Factors Affecting Microalgae Production for Biofuels and the Potentials of Chemometric Methods in Assessing and Optimizing Productivity
Abstract
:1. Introduction
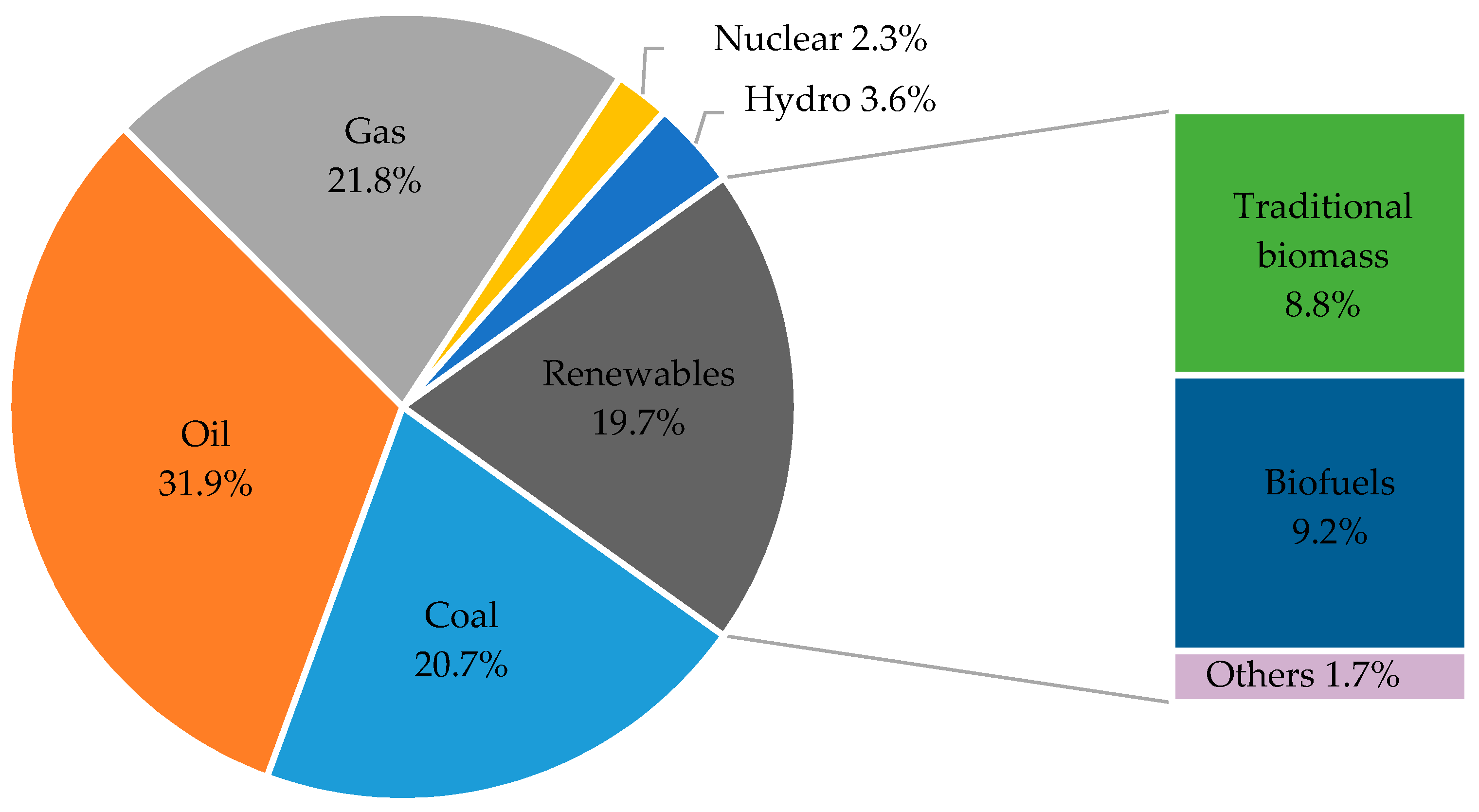
2. Renewable Energy and the Prospects of Microalgae Biofuels
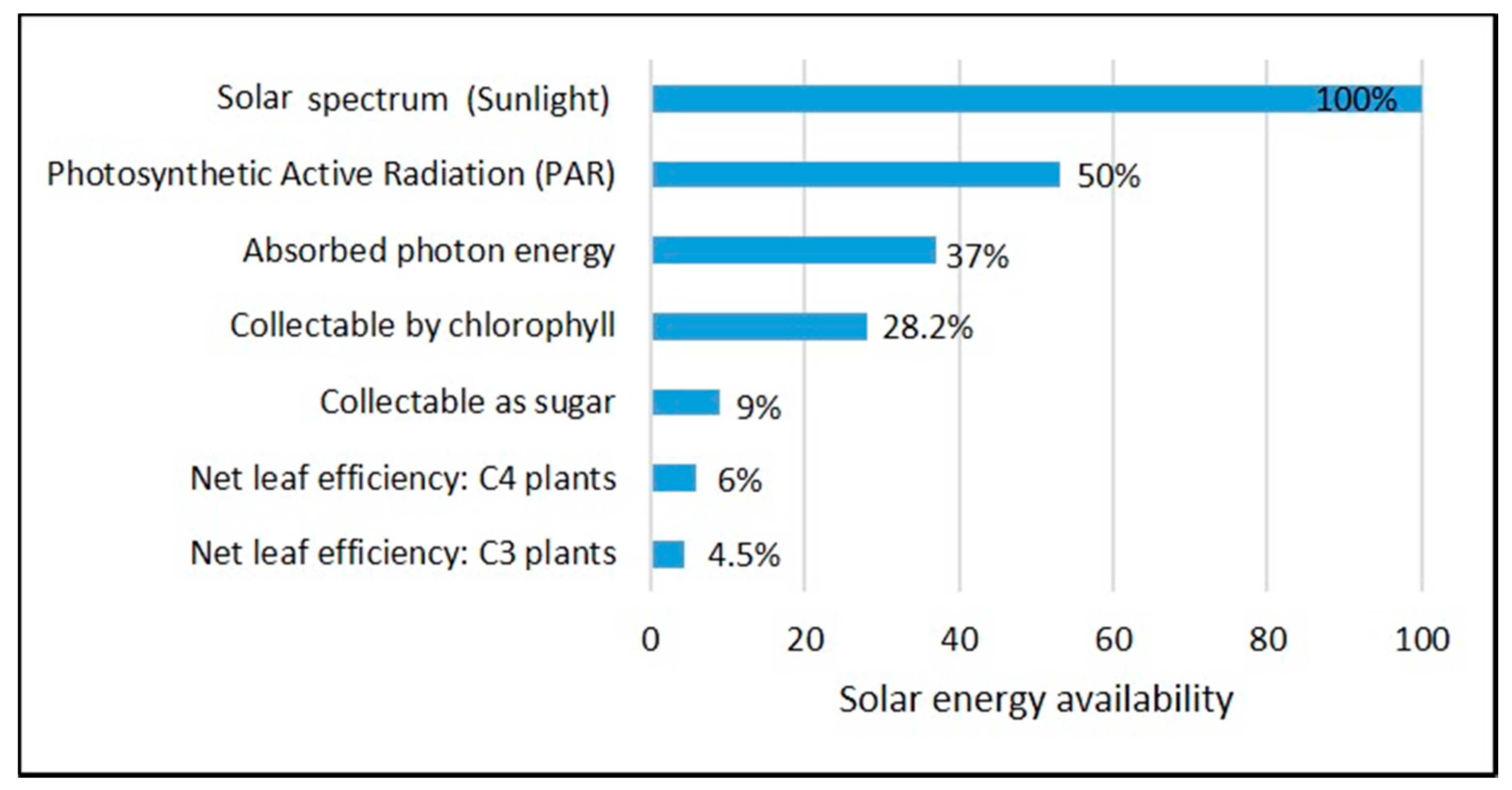
3. Microalgae Productivity: Opportunities and Challenges
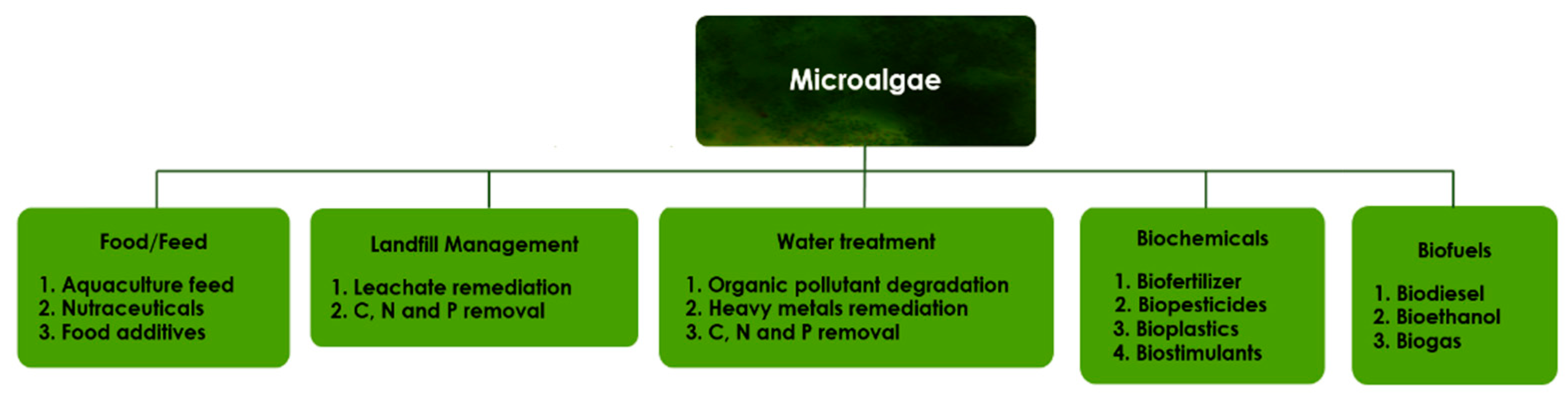
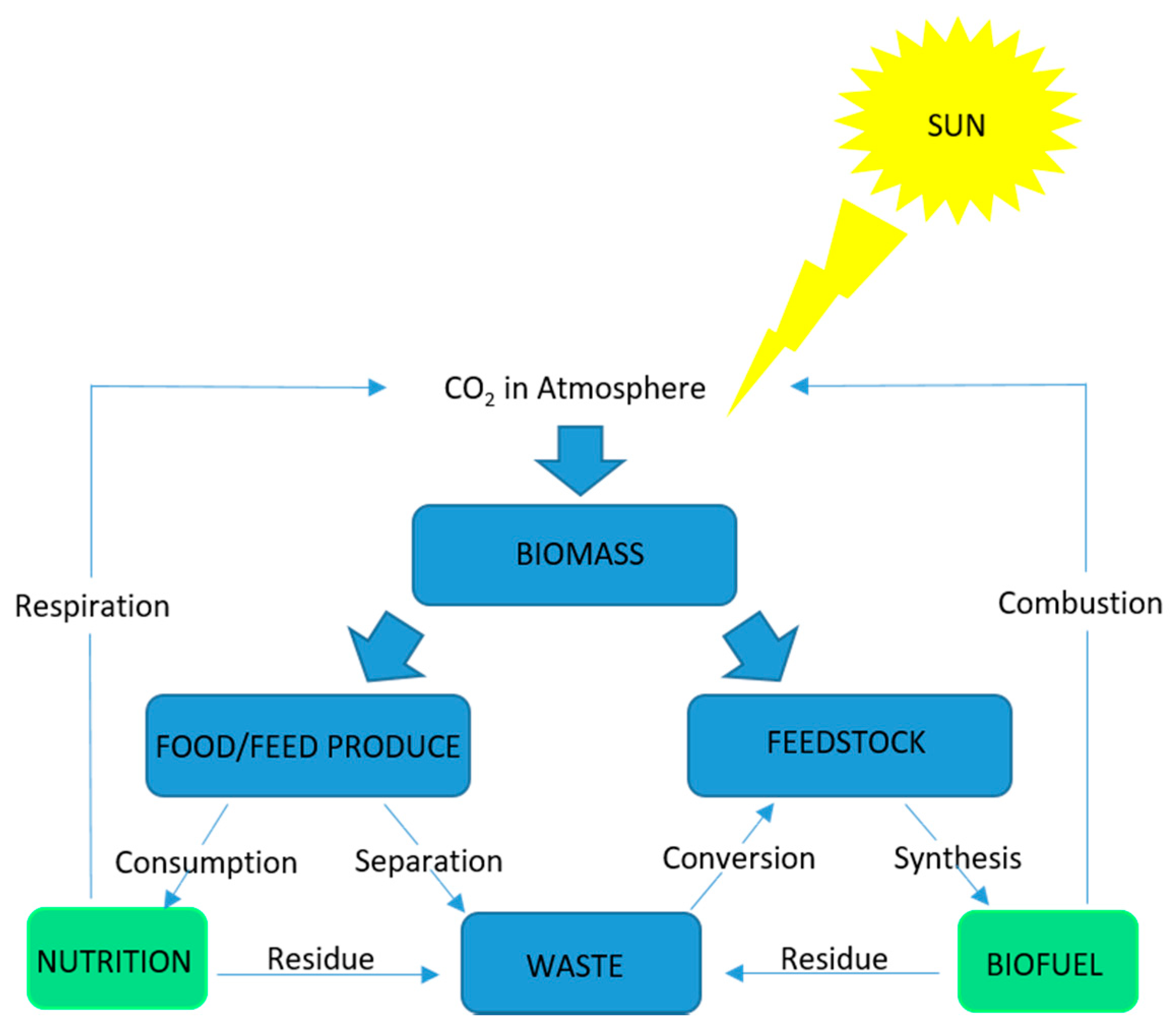
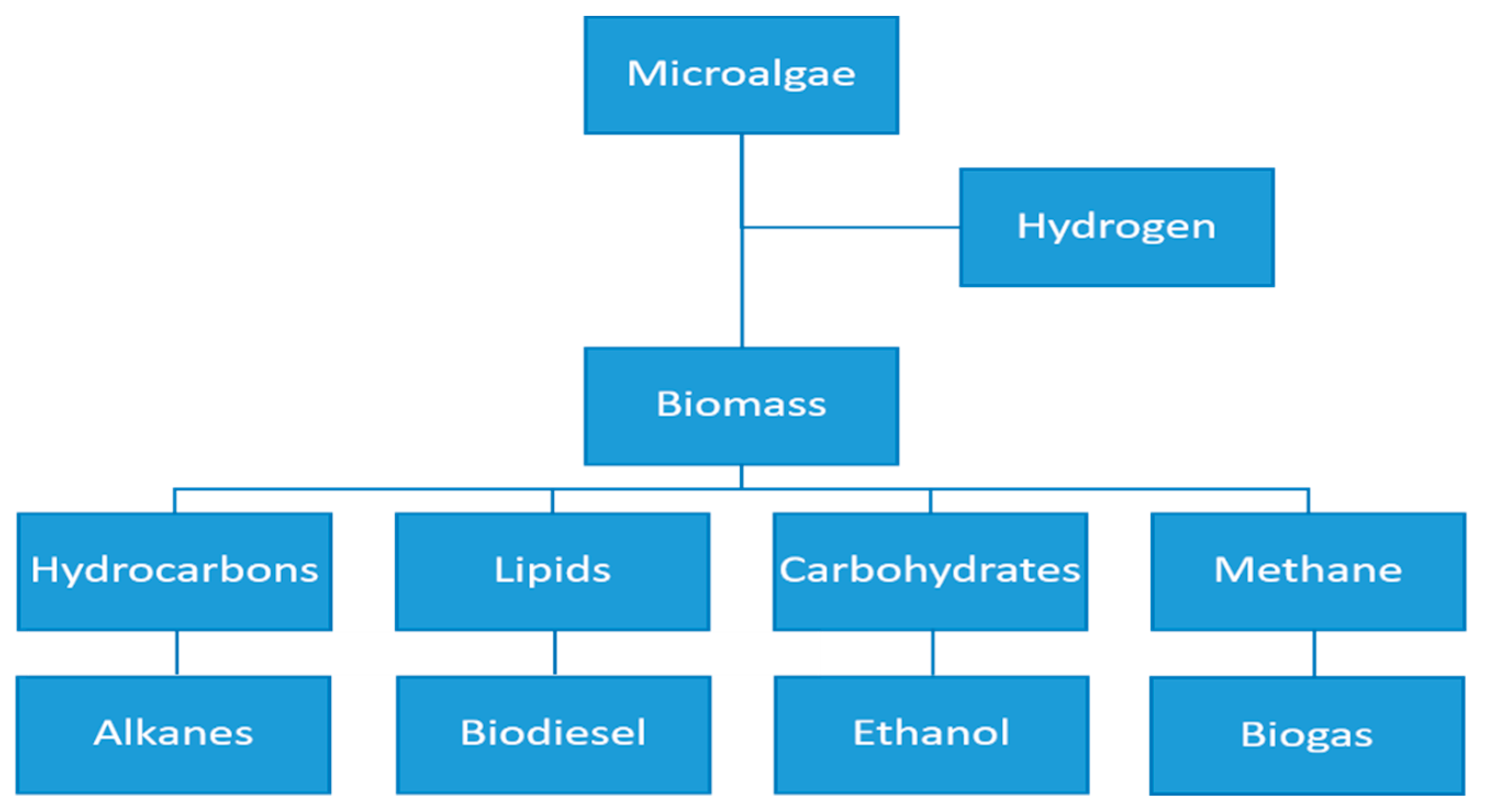
4. Microalgae Production in a Biorefinery Context
5. Chemometrics as a Tool for Multivariate Analysis (MVA)
6. Chemometrics in the Production and Processing of Microalgae
6.1. Characterization and Classification
6.2. Upstream Processes
6.2.1. Cultivation System Selection and Operation
6.2.2. Nutrient Sources and Conditions
6.2.3. Photoperiod and Trophic Conditions
6.2.4. Proteomic Metabolism
6.3. Downstream Processes
6.3.1. Dewatering
6.3.2. Lipid Disruption, Extraction and Estimation
6.3.3. Hydrothermal Liquefaction (HTL)
6.3.4. Fuel Quantity and Quality Estimation
7. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D-DIGE | Two Dimensional Differential In-Gel Electrophoresis |
