Physioxia Has a Beneficial Effect on Cartilage Matrix Production in Interleukin-1 Beta-Inhibited Mesenchymal Stem Cell Chondrogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human MSC Isolation and Harvesting
2.2. Chondrogenic Differentiation
2.3. Wet Weight and GAG Assay
2.4. Collagen I and II ELISA
2.5. Histology and Immunohistochemistry
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
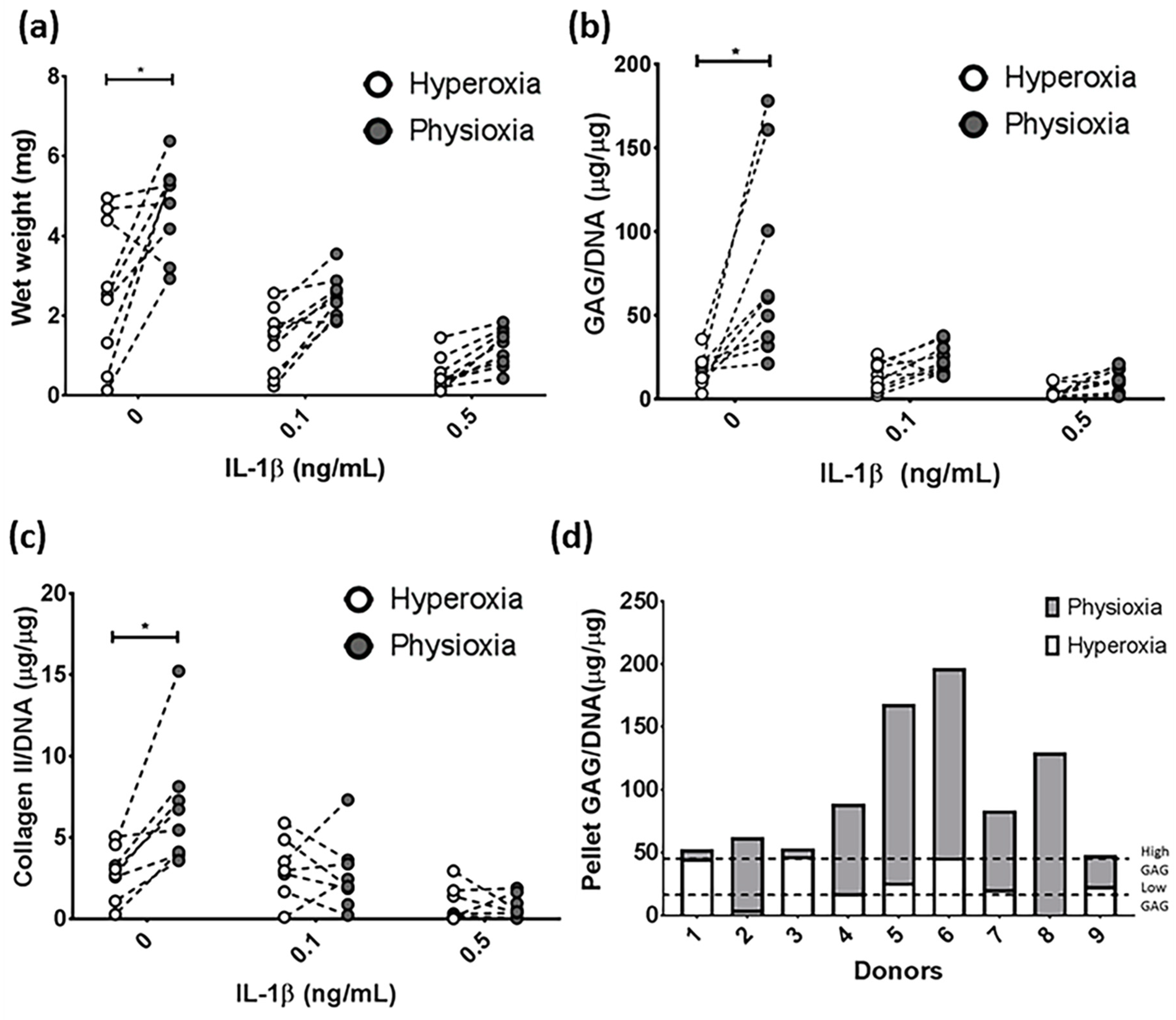
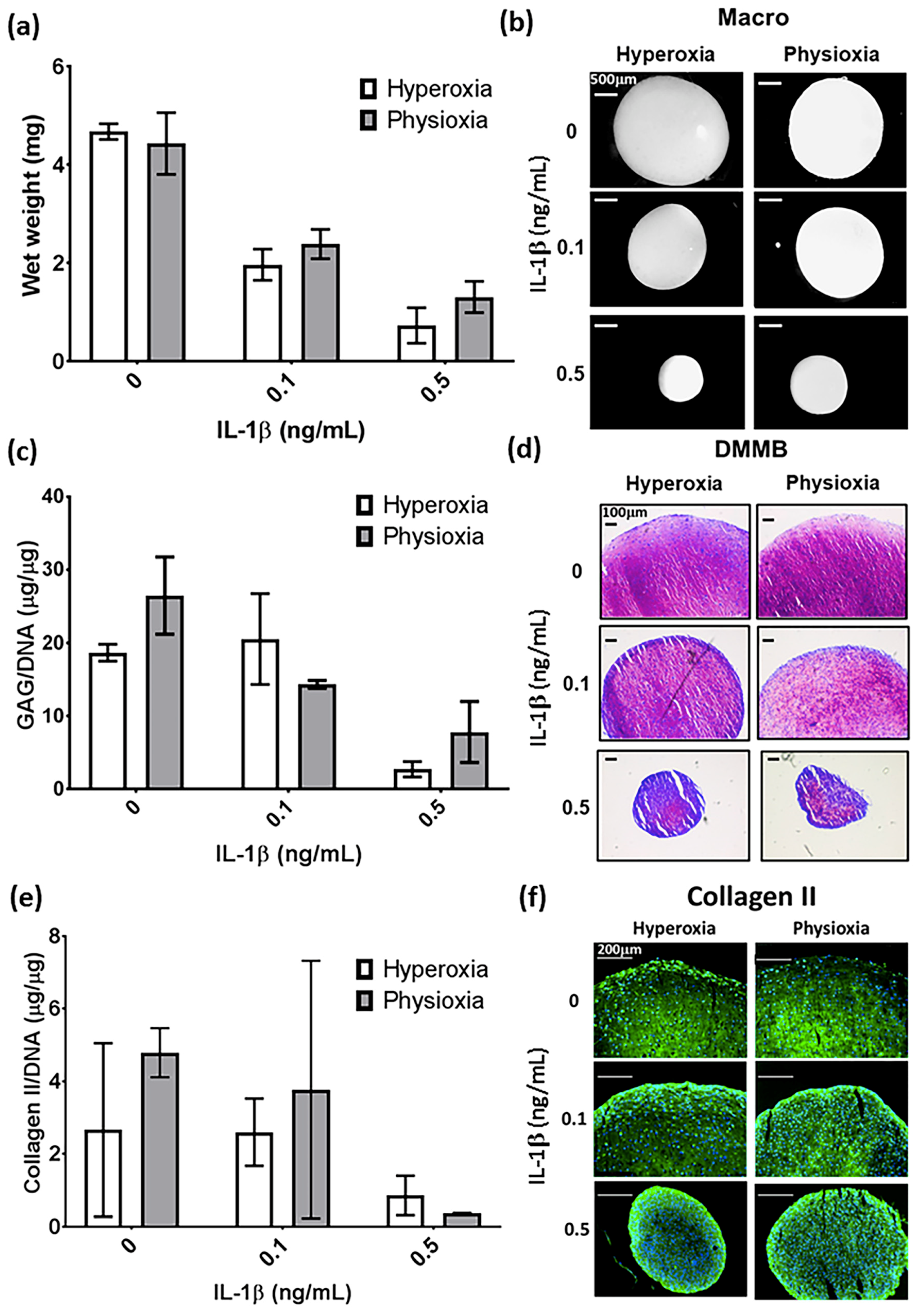
3.1. IL-1β Shows a Dose Dependant Decrease in Chondrogenic MSC Pellet Wet Weight and GAG Content
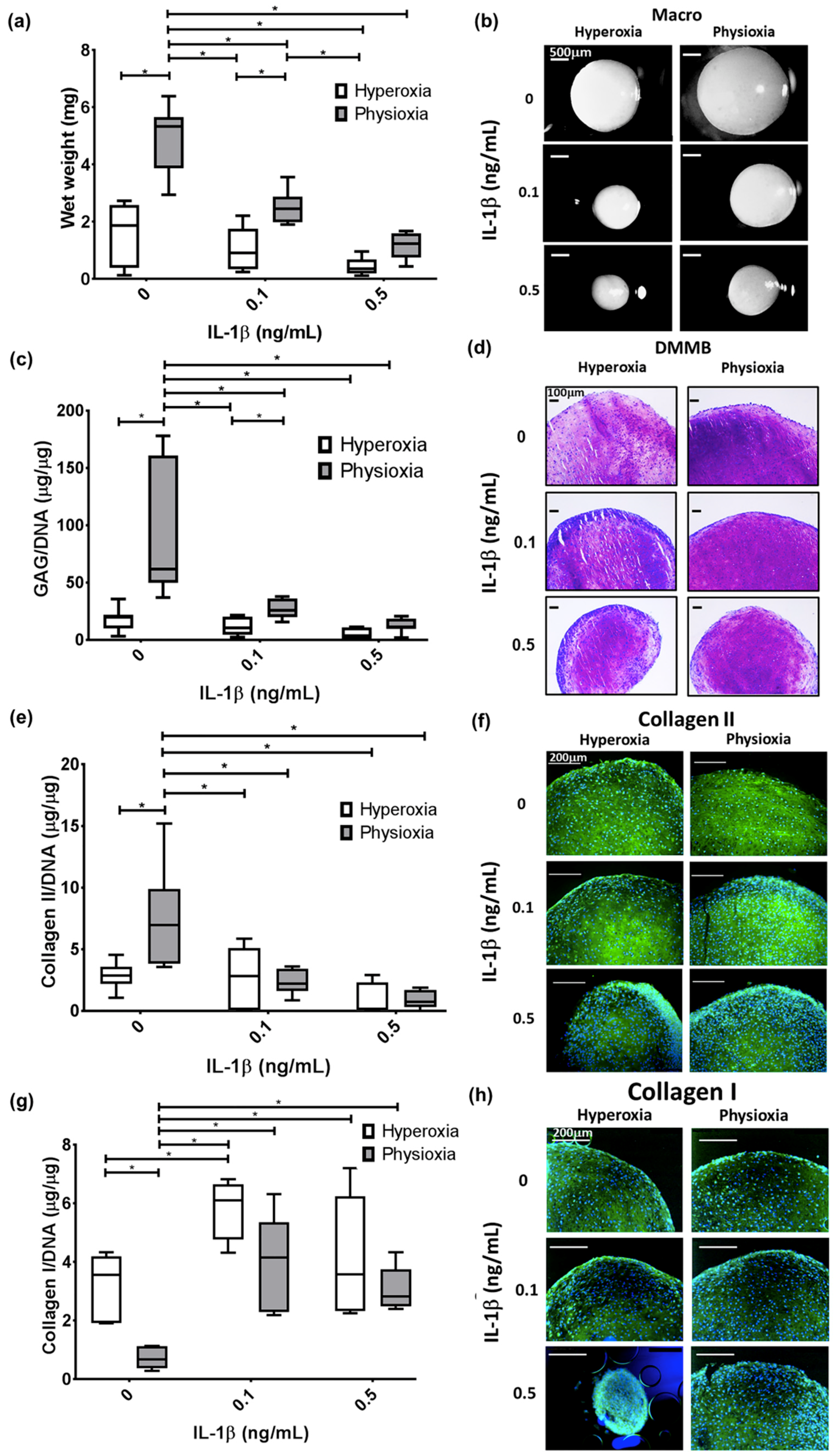
3.2. Physioxia Alone Enhances MSC Chondrogenic Matrix Expression and Content
3.3. Pellet Wet Weight and GAG Content in IL-1β Inhibited Chondrogenesis Is Suppressed under Physioxia in Responsive Donors
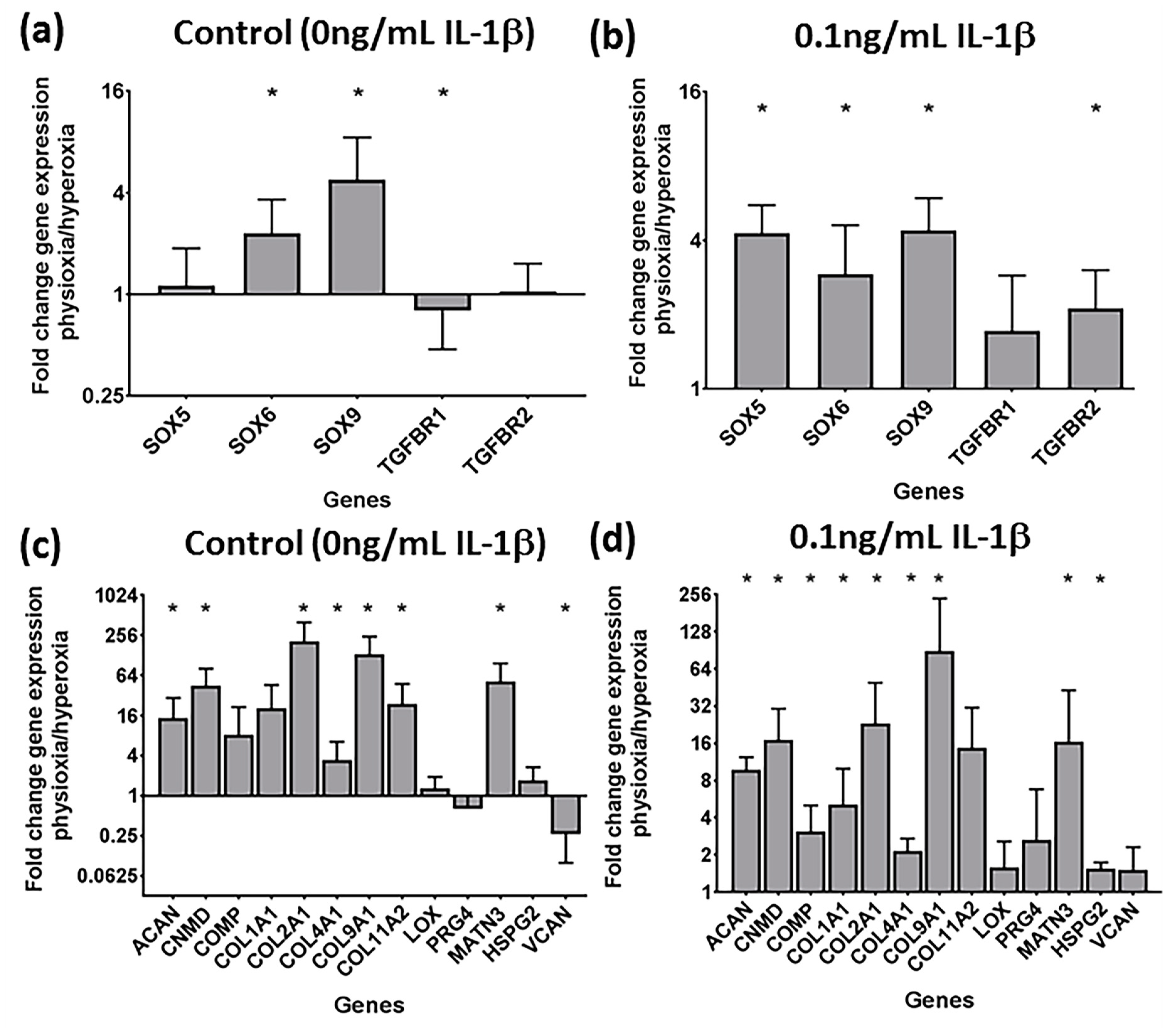
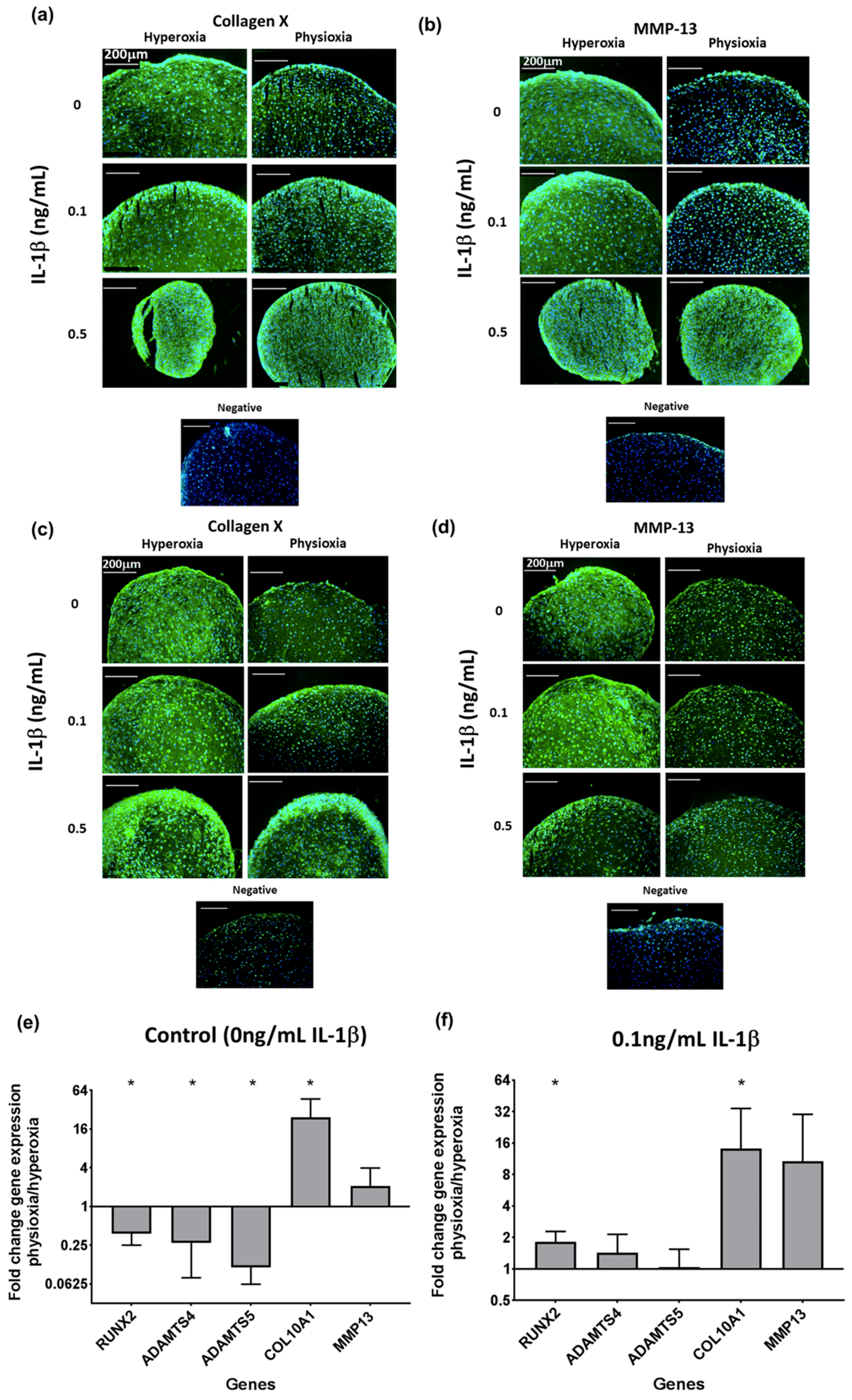
3.4. Physioxia Enhances Gene Expression of Chondrogenesis-Associated Markers and Suppresses Markers for Late Stage Hypertrophy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Fritz, J.; Albrecht, D.; Koh, J.; Zellner, J. Defect type, localization and marker gene expression determines early adverse events of matrix-associated autologous chondrocyte implantation. Injury 2015, 46 (Suppl. 4), S2–S9. [Google Scholar] [CrossRef]
- Albrecht, C.; Tichy, B.; Zak, L.; Aldrian, S.; Nurnberger, S.; Marlovits, S. Influence of cell differentiation and IL-1beta expression on clinical outcomes after matrix-associated chondrocyte transplantation. Am. J. Sports Med. 2014, 42, 59–69. [Google Scholar] [CrossRef]
- Hopkins, S.J.; Humphreys, M.; Jayson, M.I. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clin. Exp. Immunol. 1988, 72, 422–427. [Google Scholar] [PubMed]
