronal polarization through the WAVE complex. J. Neurosci. 2010, 30, 6930–6943. [Google Scholar] [CrossRef] [PubMed]
- Dietz, D.M.; Sun, H.; Lobo, M.K.; Cahill, M.E.; Chadwick, B.; Gao, V.; Koo, J.W.; Mazei-Robison, M.S.; Dias, C.; Maze, I.; et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat. Neurosci. 2012, 15, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Haditsch, U.; Anderson, M.P.; Freewoman, J.; Cord, B.; Babu, H.; Brakebusch, C.; Palmer, T.D. Neuronal Rac1 is required for learning-evoked neurogenesis. J. Neurosci. 2013, 33, 12229–12241. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, S.; D’Adamo, P.; Gualdoni, S.; Braschi, C.; Berardi, N.; de Curtis, I. Hyperactivity and novelty-induced hyperreactivity in mice lacking Rac3. Behav. Brain Res. 2008, 186, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Pennucci, R.; Tavano, S.; Tonoli, D.; Gualdoni, S.; de Curtis, I. Rac1 and Rac3 GTPases regulate the development of hilar mossy cells by affecting the migration of their precursors to the hilus. PLoS ONE 2011, 6, e24819. [Google Scholar] [CrossRef]
- Wonders, C.P.; Anderson, S.A. The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 2006, 7, 687–696. [Google Scholar] [CrossRef]
- Nadarajah, B.; Alifragis, P.; Wong, R.O.; Parnavelas, J.G. Ventricle directed migration in the developing cerebral cortex. Nat. Neurosci. 2002, 5, 218–224. [Google Scholar] [CrossRef]
- Ang, E.S., Jr.; Haydar, T.F.; Gluncic, V.; Rakic, P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J. Neurosci. 2003, 23, 5805–5815. [Google Scholar] [CrossRef]
- Tanaka, D.H.; Maekawa, K.; Yanagawa, Y.; Obata, K.; Murakami, F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development 2006, 133, 2167–2176. [Google Scholar] [CrossRef]
- Hernández-Miranda, L.R.; Parnavelas, J.G.; Chiara, F. Molecules and mechanisms involved in the generation and migration of cortical interneurons. ASN Neuro 2010, 2, e00031. [Google Scholar] [CrossRef] [PubMed]
- Vidaki, M.; Tivodar, S.; Doulgeraki, K.; Tybulewicz, V.; Kessaris, N.; Pachnis, V.; Karagogeos, D. Rac1-dependent cell cycle exit of MGE precursors and GABAergic interneuron migration to the cortex. Cereb. Cortex 2012, 22, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Vaghi, V.; Pennucci, R.; Talpo, F.; Corbetta, S.; Montinaro, V.; Barone, C.; Croci, L.; Spaiardi, P.; Consalez, G.G.; Biella, G.; et al. Rac1 and Rac3 GTPases control synergistically the development of cortical and hippocampal GABAergic interneurons. Cereb. Cortex 2014, 24, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Tivodar, S.; Kalemaki, K.; Kounoupa, Z.; Vidaki, M.; Theodorakis, K.; Denaxa, M.; Kessaris, N.; de Curtis, I.; Pachnis, V.; Karagogeos, D. Rac-GTPases regulate microtubule stability and axon growth of cortical GABAergic interneurons. Cereb. Cortex 2015, 25, 2370–2382. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ueyama, T.; Ninoyu, Y.; Sakaguchi, H.; Choijookhuu, N.; Hishikawa, Y.; Kiyonari, H.; Kohta, M.; Sakahara, M.; de Curtis, I.; et al. Novel role of Rac-Mid1 signaling in medial cerebellar development. Development 2017, 144, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Pennucci, R.; Talpo, F.; Astro, V.; Montinaro, V.; Morè, L.; Cursi, M.; Castoldi, V.; Chiaretti, S.; Bianchi, V.; Marenna, S.; et al. Loss of Either Rac1 or Rac3 GTPase Differentially Affects the Behavior of Mutant Mice and the Development of Functional GABAergic Networks. Cereb. Cortex 2016, 26, 873–890. [Google Scholar] [CrossRef]