| ANOVA | Analysis of Variance |
| BDDJ | Britt Dynamic Drainage Jar |
| BEV | Battery Electric Vehicle |
| BTEM-MLR | Band-Target Entropy Minimization Multi-Linear Regression |
| CA | Cluster Analysis |
| DC | Desalination Concentration |
| DM | Decision Maker |
| DO | Dissolved Oxygen |
| DOE | Design of Experiment |
| EEM | Excitation-Emission Matrices |
| FA | Fatty Acid |
| FAME | Fatty Acid Methyl Ester |
| FCV | Fuel Cell Vehicle |
| FPA | Focal Plane Array |
| FPB | Flat Plate Bioreactor |
| FTIR | Fourier Transform Infra-Red |
| GAIA | Graphical Analysis for Interactive Aid |
| GC | Gas Chromatography |
| GC-FID | Gas Chromatography-Flame Ionization Detection |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| GHG | Greenhouse Gas |
| HHV | Higher Heating Value |
| iLFCA | Improved Leader Follower Cluster Analysis |
| IR | Infra-Red |
| kNN | k-Nearest Neighbors |
| MCDA | Multi-Criteria Decision Analysis |
| MDS | Multi-Dimensional Scaling |
| MLR | Multi-Linear Regression |
| MUFA | Monounsaturated Fatty Acid |
| MVA | Multi-Variate Analysis |