- Hopkins, S.J.; Meager, A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin. Exp. Immunol. 1988, 73, 88–92. [Google Scholar] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- McNulty, A.L.; Rothfusz, N.E.; Leddy, H.A.; Guilak, F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J. Orthop. Res. 2013, 31, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Ning, L.; Ishijima, M.; Kaneko, H.; Kurihara, H.; Arikawa-Hirasawa, E.; Kubota, M.; Liu, L.; Xu, Z.; Futami, I.; Yusup, A.; et al. Correlations between both the expression levels of inflammatory mediators and growth factor in medial perimeniscal synovial tissue and the severity of medial knee osteoarthritis. Int. Orthop. 2011, 35, 831–838. [Google Scholar] [CrossRef]
- Panina, S.B.; Krolevets, I.V.; Milyutina, N.P.; Sagakyants, A.B.; Kornienko, I.V.; Ananyan, A.A.; Zabrodin, M.A.; Plotnikov, A.A.; Vnukov, V.V. Circulating levels of proinflammatory mediators as potential biomarkers of post-traumatic knee osteoarthritis development. J. Orthop. Traumatol. 2017, 18, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, A.I.; Beekhuizen, M.; Ct Hart, M.; Radstake, T.R.; Dhert, W.J.; Saris, D.B.; van Osch, G.J.; Creemers, L.B. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res. Ther. 2014, 16, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.; Englund, M.; Struglics, A.; Lohmander, L.S. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthr. Cartil. 2015, 23, 1906–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.U.; Johnstone, B. The role of osteochondral progenitor cells in fracture repair. Clin. Orthop. Relat. Res. 1998, 355, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Yoo, J.U. Autologous mesenchymal progenitor cells in articular cartilage repair. Clin. Orthop. Relat. Res. 1999, 367, S156–S162. [Google Scholar] [CrossRef] [PubMed]
- Felka, T.; Schafer, R.; Schewe, B.; Benz, K.; Aicher, W.K. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthr. Cartil. 2009, 17, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Wehling, N.; Palmer, G.D.; Pilapil, C.; Liu, F.; Wells, J.W.; Muller, P.E.; Evans, C.H.; Porter, R.M. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009, 60, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, N.; Krieg, J.; Weissenberger, M.; Scheller, C.; Steinert, A.F. Rescued Chondrogenesis of Mesenchymal Stem Cells under Interleukin 1 Challenge by Foamyviral Interleukin 1 Receptor Antagonist Gene Transfer. Front. Pharmacol. 2017, 8, 255. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Curcumin mediated suppression of nuclear factor-kappaB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 2010, 12, R127. [Google Scholar] [CrossRef]
- Glass, K.A.; Link, J.M.; Brunger, J.M.; Moutos, F.T.; Gersbach, C.A.; Guilak, F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 2014, 35, 5921–5931. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.E.; Koh, P.S.; Seo, B.K.; Park, Y.C.; Baek, Y.H.; Lee, J.D.; Park, D.S. Mangiferin reduces the inhibition of chondrogenic differentiation by IL-1beta in mesenchymal stem cells from subchondral bone and targets multiple aspects of the Smad and SOX9 pathways. Int. J. Mol. Sci. 2014, 15, 16025–16042. [Google Scholar] [CrossRef]
- Lei, M.; Liu, S.Q.; Liu, Y.L. Resveratrol protects bone marrow mesenchymal stem cell derived chondrocytes cultured on chitosan-gelatin scaffolds from the inhibitory effect of interleukin-1beta. Acta Pharmacol. Sin. 2008, 29, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Glass, K.A.; Compton, S.A.; Ross, A.K.; Gersbach, C.A.; Guilak, F.; Estes, B.T. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc. Natl. Acad. Sci. USA 2016, 113, E4513–E4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, S.M.; Richbourgh, B.; Ding, Y.; Hettinghouse, A.; Komatsu, D.E.; Qin, Y.X.; Liu, C.J. Chondro-protective effects of low intensity pulsed ultrasound. Osteoarthr. Cartil. 2016, 24, 1989–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafont, J.E. Lack of oxygen in articular cartilage: Consequences for chondrocyte biology. Int. J. Exp. Pathol. 2010, 91, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lund-Olesen, K. Oxygen tension in synovial fluids. Arthritis Rheum. 1970, 13, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Lafont, J.E.; Talma, S.; Hopfgarten, C.; Murphy, C.L. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J. Biol. Chem. 2008, 283, 4778–4786. [Google Scholar] [CrossRef] [PubMed]