- Ito, H.; Morishita, R.; Mizuno, M.; Tabata, H.; Nagata, K.I. Rho family GTPases, Rac and Cdc42, control the localization of neonatal dentate granule cells during brain development. Hippocampus 2019, 29, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, V.; Jones, R.; Umbach, A.; Ammoni, A.; Passafaro, M.; Hirsch, E.; Merlo, G.R. Rho GTPases in ntellectual Disability: From Genetics to Therapeutic Opportunities. Int. J. Mol. Sci. 2018, 19, 1821. [Google Scholar] [CrossRef]
- Reijnders, M.R.F.; Ansor, N.M.; Kousi, M.; Yue, W.W.; Tan, P.L.; Clarkson, K.; Clayton-Smith, J.; Corning, K.; Jones, J.R.; Lam, W.W.K.; et al. Deciphering Developmental Disorders Study, Millard TH, Katsanis N, Brunner HG, Banka S. RAC1 missense mutations in developmental disorders with diverse phenotypes. Am. J. Hum. Genet. 2017, 101, 466–477. [Google Scholar] [CrossRef]
- Kawazu, M.; Ueno, T.; Kontani, K.; Ogita, Y.; Ando, M.; Fukumura, K.; Yamato, A.; Soda, M.; Takeuchi, K.; Miki, Y.; et al. Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc. Natl. Acad. Sci. USA 2013, 110, 3029–3034. [Google Scholar] [CrossRef]
- Costain, G.; Callewaert, B.; Gabriel, H.; Tan, T.Y.; Walker, S.; Christodoulou, J.; Lazar, T.; Menten, B.; Orkin, J.; Sadedin, S.; et al. De novo missense variants in RAC3 cause a novel neurodevelopmental syndrome. Genet. Med. 2019, 21, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.J.; Gil-Henn, H.; Mader, C.C.; Halo, T.; Yin, T.; Condeelis, J.; Machida, K.; Wu, Y.I.; Koleske, A.J. Phosphorylated cortactin recruits Vav2 guanine nucleotide exchange factor to activate Rac3 and promote invadopodial function in invasive breast cancer cells. Mol. Biol. Cell 2017, 28, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, T.; Kaba Yasui, H.; Kato, M.; Nakashima, M.; Saitsu, H. A de novo variant in RAC3 causes severe global developmental delay and a middle interhemispheric variant of holoprosencephaly. J. Hum. Genet. 2019. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Svensmark, J.H.; Brakebusch, C. Rho GTPases in cancer: Friend or foe? Oncogene 2019. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Gómez del Pulgar, T.; Benitah, S.A.; Valerón, P.F.; Espina, C.; Lacal, J.C. Rho GTPase expression in tumourigenesis: Evidence for a significant link. Bioessays 2005, 27, 602–613. [Google Scholar] [CrossRef]
- De, P.; Aske, J.C.; Dey, N. RAC1 Takes the Lead in Solid Tumors. Cells 2019, 8, 382. [Google Scholar] [CrossRef]
- Mira, J.P.; Benard, V.; Groffen, J.; Sanders, L.C.; Knaus, U.G. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 185–189. [Google Scholar] [CrossRef]
- Hwang, S.; Chang, J.H.; Cheng, T.S.; Sy, W.D.; Lieu, A.S.; Lin, C.L.; Lee, K.S.; Howng, S.L.; Hong, Y.R. Expression of Rac3 in human brain tumors. J. Clin. Neurosci. 2005, 12, 571–574. [Google Scholar] [CrossRef]
- Leung, K.; Nagy, A.; Gonzalez-Gomez, I.; Groffen, J.; Heisterkamp, N.; Kaartinen, V. Targeted expression of activated Rac3 in mammary epithelium leads to defective postlactational involution and benign mammary gland lesions. Cells Tissues Organs 2003, 175, 72–83. [Google Scholar] [CrossRef]