| NMR | Nuclear Magnetic Resonance |
| NREL | National Renewable Energy Laboratory |
| OFAT | One Factor At a Time |
| OR | Operations Research |
| ORP | Open Raceway Pond |
| PAR | Photosynthetic Active Radiation |
| PBR | Photobioreactor |
| PCA | Principal Component Analysis |
| PCE | Photon Conversion Efficiency |
| PHEV | Plug-in Hybrid Electric Vehicle |
| PLSDA | Partial List Squares Discriminant Analysis |
| PLSR | Partial Least Square Regression |
| PROMETHEE | Preference Ranking Organization Method for Enrichment Evaluation |
| RDB | Renewable Diesel Blendstock |
| SIMCA | Soft Independent Modeling of Class Analogy |
| SVM | Support Vector Machine |
| TAG | Triacylglycerol |
| TAPPI | Technical Association of the Pulp and Paper Industry |
| TWIM–MS | Traveling Wave Ion Mobility–Mass Spectrometry |
| UHPLC–HDMS | Ultrahigh-Performance Liquid Chromatography–High Definition Mass Spectrometry |
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal Biofuels: Current Status and Key Challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Sivakaminathan, S.; Hankamer, B.; Wolf, J.; Yarnold, J. High-throughput optimisation of light-driven microalgae biotechnologies. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokot, S.; Ayoko, G.A. CHEMOMETRICS AND STATISTICS|Multicriteria Decision Making. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 40–45. [Google Scholar]
- Wold, S. Chemometrics, why, what and where to next? J. Pharm. Biomed. Anal. 1991, 9, 589–596. [Google Scholar] [CrossRef]
- Keller, H.R.; Massart, D.L.; Brans, J.P. Multicriteria decision making: A case study. Chemom. Intell. Lab. Syst. 1991, 11, 175–189. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments; Productivity Press: New York, NY, USA, 2005. [Google Scholar]
- Kokot, S.; Grigg, M.; Panayiotou, H.; Phuong, T.D. Data Interpretation by some Common Chemometrics Methods. Electroanalysis 1998, 10, 1081–1088. [Google Scholar] [CrossRef]
- Painuly, J.P. Barriers to renewable energy penetration: A framework for analysis. Renew. Energy 2001, 24, 73–89. [Google Scholar] [CrossRef]
- BP Plc. BP Statistical Review of World Energy. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf (accessed on 21 July 2019).
