Tissue Specific Differentiation of Human Chondrocytes Depends on Cell Microenvironment and Serum Selection
Abstract
1. Introduction
2. Materials and Methods
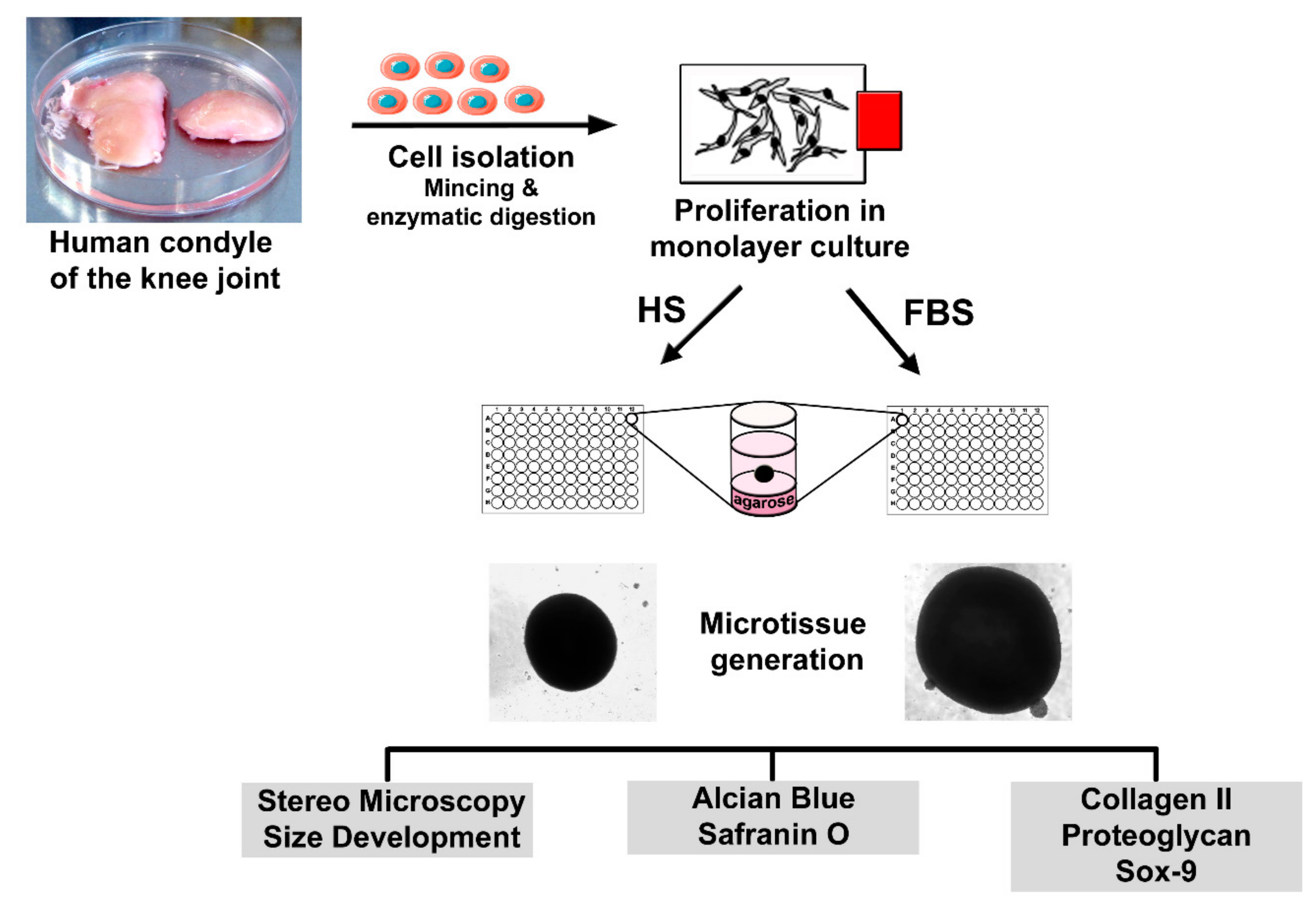
2.1. Isolation of Human Chondrocytes and Cultivation as a Monolayer (2D)
2.2. Generation of Microtissues (3D) and Sample Preparation for Analyses
2.3. Immunochemical and Histological Analysis
2.4. Visualization of Actin Structures in Chondrocytes
2.5. Viability Analysis of Cells in Microtissues
2.6. Microscopic Analysis
2.7. Statistical Analysis
3. Results
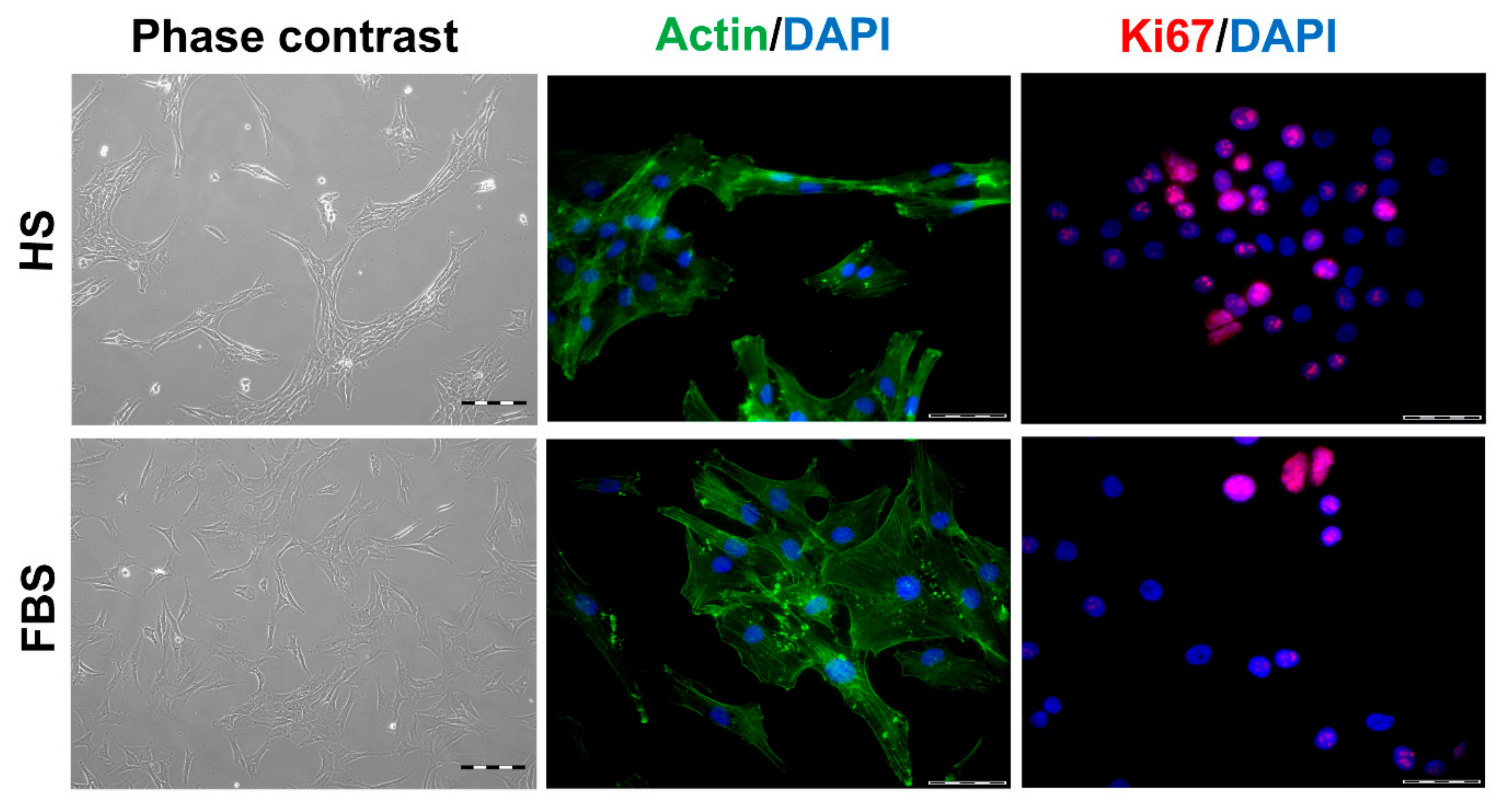
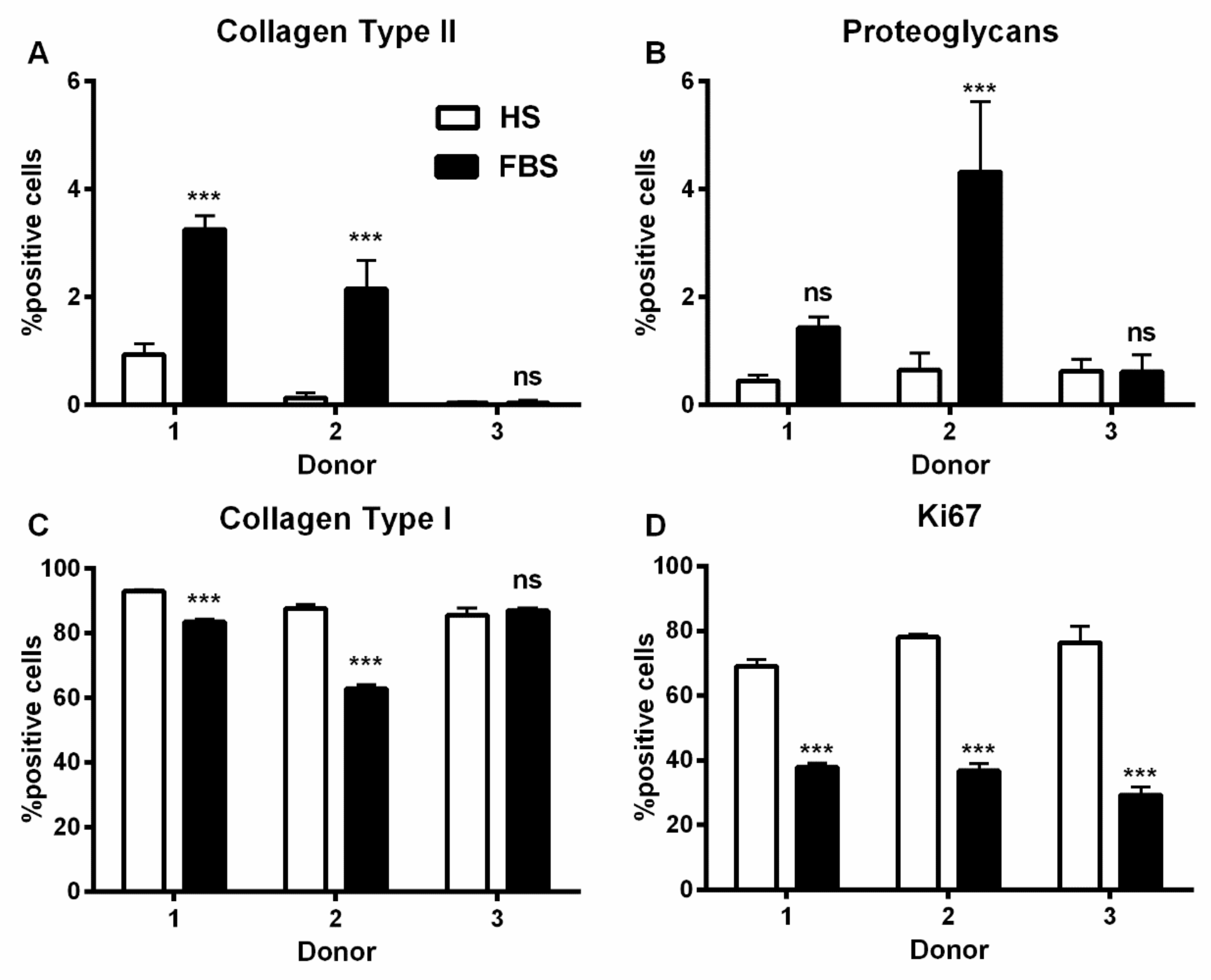
3.1. Chondrocytes in 2D: Reduction of Chondrogenic Markers Paralleled by Augmentation of Proliferation
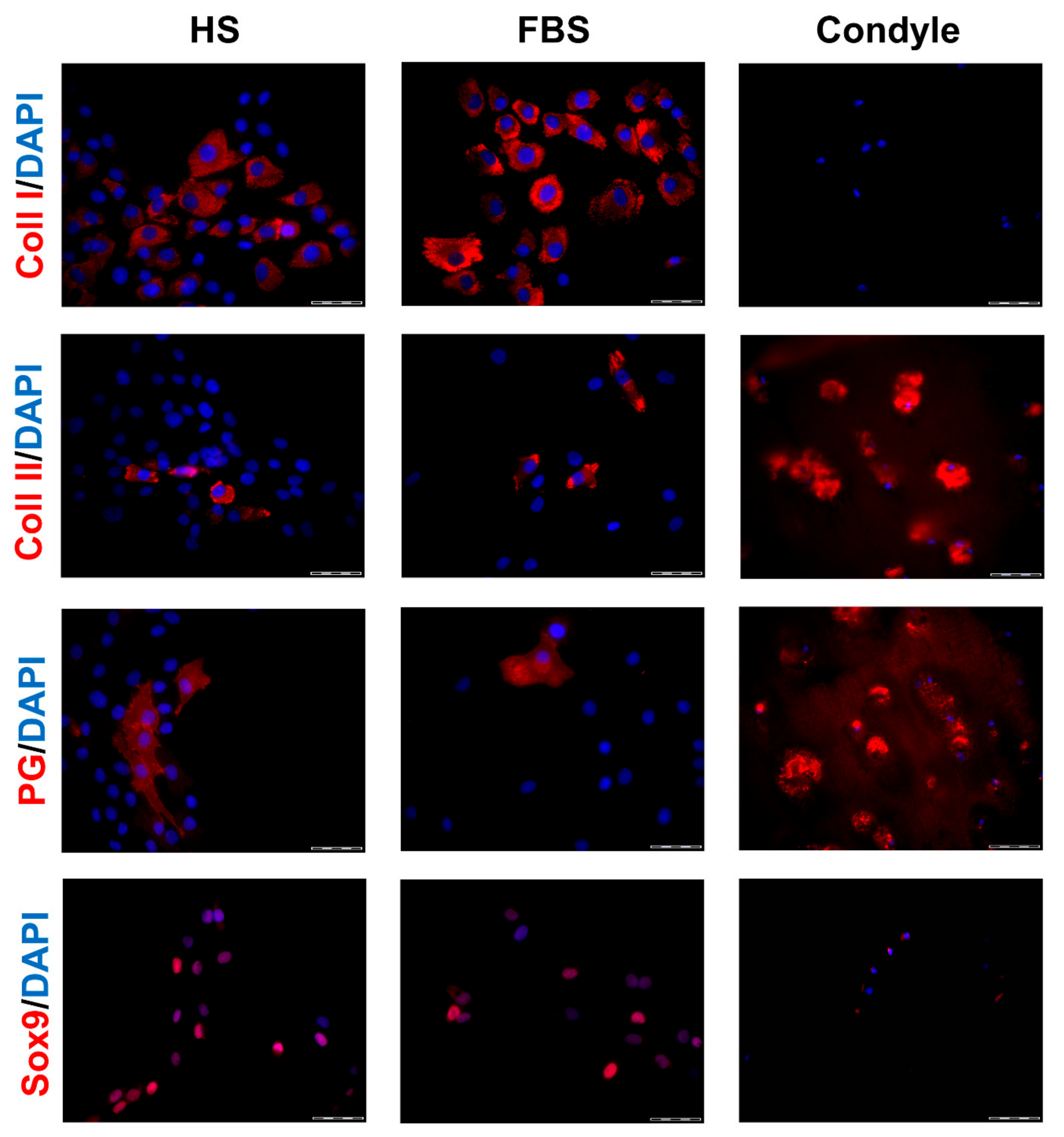
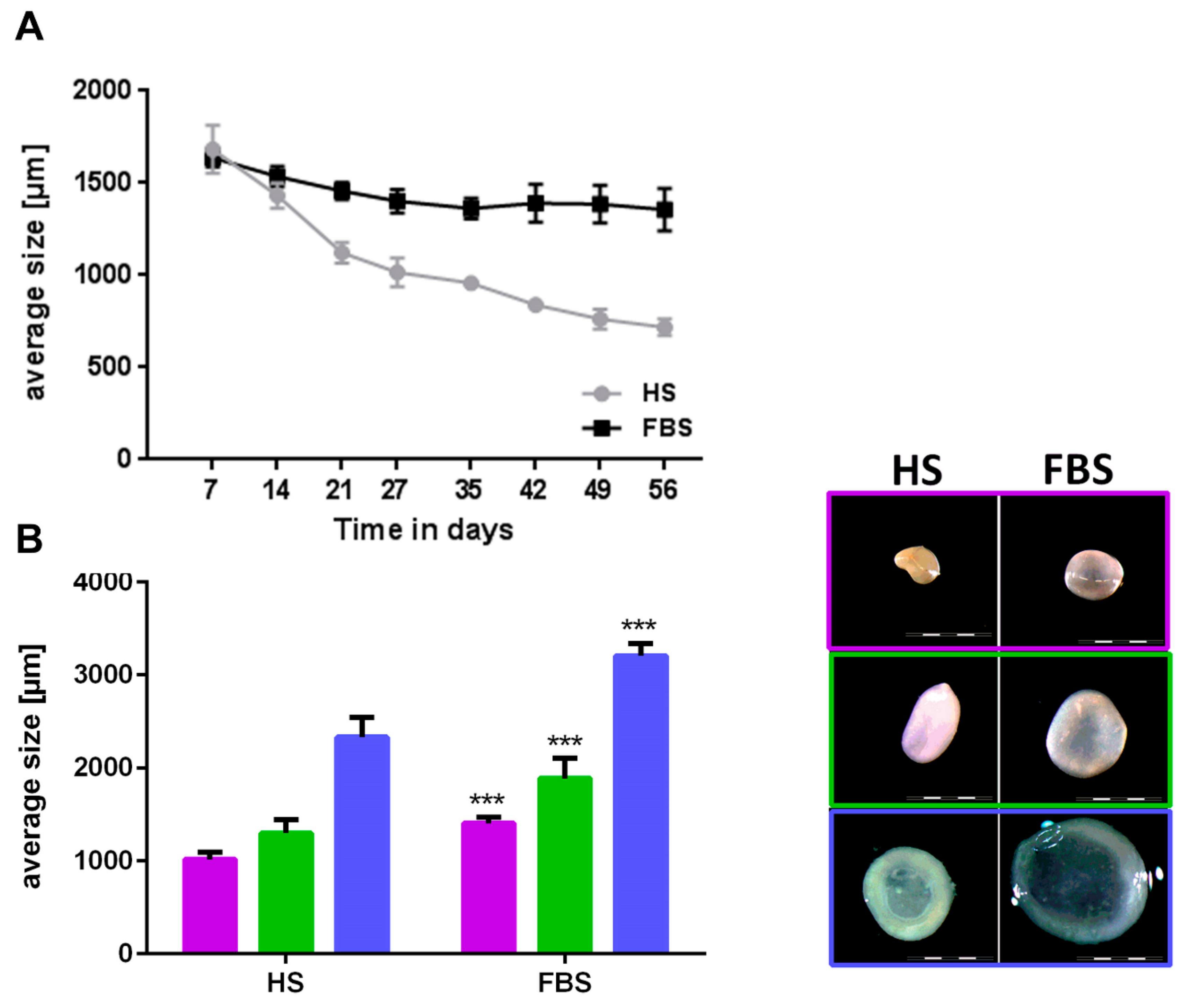
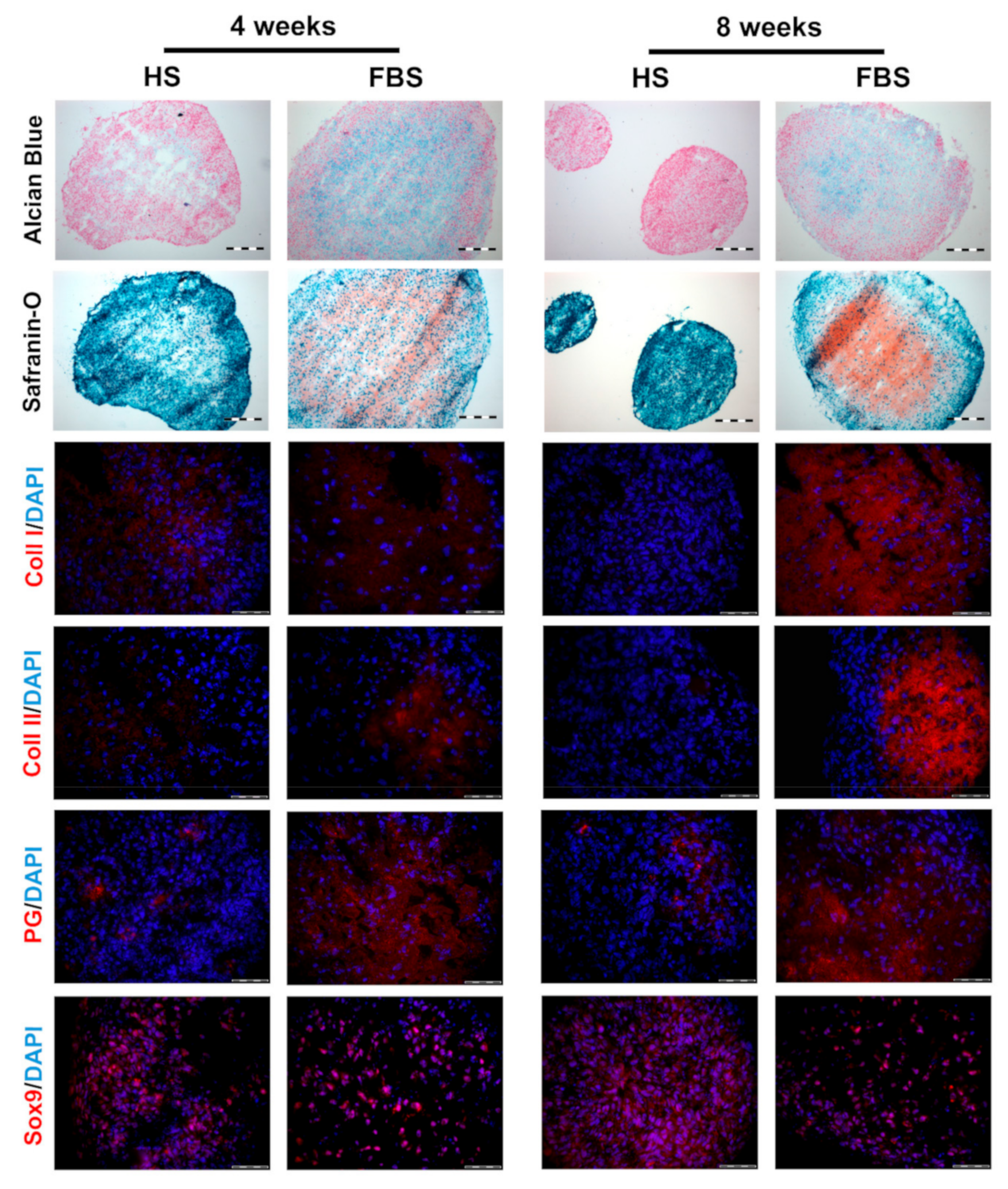
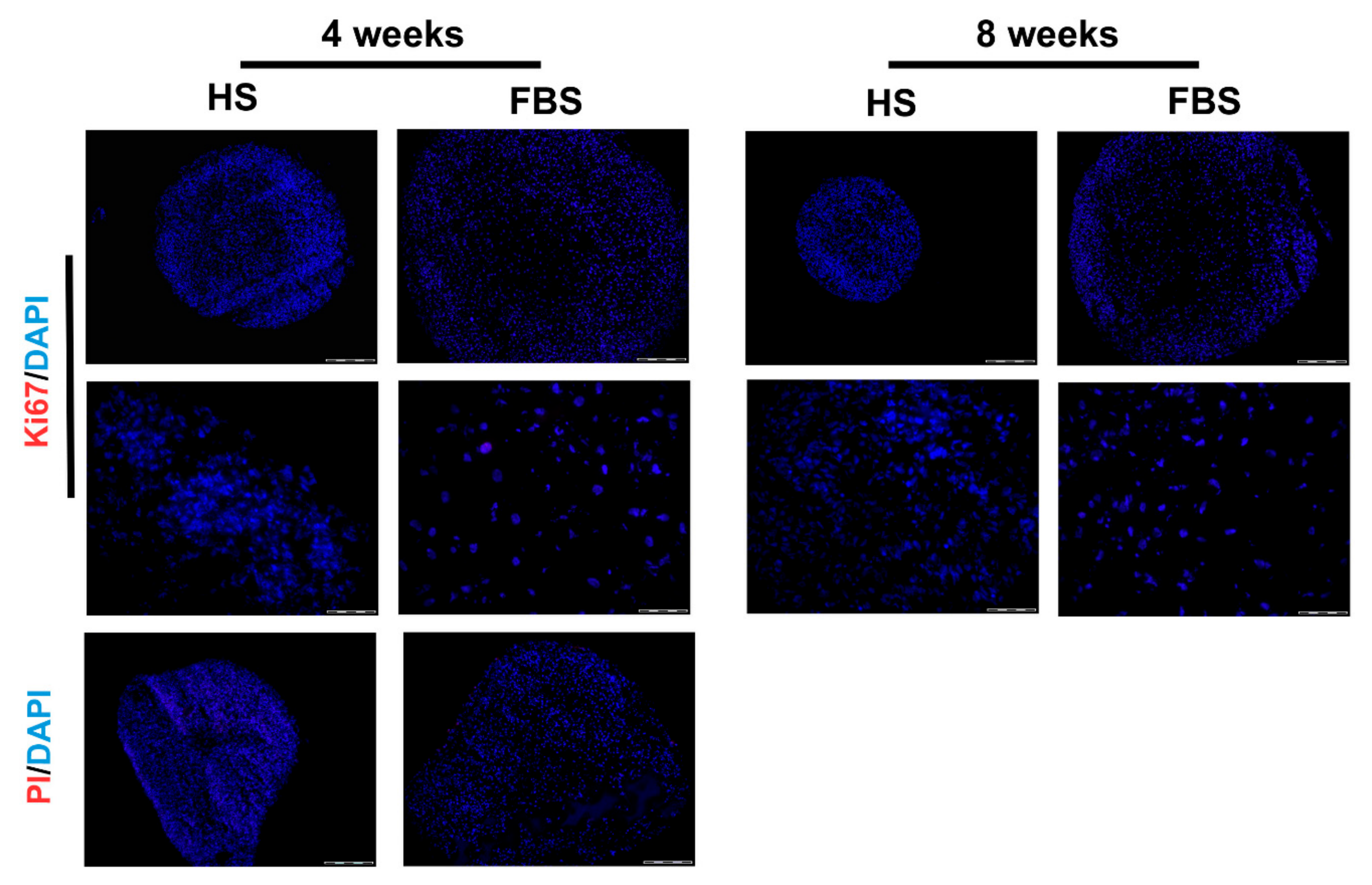
3.2. Chondrocytes in 3D: Differentiation Depends on Serum Type
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Losina, E.; Weinstein, A.M.; Reichmann, W.M.; Burbine, S.A.; Solomon, D.H.; Daigle, M.E.; Rome, B.N.; Chen, S.P.; Hunter, D.J.; Suter, L.G.; et al. Lifetime Risk and Age at Diagnosis of Symptomatic Knee Osteoarthritis in the US: Incidence and Lifetime Risk of Diagnosed Symptomatic Knee OA. Arthritis