- Chan, A.Y.; Coniglio, S.J.; Chuang, Y.Y.; Michaelson, D.; Knaus, U.G.; Philips, M.R.; Symons, M. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene 2005, 24, 7821–7829. [Google Scholar] [CrossRef] [PubMed]
- Keller, P.J.; Gable, C.M.; Wing, M.R.; Cox, A.D. Rac3-mediated transformation requires multiple effector pathways. Cancer Res. 2005, 65, 9883–9890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, M.P.; Zhang, M.; Le, T.P.; Wu, P.; Lainé, M.; Greene, G.L. RAC3 is a pro-migratory co-activator of ERα. Oncogene 2011, 30, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Morcillo-Garcia, S.; Noblejas-Lopez, M.D.M.; Nieto-Jimenez, C.; Perez-Peña, J.; Nuncia-Cantarero, M.; Győrffy, B.; Amir, E.; Pandiella, A.; Galan-Moya, E.M.; Ocana, A. Genetic mutational status of genes regulating epigenetics: Role of the histone methyltransferase KMT2D in triple negative breast tumors. PLoS ONE 2019, 14, e0209134. [Google Scholar] [CrossRef] [PubMed]
- Gest, C.; Joimel, U.; Huang, L.; Pritchard, L.L.; Petit, A.; Dulong, C.; Buquet, C.; Hu, C.Q.; Mirshahi, P.; Laurent, M.; et al. Rac3 induces a molecular pathway triggering breast cancer cell aggressiveness: Differences in MDA-MB-231 and MCF-7 breast cancer cell lines. BMC Cancer 2013, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Engers, R.; Ziegler, S.; Mueller, M.; Walter, A.; Willers, R.; Gabbert, H.E. Prognostic relevance of increased Rac GTPase expression in prostate carcinomas. Endocr. Relat. Cancer 2007, 14, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Lehr, J.E.; Pienta, K.J. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J. Natl. Cancer Inst. 1998, 90, 118–123. [Google Scholar] [CrossRef]
- Chatterjee, M.; Sequeira, L.; Jenkins-Kabaila, M.; Dubyk, C.W.; Pathak, S.; van Golen, K.L. Individual rac GTPases mediate aspects of prostate cancer cell and bone marrow endothelial cell interactions. J. Signal. Transduct. 2011, 2011, 541851. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, T.; Wang, G.; Wang, H.; Che, X.; Gao, X.; Liu, H. Rac3 Regulates Cell Invasion, Migration and EMT in Lung Adenocarcinoma through p38 MAPK Pathway. J. Cancer 2017, 8, 2511–2522. [Google Scholar] [CrossRef]
- Liu, T.Q.; Wang, G.B.; Li, Z.J.; Tong, X.D.; Liu, H.X. Silencing of Rac3 inhibits proliferation and induces apoptosis of human lung cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 3061–3065. [Google Scholar] [CrossRef]
- Lai, Y.J.; Tsai, J.C.; Tseng, Y.T.; Wu, M.S.; Liu, W.S.; Lam, H.I.; Yu, J.H.; Nozell, S.E.; Benveniste, E.N. Small G protein Rac GTPases regulate the maintenance of glioblastoma stem-like cells in vitro and in vivo. Oncotarget 2017, 8, 18031–18049. [Google Scholar] [CrossRef] [PubMed]
- Hantschel, O.; Superti-Furga, G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2004, 5, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Hajdo-Milasinović, A.; Ellenbroek, S.I.; van Es, S.; van der Vaart, B.; Collard, J.G. Rac1 and Rac3 have opposing functions in cell adhesion and differentiation of neuronal cells. J. Cell Sci. 2007, 120, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Hajdo-Milasinovic, A.; van der Kammen, R.A.; Moneva, Z.; Collard, J.G. Rac3 inhibits adhesion and differentiation of neuronal cells by modifying GIT1 downstream signaling. J. Cell Sci. 2009, 122, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Hoefen, R.J.; Berk, B.C. The multifunctional GIT family of proteins. J. Cell Sci. 2006, 119, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Albiges-Rizo, C.; Destaing, O.; Fourcade, B.; Planus, E.; Block, M.R. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J. Cell Sci. 2009, 122, 3037–3049. [Google Scholar] [CrossRef]