- Luthra, S.; Kumar, S.; Garg, D.; Haleem, A. Barriers to renewable/sustainable energy technologies adoption: Indian perspective. Renew. Sustain. Energy Rev. 2015, 41, 762–776. [Google Scholar] [CrossRef]
- Gürtürk, M.; Benli, H.; Ertürk, N.K. Effects of different parameters on energy—Exergy and power conversion efficiency of PV modules. Renew. Sustain. Energy Rev. 2018, 92, 426–439. [Google Scholar] [CrossRef]
- Hill, J.S. Are Photovoltaics or Biofuels Better at Energy Conversion? Available online: https://cleantechnica.com/2013/01/18/are-photovoltaics-or-biofuels-better-at-energy-conversion/ (accessed on 12 December 2016).
- Sandy Thomas, C.E. Transportation options in a carbon-constrained world: Hybrids, plug-in hybrids, biofuels, fuel cell electric vehicles, and battery electric vehicles. Int. J. Hydrog. Energy 2009, 34, 9279–9296. [Google Scholar] [CrossRef]
- Contestabile, M.; Offer, G.J.; Slade, R.; Jaeger, F.; Thoennes, M. Battery electric vehicles, hydrogen fuel cells and biofuels. Which will be the winner? Energy Environ. Sci. 2011, 4, 3754–3772. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.J.; Yoo, S.H. Public value of marine biodiesel technology development in South Korea. Sustainability 2018, 10, 4252. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef] [Green Version]
- Klass, D.L. Biomass for renewable energy and fuels. Encycl. Energy 2004, 1, 193–212. [Google Scholar]
- Novozymes Australia Pty Ltd. Review of EU Biofuels Directive, Public Consultation Exercise, April–July 2006 Input from Novozymes A/S. Available online: http://news.bio-based.eu/media/news-images/20060613-08/novozymes.pdf (accessed on 12 March 2019).
- Musa, M.; Doshi, A.; Brown, R.; Rainey, T.J. Microalgae dewatering for biofuels: A comparative techno-economic assessment using single and two-stage technologies. J. Clean. Prod. 2019, 229, 325–336. [Google Scholar] [CrossRef]
- Um, B.H.; Kim, Y.S. Review: A chance for Korea to advance algal-biodiesel technology. J. Ind. Eng. Chem. 2009, 15, 1–7. [Google Scholar] [CrossRef]
- Lee, R.A.; Lavoie, J.-M. From first-to third-generation biofuels: Challenges of producing a commodity from a biomass of increasing complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life cycle assessment of biofuel production from brown seaweed in Nordic conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.J.; Sarker, S.; Hjelme, D.R.; Lien, K.M. Fermentative Bioethanol Production Using Enzymatically Hydrolysed Saccharina latissima. Adv. Microbiol. 2018, 8, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Deng, C.; Ding, L.; Bose, A.; Murphy, J.D. Improving gaseous biofuel production from seaweed Saccharina latissima: The effect of hydrothermal pretreatment on energy efficiency. Energy Convers. Manag. 2019, 196, 1385–1394. [Google Scholar] [CrossRef]
- Sánchez, J.; Curt, M.D.; Robert, N.; Fernández, J. Biomass Resources. Role Bioenergy Bioecon. 2019, 25–111. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). State of Technology Review—Algae Bioenergy An IEA Bioenergy Inter-Task Strategic Project. Available online: http://www.ieabioenergy.com/wp-content/uploads/2017/01/IEA-Bioenergy-Algae-report-update-20170114.pdf (accessed on 9 August 2018).