- Lafont, J.E.; Talma, S.; Murphy, C.L. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007, 56, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.L.; Polak, J.M. Control of human articular chondrocyte differentiation by reduced oxygen tension. J. Cell. Physiol. 2004, 199, 451–459. [Google Scholar] [CrossRef]
- Murphy, C.L.; Sambanis, A. Effect of oxygen tension on chondrocyte extracellular matrix accumulation. Connect. Tissue Res. 2001, 42, 87–96. [Google Scholar] [CrossRef]
- Murphy, C.L.; Sambanis, A. Effect of oxygen tension and alginate encapsulation on restoration of the differentiated phenotype of passaged chondrocytes. Tissue Eng. 2001, 7, 791–803. [Google Scholar] [CrossRef]
- Murphy, C.L.; Thoms, B.L.; Vaghjiani, R.J.; Lafont, J.E. Hypoxia. HIF-mediated articular chondrocyte function: Prospects for cartilage repair. Arthritis Res. Ther. 2009, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Strobel, S.; Loparic, M.; Wendt, D.; Schenk, A.D.; Candrian, C.; Lindberg, R.L.; Moldovan, F.; Barbero, A.; Martin, I. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res. Ther. 2010, 12, R34. [Google Scholar] [CrossRef] [PubMed]
- Thoms, B.L.; Dudek, K.A.; Lafont, J.E.; Murphy, C.L. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheum. 2013, 65, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Cho, H.; Anderson, D.E.; Holden, P.; Ravi, V.; Little, C.B.; Johnstone, B. Reoxygenation enhances tumour necrosis factor alpha-induced degradation of the extracellular matrix produced by chondrogenic cells. Eur. Cell Mater. 2016, 31, 425–439. [Google Scholar] [CrossRef]
- Mennan, C.; Garcia, J.; McCarthy, H.; Owen, S.; Perry, J.; Wright, K.; Banerjee, R.; Richardson, J.B.; Roberts, S. Human Articular Chondrocytes Retain Their Phenotype in Sustained Hypoxia While Normoxia Promotes Their Immunomodulatory Potential. Cartilage 2018, 1947603518769714. [Google Scholar] [CrossRef]