Care Res. 2013, 65, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthritis Cartilage 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Harvey, W.; Yang, M.; Cooke, T.; Segal, N.; Lane, N.; Lewis, C.; Felson, D. Associations Of Leg Length Inequality With Prevalent, Incident, And Progressive Knee Osteoarthritis: A Cohort Study. Ann. Intern. Med. 2010, 152, 287–295. [Google Scholar] [CrossRef]
- Øiestad, B.E.; Juhl, C.B.; Eitzen, I.; Thorlund, J.B. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage 2015, 23, 171–177. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Ademi, Z.; Osborne, R.H.; Liew, D. Comparison of Health-Related Quality of Life, Work Status, and Health Care Utilization and Costs According to Hip and Knee Joint Disease Severity: A National Australian Study. Phys. Ther. 2013, 93, 889–899. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J. Osteoarthritis year in review 2018: Biology. Osteoarthritis Cartilage 2019, 27, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.; Hübscher, M.; O’Leary, H.; Moloney, N. Current concepts in joint pain in knee osteoarthritis. Schmerz 2019, 33, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Chan, P.; Chiu, K.; Yan, C.; Yeung, S.; Ng, Y.; Shiu, K.; Ho, T. Non-surgical treatment of knee osteoarthritis. Hong Kong Med. J. 2019, 25, 127–133. [Google Scholar]