- Williams, P.J.L.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 554–590. [Google Scholar] [CrossRef]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Optimisation of tray drier microalgae dewatering techniques using response surface methodology. Energies 2018, 11, 2327. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Davis, R.; Laurens, L.M.L.; Van Wychen, S.; Pienkos, P.T.; Nagle, N. Combined algal processing: A novel integrated biorefinery process to produce algal biofuels and bioproducts. Algal Res. 2016, 19, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Sharma, K.K.; Garg, S.; Li, Y.; Malekizadeh, A.; Schenk, P.M. Critical analysis of current microalgae dewatering techniques. Biofuels 2013, 4, 397–407. [Google Scholar] [CrossRef]
- Dogan-Subasi, E.; Demirer, G.N. Anaerobic Digestion of Microalgal (Chlorella vulgaris) Biomass as a Source of Biogas and Biofertilizer. Environ. Prog. Sustain. Energy 2016, 35, 936–941. [Google Scholar] [CrossRef]
- Mirsiaghi, M.; Reardon, K.F. Conversion of lipid-extracted Nannochloropsis salina biomass into fermentable sugars. Algal Res. 2015, 8, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Yarnold, J.; Ross, I.L.; Hankamer, B. Photoacclimation and productivity of Chlamydomonas reinhardtii grown in fluctuating light regimes which simulate outdoor algal culture conditions. Algal Res. 2016, 13, 182–194. [Google Scholar] [CrossRef]
- González-Garcinuño, Á.; Tabernero, A.; Sánchez-Álvarez, J.M.; Martin del Valle, E.M.; Galán, M.A. Effect of nitrogen source on growth and lipid accumulation in Scenedesmus abundans and Chlorella ellipsoidea. Bioresour. Technol. 2014, 173, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Du, K.; Wang, Z.; Peng, X.; Luo, L.; Tao, H.; Xu, Y.; Zhang, D.; Geng, Y.; Li, Y. Effective cultivation of microalgae for biofuel production: A pilot-scale evaluation of a novel oleaginous microalga Graesiella sp. WBG-1. Biotechnol. Biofuels 2016, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.; Rosa, G.M.; Morillas España, A.; Santos, L.O.; Morais, M.G.; Molina Grima, E.; Costa, J.A.V.; Acién Fernández, F.G. Engineering strategies for the enhancement of Nannochloropsis gaditana outdoor production: Influence of the CO2 flow rate on the culture performance in tubular photobioreactors. Process Biochem. 2019, 76, 171–177. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Wolf, J.; Stephens, E.; Steinbusch, S.; Yarnold, J.; Ross, I.L.; Steinweg, C.; Doebbe, A.; Krolovitsch, C.; Müller, S.; Jakob, G.; et al. Multifactorial comparison of photobioreactor geometries in parallel microalgae cultivations. Algal Res. 2016, 15, 187–201. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Tan, X.B.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Wong, C.Y.; Lee, K.T. Cultivation of microalgae for biodiesel production: A review on upstream and downstream processing. Chin. J. Chem. Eng. 2018, 26, 17–30. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Yen, H.-W.; Hu, I.-C.; Chen, C.-Y.; Nagarajan, D. Design of photobioreactors for algal cultivation. Biofuels Algae 2019, 225–256. [Google Scholar] [CrossRef]
- Berner, F.; Heimann, K.; Sheehan, M. Microalgal biofilms for biomass production. J. Appl. Phycol. 2015, 27, 1793–1804. [Google Scholar] [CrossRef]
- Roostaei, J.; Zhang, Y.; Gopalakrishnan, K.; Ochocki, A.J. Mixotrophic Microalgae Biofilm: A Novel Algae Cultivation Strategy for Improved Productivity and Cost-efficiency of Biofuel Feedstock Production. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Dahms, H.-U.; Ponnusamy, V.K. Potential of two-stage cultivation in microalgae biofuel production. Fuel 2019, 252, 339–349. [Google Scholar] [CrossRef]
- Krasowska, A.; Jablonski, S.; Biniarz, P.; Plachetka, M.; Lukaszewicz, M. Microalgae—Biodiesel Potential Producers: A Review. Eur. Sci. J. 2013, 9, 24–26. [Google Scholar]
- International Energy Agency (IEA). Task 42 Biorefinery: Bio Based Chemicals Value Added Products from Biorefineries. Available online: http://www.ieabioenergy.com/wp-content/uploads/2013/10/Task-42-Booklet.pdf (accessed on 9 August 2018).