- Ruiz-Romero, C.; Calamia, V.; Rocha, B.; Mateos, J.; Fernandez-Puente, P.; Blanco, F.J. Hypoxia conditions differentially modulate human normal and osteoarthritic chondrocyte proteomes. J. Proteome Res. 2010, 9, 3035–3045. [Google Scholar] [CrossRef] [PubMed]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef]
- Bornes, T.D.; Jomha, N.M.; Mulet-Sierra, A.; Adesida, A.B. Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res. Ther. 2015, 6, 84. [Google Scholar] [CrossRef]
- Anderson, D.E.; Markway, B.D.; Bond, D.; McCarthy, H.E.; Johnstone, B. Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem Cell Res. Ther. 2016, 7, 154. [Google Scholar] [CrossRef]
- Lee, H.H.; Chang, C.C.; Shieh, M.J.; Wang, J.P.; Chen, Y.T.; Young, T.H.; Hung, S.C. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci. Rep. 2013, 3, 2683. [Google Scholar] [CrossRef]
- Leijten, J.; Georgi, N.; Moreira Teixeira, L.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc. Natl. Acad. Sci. USA 2014, 111, 13954–13959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Leduc, T.; Desance, M.; Hervieu, M.; Legendre, F.; Ollitrault, D.; de Vienne, C.; Herlicoviez, M.; Galera, P.; Demoor, M. Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges. Int. J. Mol. Sci. 2017, 18, 1933. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.C.; Moreira Teixeira, L.S.; Landman, E.B.; van Blitterswijk, C.A.; Karperien, M. Hypoxia inhibits hypertrophic differentiation and endochondral ossification in explanted tibiae. PLoS ONE 2012, 7, e49896. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Tan, G.K.; Brooke, G.; Hudson, J.E.; Cooper-White, J.J.; Doran, M.R. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant 2010, 19, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zellner, J.; Grechenig, S.; Pfeifer, C.G.; Krutsch, W.; Koch, M.; Welsch, G.; Scherl, M.; Seitz, J.; Zeman, F.; Nerlich, M.; et al. Clinical and Radiological Regeneration of Large and Deep Osteochondral Defects of the Knee by Bone Augmentation Combined With Matrix-Guided Autologous Chondrocyte Transplantation. Am. J. Sports Med. 2017, 45, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Thorpe, S.D.; Jegard, N.C.