- Shi, Y.; Hu, X.; Cheng, J.; Zhang, X.; Zhao, F.; Shi, W.; Ren, B.; Yu, H.; Yang, P.; Li, Z.; et al. A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development. Nat. Commun. 2019, 10, 1914. [Google Scholar] [CrossRef] [PubMed]
- Nehrer, S.; Minas, T. Treatment of Articular Cartilage Defects. Invest. Radiol. 2000, 35, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Bunpetch, V.; Zhou, W.; Ouyang, H. Optimization strategies for ACI: A step-chronicle review. J. Orthop. Translat. 2019, 17, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gionet-Gonzales, M.A.; Leach, J.K. Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 2018, 13, 034109. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Martin, F.; Lehmann, M.; Sack, U.; Anderer, U. Featured Article: In vitro development of personalized cartilage microtissues uncovers an individualized differentiation capacity of human chondrocytes. Exp. Biol. Med. 2017, 242, 1746–1756. [Google Scholar] [CrossRef]
- Lehmann, M.; Martin, F.; Mannigel, K.; Kaltschmidt, K.; Sack, U.; Anderer, U. Three-dimensional scaffold-free fusion culture: The way to enhance chondrogenesis of in vitro propagated human articular chondrocytes. Eur. J. Histochem. 2013, 57, e31. [Google Scholar] [CrossRef] [PubMed]