- Doshi, A.; Pascoe, S.; Coglan, L.; Rainey, T. The financial feasibility of microalgae biodiesel in an integrated, multi-output production system. Biofuels Bioprod. Biorefin. 2017, 11, 991–1006. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: Essex, UK, 2010. [Google Scholar]
- Brereton, R.G. Chemometrics for Pattern Recognition; John Wiley & Sons: Bristol, UK, 2009. [Google Scholar]
- Liu, G.; Ma, F.; Liu, G.; Zhao, H.; Guo, J.; Cao, J. Application of Multivariate Statistical Analysis to Identify Water Sources in A Coastal Gold Mine, Shandong, China. Sustainability 2019, 11, 3345. [Google Scholar] [CrossRef]
- Musa, M.; Kikuchi, A.; Ismail, N.E.; Jaafar, J.; Abdul Majid, Z.; Salim, M.R.; Tanaka, K. Application of ion chromatography for the assessment of cadmium adsorption in simulated wastewater by activated carbon. Desalin. Water Treat. 2014, 52, 3616–3622. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648. [Google Scholar] [CrossRef]
- Von der Kammer, F.; Ferguson, P.L.; Holden, P.A.; Masion, A.; Rogers, K.R.; Klaine, S.J.; Koelmans, A.A.; Horne, N.; Unrine, J.M. Analysis of engineered nanomaterials in complex matrices (environment and biota): General considerations and conceptual case studies. Environ. Toxicol. Chem. 2012, 31, 32–49. [Google Scholar] [CrossRef]
- Ayoko, G.A.; Bonire, J.J.; Abdulkadir, S.S.; Olurinola, P.F.; Ehinmidu, J.O.; Kokot, S.; Yiasel, S. A multicriteria ranking of organotin (IV) compounds with fungicidal properties. Appl. Organomet. Chem. 2003, 17, 749–758. [Google Scholar] [CrossRef]
- Xiong, X.; He, Y.; Feng, B.; Pan, Y.; Ke, X.; Han, L.; Zhang, D.; Zhang, Y. Identification of quality markers of Xiaojin Pills using a combination of high-performance liquid chromatographtandem mass spectrometry and multivariate analysis. Trop. J. Pharm. Res. 2018, 17, 1663–1672. [Google Scholar] [CrossRef]
- Jiménez-Carvelo, A.M.; Pérez-Castaño, E.; González-Casado, A.; Cuadros-Rodríguez, L. One input-class and two input-class classifications for differentiating olive oil from other edible vegetable oils by use of the normal-phase liquid chromatography fingerprint of the methyl-transesterified fraction. Food Chem. 2017, 221, 1784–1791. [Google Scholar] [CrossRef]
- Raponi, F.; Moscetti, R.; Monarca, D.; Colantoni, A.; Massantini, R. Monitoring and optimization of the process of drying fruits and vegetables using computer vision: A review. Sustainability 2017, 9, 2009. [Google Scholar] [CrossRef]
- Taguchi, Y.; Wang, H. Exploring MicroRNA Biomarkers for Parkinson’s Disease from mRNA Expression Profiles. Cells 2018, 7, 245. [Google Scholar] [CrossRef]
- Purcell, D.E.; Leonard, G.J.; O’Shea, M.G.; Kokot, S. A chemometrics investigation of sugarcane plant properties based on the molecular composition of epicuticular wax. Chemom. Intell. Lab. Syst. 2005, 76, 135–147. [Google Scholar] [CrossRef]
- Alzagameem, A.; Bergs, M.; Do, X.T.; Klein, S.E.; Rumpf, J.; Larkins, M.; Monakhova, Y.; Pude, R.; Schulze, M. Low-Input Crops as Lignocellulosic Feedstock for Second-Generation Biorefineries and the Potential of Chemometrics in Biomass Quality Control. Appl. Sci. 2019, 9, 2252. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Ross, I.L.; Kruse, O.; Hankamer, B. Selection, breeding and engineering of microalgae for bioenergy and biofuel production. Trends Biotechnol. 2012, 30, 198–204. [Google Scholar] [CrossRef]
- Fasciotti, M.; Souza, G.H.M.F.; Astarita, G.; Costa, I.C.R.; Monteiro, T.V.C.; Teixeira, C.M.L.L.; Eberlin, M.N.; Sarpal, A.S. Investigating the Potential of Ion Mobility-Mass Spectrometry for Microalgae Biomass Characterization. Anal. Chem. 2019, 91, 9266–9276. [Google Scholar] [CrossRef]
- Tan, S.T.; Balasubramanian, R.K.; Das, P.; Obbard, J.P.; Chew, W. Application of mid-infrared chemical imaging and multivariate chemometrics analyses to characterise a population of microalgae cells. Bioresour. Technol. 2013, 134, 316–323. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodriguez, L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: Modern lipid analysis. TrAC Trends Anal. Chem. 2009, 28, 263–278. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef]
- Giordano, M.; Kansiz, M.; Heraud, P.; Beardall, J.; Wood, B.; McNaughton, D. Fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalga Chaetoceros muellerii (Bacillariophyceae). J. Phycol. 2001, 37, 271–279. [Google Scholar] [CrossRef]
- Li, X. Infrared Application. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Spectroscopy/Vibrational_Spectroscopy/Infrared_Spectroscopy/Infrared%3A_Application (accessed on 25 May 2019).