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng. Part C Methods 2013, 19, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Studle, C.; Occhetta, P.; Geier, F.; Mehrkens, A.; Barbero, A.; Martin, I. Challenges Toward the Identification of Predictive Markers for Human Mesenchymal Stromal Cells Chondrogenic Potential. Stem Cells Transl. Med. 2019, 8, 194–204. [Google Scholar] [CrossRef]
- Both, S.K.; van der Muijsenberg, A.J.; van Blitterswijk, C.A.; de Boer, J.; de Bruijn, J.D. A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 2007, 13, 3–9. [Google Scholar] [CrossRef]
- Yin, H.; Yan, Z.; Bauer, R.J.; Peng, J.; Schieker, M.; Nerlich, M.; Docheva, D. Functionalized thermosensitive hydrogel combined with tendon stem/progenitor cells as injectable cell delivery carrier for tendon tissue engineering. Biomed. Mater. 2018, 13, 034107. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Galeano-Garces, C.; Camilleri, E.T.; Riester, S.M.; Dudakovic, A.; Larson, D.R.; Qu, W.; Smith, J.; Dietz, A.B.; Im, H.J.; Krych, A.J.; et al. Molecular Validation of Chondrogenic Differentiation and Hypoxia Responsiveness of Platelet-Lysate Expanded Adipose Tissue-Derived Human Mesenchymal Stromal Cells. Cartilage 2017, 8, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, S.K.; Jung, B.J.; Choi, S.B.; Choi, E.Y.; Kim, C.S. Enhancing proliferation and optimizing the culture condition for human bone marrow stromal cells using hypoxia and fibroblast growth factor-2. Stem Cell Res. 2018, 28, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Gawlitta, D.; van Rijen, M.H.; Schrijver, E.J.; Alblas, J.; Dhert, W.J. Hypoxia impedes hypertrophic chondrogenesis of human multipotent stromal cells. Tissue Eng. Part A 2012, 18, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, S.; Graf, F.; Fischer, J.; Moradi, B.; Little, C.B.; Richter, W. Regulation of aggrecanases from the ADAMTS family and aggrecan neoepitope formation during in vitro chondrogenesis of human mesenchymal stem cells. Eur. Cell Mater. 