- Anderer, U.; Libera, J. In Vitro Engineering of Human Autogenous Cartilage. J. Bone Miner. Res. 2002, 17, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Fickert, S.; Gerwien, P.; Helmert, B.; Schattenberg, T.; Weckbach, S.; Kaszkin-Bettag, M.; Lehmann, L. One-Year Clinical and Radiological Results of a Prospective, Investigator-Initiated Trial Examining a Novel, Purely Autologous 3-Dimensional Autologous Chondrocyte Transplantation Product in the Knee. Cartilage 2012, 3, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, J.; Brunner, D.; De Smet, K.; Fex Svenningsen, Å.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.L.; et al. Optimization of chemically defined cell culture media – Replacing fetal bovine serum in mammalian in vitro methods. Toxicol. In Vitro 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Dessels, C.; Potgieter, M.; Pepper, M.S. Making the Switch: Alternatives to Fetal Bovine Serum for Adipose-Derived Stromal Cell Expansion. Front. Cell Dev. Biol. 2016, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Heger, J.I.; Froehlich, K.; Pastuschek, J.; Schmidt, A.; Baer, C.; Mrowka, R.; Backsch, C.; Schleußner, E.; Markert, U.R.; Schmidt, A. Human serum alters cell culture behavior and improves spheroid formation in comparison to fetal bovine serum. Exp. Cell Res. 2018, 365, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, J.; Mellor, D.; Brands, R.; Fischer, R.; Gruber, F.; Gstraunthaler, G.; Hellebrekers, L.; Hyllner, J.; Jonker, F.H.; Prieto, P.; et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol. In Vitro 2004, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Lehmann, M.; Anderer, U. Generation of scaffold free 3-D cartilage-like microtissues from human chondrocytes. In Medical Advancements in Aging and Regenerative Technologies: Clinical Tools and Applications; Daskalaki, A., Das, P., Eds.; Advances in Medical Technologies and Clinical Practice; IGI Global: Hershey, PA, USA, 2013; ISBN 978-1-4666-2506-8. [Google Scholar]
- Martin, F.; Lehmann, M.; Schlaeger, P.; Sack, U.; Anderer, U. Differentiation Capacity of Chondrocytes in Microtissues Depends on TGF-Beta Subtype. J. Biochip. Tissue Chip. 2015, s2. [Google Scholar] [CrossRef]
- Rosenberg, L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J. Bone Joint Surg. Am. 1971, 53, 69–82. [Google Scholar] [CrossRef]
- Steedman, H.F. Alcian blue 8GS: A new stain for mucin. Q. J. Microsc. Sci. 1950, 91, 477–479. [Google Scholar]
- Dell’Accio, F.; De Bari, C.; Luyten, F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001, 44, 1608–1619. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Growth factor impact on articular cartilage subpopulations. Cell Tissue Res. 2005, 322, 463–473. [Google Scholar] [CrossRef]