- Artym, V.V.; Zhang, Y.; Seillier-Moiseiwitsch, F.; Yamada, K.M.; Mueller, S.C. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: Defining the stages of invadopodia formation and function. Cancer Res. 2006, 66, 3034–3043. [Google Scholar] [CrossRef]
- Abe, K.; Rossman, K.; Liu, B.; Ritola, K.D.; Chiang, D.; Campbell, S.L.; Burridge, K.; Der, C.J. Vav2 is an activator of Cdc42, Rac1, and RhoA. J. Biol. Chem. 2000, 275, 10141–10149. [Google Scholar] [CrossRef]
- Donnelly, S.K.; Cabrera, R.; Mao, S.P.H.; Christin, J.R.; Wu, B.; Guo, W.; Bravo-Cordero, J.J.; Condeelis, J.S.; Segall, J.E.; Hodgson, L. Rac3 regulates breast cancer invasion and metastasis by controlling adhesion and matrix degradation. J. Cell Biol. 2017, 216, 4331–4349. [Google Scholar] [CrossRef]
- Haataja, L.; Kaartinen, V.; Groffen, J.; Heisterkamp, N. The small GTPase Rac3 interacts with the integrin-binding protein CIB and promotes integrin αIIbβ3-mediated adhesion and spreading. J. Biol. Chem. 2002, 277, 8321–8328. [Google Scholar] [CrossRef]
- Naik, U.P.; Patel, P.M.; Parise, L.V. Identification of a novel calcium-binding protein that interacts with the integrin αIIb cytoplasmic domain. J. Biol. Chem. 1997, 272, 4651–4654. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Hossain, M.S.; Guo, D.Y.; Liu, S.; Tong, H.; Khakpoor, A.; Casey, P.J.; Wang, M. A role for Rac3 GTPase in the regulation of autophagy. J. Biol. Chem. 2011, 286, 35291–35298. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, D.; Craig, K.L.; Tyers, M.; Elledge, S.J.; Harper, J.W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 1997, 91, 209–219. [Google Scholar] [CrossRef]
- Zheng, N.; Schulman, B.A.; Song, L.; Miller, J.J.; Jeffrey, P.D.; Wang, P.; Chu, C.; Koepp, D.M.; Elledge, S.J.; Pagano, M.; et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 2002, 416, 703–709. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, J.; Wei, J.; Bowser, R.K.; Khoo, A.; Liu, Z.; Luketich, J.D.; Pennathur, A.; Ma, H.; Zhao, Y. F-box protein complex FBXL19 regulates TGFβ1-induced E-cadherin down-regulation by mediating Rac3 ubiquitination and degradation. Mol. Cancer 2014, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Finkielstein, C.V.; Overduin, M.; Capelluto, D.G. Cell migration and signaling specificity is determined by the phosphatidylserine recognition motif of Rac1. J. Biol. Chem. 2006, 281, 27317–27326. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Curtis, I. The Rac3 GTPase in Neuronal Development, Neurodevelopmental Disorders, and Cancer. Cells 2019, 8, 1063. https://doi.org/10.3390/cells8091063
de Curtis I. The Rac3 GTPase in Neuronal Development, Neurodevelopmental Disorders, and Cancer. Cells. 2019; 8(9):1063. https://doi.org/10.3390/cells8091063
Chicago/Turabian Stylede Curtis, Ivan. 2019. "The Rac3 GTPase in Neuronal Development, Neurodevelopmental Disorders, and Cancer" Cells 8, no. 9: 1063. https://doi.org/10.3390/cells8091063
APA Stylede Curtis, I. (2019). The Rac3 GTPase in Neuronal Development, Neurodevelopmental Disorders, and Cancer. Cells, 8(9), 1063. https://doi.org/10.3390/cells8091063