2012, 23, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Huang, W.; Harley, V.R.; Goodfellow, P.N.; de Crombrugghe, B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell. Biol. 1997, 17, 2336–2346. [Google Scholar] [CrossRef] [Green Version]
- Bauge, C.; Beauchef, G.; Leclercq, S.; Kim, S.J.; Pujol, J.P.; Galera, P.; Boumediene, K. NFkappaB mediates IL-1beta-induced down-regulation of TbetaRII through the modulation of Sp3 expression. J. Cell. Mol. Med. 2008, 12, 1754–1766. [Google Scholar] [CrossRef]
- Bauge, C.; Legendre, F.; Leclercq, S.; Elissalde, J.M.; Pujol, J.P.; Galera, P.; Boumediene, K. Interleukin-1beta impairment of transforming growth factor beta1 signaling by down-regulation of transforming growth factor beta receptor type II and up-regulation of Smad7 in human articular chondrocytes. Arthritis Rheum. 2007, 56, 3020–3032. [Google Scholar] [CrossRef]
- Bauge, C.; Attia, J.; Leclercq, S.; Pujol, J.P.; Galera, P.; Boumediene, K. Interleukin-1beta up-regulation of Smad7 via NF-kappaB activation in human chondrocytes. Arthritis Rheum. 2008, 58, 221–226. [Google Scholar] [CrossRef]
- Bornes, T.D.; Adesida, A.B.; Jomha, N.M. Articular Cartilage Repair with Mesenchymal Stem Cells After Chondrogenic Priming: A Pilot Study. Tissue Eng. Part A 2018, 24, 761–774. [Google Scholar] [CrossRef]
- Portron, S.; Merceron, C.; Gauthier, O.; Lesoeur, J.; Sourice, S.; Masson, M.; Fellah, B.H.; Geffroy, O.; Lallemand, E.; Weiss, P.; et al. Effects of in vitro low oxygen tension preconditioning of adipose stromal cells on their in vivo chondrogenic potential: Application in cartilage tissue repair. PLoS ONE 2013, 8, e62368. [Google Scholar] [CrossRef]