- Gruber, R.; Sittinger, M.; BujIa, J. Untersuchungen zur In-vitro-Kulti- vierung von Humanchondrozyten bei Einsatz von autologem Human- serum als Mediumzusatz: Minimierung des moglichen Risikos einer lnfektion mit Erregern von Prionen-Erkrankungen. Laryngo. Rhino. Otol. 1996, 75, 105–108. [Google Scholar] [CrossRef]
- Tallheden, T.; Van Der Lee, J.; Brantsing, C.; Månsson, J.-E.; Sjögren-Jansson, E.; Lindahl, A. Human Serum for Culture of Articular Chondrocytes. Cell Transplant. 2005, 14, 469–479. [Google Scholar] [CrossRef]
- Choi, Y.C.; Morris, G.M.; Lee, F.S.; Sokoloff, L. The Effect of Serum on Monolayer Cell Culture of Mammalian Articular Chondrocytes. Connect. Tissue Res. 1980, 7, 105–112. [Google Scholar] [CrossRef]
- Emerman, J.T.; Fiedler, E.E.; Tolcher, A.W.; Rebbeck, P.M. Effects of defined medium, fetal bovine serum, and human serum on growth and chemosensitivities of human breast cancer cells in primary culture: Inference for in vitro assays. In Vitro Cell. Dev. Biol. 1987, 23, 134–140. [Google Scholar] [CrossRef]
- Cánovas, D. Human AB serum as an alternative to fetal bovine serum for endothelial and cancer cell culture. ALTEX 2012, 29, 426–428. [Google Scholar] [CrossRef]