- Pattappa, G.; Johnstone, B.; Zellner, J.; Docheva, D.; Angele, P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. Int. J. Mol. Sci. 2019, 20, 484. [Google Scholar] [CrossRef] [PubMed]

| Transcription Factors | Cartilage Matrix |
|---|---|
| L-SOX5 L-SOX6 SOX9 | Aggrecan (ACAN) Chondromodulin-1 (LECT1) Cartilage oligomeric matrix protein (COMP) Collagen type I α1 (COL1A1) Collagen type II α1 (COL2A1) Collagen type VI α1 (COL6A1) Collagen type IX α1 (COL9A1) Collagen type XI α2 (COL11A2) Lysl Oxidase (LOX) Lubricin (PRG4) Matrillin-3 (MATN3) Perlecan (HSPG2) Versican (VCAN) |
| Transforming growth factor-β receptors | Hypertrophy |
| TGF-β receptor I (TGFBR1) TGF-β receptor II (TGFBR2) | Collagen type X α1 (COL10A1) MMP-9 MMP-13 ADAMTS-4 ADAMTS-5 RUNX2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattappa, G.; Schewior, R.; Hofmeister, I.; Seja, J.; Zellner, J.; Johnstone, B.; Docheva, D.; Angele, P. Physioxia Has a Beneficial Effect on Cartilage Matrix Production in Interleukin-1 Beta-Inhibited Mesenchymal Stem Cell Chondrogenesis. Cells 2019, 8, 936. https://doi.org/10.3390/cells8080936
Pattappa G, Schewior R, Hofmeister I, Seja J, Zellner J, Johnstone B, Docheva D, Angele P. Physioxia Has a Beneficial Effect on Cartilage Matrix Production in Interleukin-1 Beta-Inhibited Mesenchymal Stem Cell Chondrogenesis. Cells. 2019; 8(8):936. https://doi.org/10.3390/cells8080936
Chicago/Turabian StylePattappa, Girish, Ruth Schewior, Isabelle Hofmeister, Jennifer Seja, Johannes Zellner, Brian Johnstone, Denitsa Docheva, and Peter Angele. 2019. "Physioxia Has a Beneficial Effect on Cartilage Matrix Production in Interleukin-1 Beta-Inhibited Mesenchymal Stem Cell Chondrogenesis" Cells 8, no. 8: 936. https://doi.org/10.3390/cells8080936
APA StylePattappa, G., Schewior, R., Hofmeister, I., Seja, J., Zellner, J., Johnstone, B., Docheva, D., & Angele, P. (2019). Physioxia Has a Beneficial Effect on Cartilage Matrix Production in Interleukin-1 Beta-Inhibited Mesenchymal Stem Cell Chondrogenesis. Cells, 8(8), 936. https://doi.org/10.3390/cells8080936