- Dehne, T.; Karlsson, C.; Ringe, J.; Sittinger, M.; Lindahl, A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res. Ther. 2009, 11, R133. [Google Scholar] [CrossRef]
- Chaipinyo, K.; Oakes, B.W.; Van Damme, M.-P.I. The use of debrided human articular cartilage for autologous chondrocyte implantation: Maintenance of chondrocyte differentiation and proliferation in type I collagen gels. J. Orthop. Res. 2004, 22, 446–455. [Google Scholar] [CrossRef]
- Meretoja, V.V.; Dahlin, R.L.; Wright, S.; Kasper, F.K.; Mikos, A.G. Articular Chondrocyte Redifferentiation in 3D Co-cultures with Mesenchymal Stem Cells. Tissue Eng. Part C Methods 2014, 20, 514–523. [Google Scholar] [CrossRef]
- Akiyama, H. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef]
- Kamil, S.H.; Kojima, K.; Vacanti, M.P.; Zaporojan, V.; Vacanti, C.A.; Eavey, R.D. Tissue engineered cartilage: Utilization of autologous serum and serum-free media for chondrocyte culture. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 71–75. [Google Scholar] [CrossRef]
- Wolf, F.; Haug, M.; Farhadi, J.; Candrian, C.; Martin, I.; Barbero, A. A low percentage of autologous serum can replace bovine serum to engineer human nasal cartilage. Eur. Cell. Mater. 2008, 15, 1–10. [Google Scholar] [CrossRef]
| Donor | Gender | Age | Macroscopic Appearance | Cell Yield | Cell Viability (%) |
|---|---|---|---|---|---|
| 1 | Female | 57 | Smooth, intact surface | 5.3 × 106 | 92.80 |
| 2 | Male | 49 | Smooth, intact surface | 3.34 × 106 | 92.40 |
| 3 | Female | 73 | Surface discontinuity, fibrillation visible | 6.44 × 106 | 91.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ecke, A.; Lutter, A.-H.; Scholka, J.; Hansch, A.; Becker, R.; Anderer, U. Tissue Specific Differentiation of Human Chondrocytes Depends on Cell Microenvironment and Serum Selection. Cells 2019, 8, 934. https://doi.org/10.3390/cells8080934
Ecke A, Lutter A-H, Scholka J, Hansch A, Becker R, Anderer U. Tissue Specific Differentiation of Human Chondrocytes Depends on Cell Microenvironment and Serum Selection. Cells. 2019; 8(8):934. https://doi.org/10.3390/cells8080934
Chicago/Turabian StyleEcke, Annemarie, Anne-Helen Lutter, Jenny Scholka, Anna Hansch, Roland Becker, and Ursula Anderer. 2019. "Tissue Specific Differentiation of Human Chondrocytes Depends on Cell Microenvironment and Serum Selection" Cells 8, no. 8: 934. https://doi.org/10.3390/cells8080934
APA StyleEcke, A., Lutter, A.-H., Scholka, J., Hansch, A., Becker, R., & Anderer, U. (2019). Tissue Specific Differentiation of Human Chondrocytes Depends on Cell Microenvironment and Serum Selection. Cells, 8(8), 934. https://doi.org/10.3390/cells8080934