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Henrion, R.; Henrion, G.; Böhme, M.; Behrendt, H. Three-way Principal Components Analysis for fluorescence spectroscopic classification of algae species. Fresenius. J. Anal. Chem. 1997, 357, 522–526. [Google Scholar] [CrossRef]
- Lopes, J.A.; Costa, P.F.; Alves, T.P.; Menezes, J.C. Chemometrics in bioprocess engineering: Process analytical technology (PAT) applications. Chemom. Intell. Lab. Syst. 2004, 74, 269–275. [Google Scholar] [CrossRef]
- Tescione, L.; Lambropoulos, J.; Paranandi, M.R.; Makagiansar, H.; Ryll, T. Application of bioreactor design principles and multivariate analysis for development of cell culture scale down models. Biotechnol. Bioeng. 2015, 112, 84–97. [Google Scholar] [CrossRef]
- Thomassen, Y.E.; van Sprang, E.N.M.; van der Pol, L.A.; Bakker, W.A.M. Multivariate data analysis on historical IPV production data for better process understanding and future improvements. Biotechnol. Bioeng. 2010, 107, 96–104. [Google Scholar] [CrossRef]
- San Pedro, A.; González-López, C.V.; Acién, F.G.; Molina-Grima, E. Outdoor pilot production of Nannochloropsis gaditana: Influence of culture parameters and lipid production rates in raceway ponds. Algal Res. 2015, 8, 205–213. [Google Scholar] [CrossRef]
- San Pedro, A.; González-López, C.V.; Acién, F.G.; Molina-Grima, E. Outdoor pilot-scale production of Nannochloropsis gaditana: Influence of culture parameters and lipid production rates in tubular photobioreactors. Bioresour. Technol. 2014, 169, 667–676. [Google Scholar] [CrossRef]
- San Pedro, A.; González-López, C.V.V.; Acién, F.G.G.; Molina-Grima, E. Outdoor pilot production of Nannochloropsis gaditana: Influence of culture parameters and lipid production rates in flat-panel photobioreactors. Algal Res. 2016, 18, 156–165. [Google Scholar] [CrossRef]
- He, S.; Fang, S.; Xie, W.; Zhang, P.; Li, Z.; Zhou, D.; Zhang, Z.; Guo, J.; Du, C.; Du, J.; et al. Assessment of physiological responses and growth phases of different microalgae under environmental changes by Raman spectroscopy with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 287–294. [Google Scholar] [CrossRef]
- Obeid, F.; Chu Van, T.; Brown, R.; Rainey, T. Nitrogen and sulphur in algal biocrude: A review of the HTL process, upgrading, engine performance and emissions. Energy Convers. Manag. 2019, 181, 105–119. [Google Scholar] [CrossRef]
- Jiménez, C.; Cossío, B.R.; Niell, F.X. Relationship between physicochemical variables and productivity in open ponds for the production of Spirulina: A predictive model of algal yield. Aquaculture 2003, 221, 331–345. [Google Scholar] [CrossRef]
- Tan, X.B.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Wong, C.Y.; Ramli, A.; Kiew, P.L.; Lee, K.T. Semi-continuous cultivation of Chlorella vulgaris using chicken compost as nutrients source: Growth optimization study and fatty acid composition analysis. Energy Convers. Manag. 2018, 164, 363–373. [Google Scholar] [CrossRef]
- Yodsuwan, N.; Sawayama, S.; Sirisansaneeyakul, S. Effect of nitrogen concentration on growth, lipid production and fatty acid profiles of the marine diatom Phaeodactylum tricornutum. Agric. Nat. Resour. 2017, 51, 190–197. [Google Scholar] [CrossRef]
- Difusa, A.; Mohanty, K.; Goud, V.V. The chemometric approach applied to FTIR spectral data for the analysis of lipid content in microalgae cultivated in different nitrogen sources. Biomass Convers. Biorefin. 2016, 6, 427–433. [Google Scholar] [CrossRef]
- Li, X.; Sha, J.; Chu, B.; Wei, Y.; Huang, W.; Zhou, H.; Xu, N.; He, Y. Quantitative visualization of intracellular lipids concentration in a microalgae cell based on Raman micro-spectroscopy coupled with chemometrics. Sens. Actuators B Chem. 2019, 292, 7–15. [Google Scholar] [CrossRef]
- Peng, Y.; Zou, T.; Li, L.; Tang, S.; Li, Q.; Zhang, J.; Chen, Y.; Wang, X.; Yang, G.; Hu, Y. Map-Based Cloning and Functional Analysis of YE1 in Rice, Which Is Involved in Light-Dependent Chlorophyll Biogenesis and Photoperiodic Flowering Pathway. Int. J. Mol. Sci. 2019, 20, 758. [Google Scholar] [CrossRef]
- Hanelt, D.; Figueroa, F.L. Physiological and photomorphogenic effects of light on marine macrophytes. In Seaweed Biology; Springer: Berlin, Germany, 2012; pp. 3–23. [Google Scholar]
- Matos, Â.P.; Cavanholi, M.G.; Moecke, E.H.S.; Sant’Anna, E.S. Effects of different photoperiod and trophic conditions on biomass, protein and lipid production by the marine alga Nannochloropsis gaditana at optimal concentration of desalination concentrate. Bioresour. Technol. 2016, 224, 490–497. [Google Scholar] [CrossRef]
- Simionato, D.; Sforza, E.; Corteggiani Carpinelli, E.; Bertucco, A.; Giacometti, G.M.; Morosinotto, T. Acclimation of Nannochloropsis gaditana to different illumination regimes: Effects on lipids accumulation. Bioresour. Technol. 2011, 102, 6026–6032. [Google Scholar] [CrossRef]
- Stewart, M.; Mulenos, M.; Steele, L.; Sayes, C. Differences among Unique Nanoparticle Protein Corona Constructs: A Case Study Using Data Analytics and Multi-Variant Visualization to Describe Physicochemical Characteristics. Appl. Sci. 2018, 8, 2669. [Google Scholar] [CrossRef]
- Kadar, E.; Rooks, P.; Lakey, C.; White, D.A. The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures. Sci. Total Environ. 2012, 439, 8–17. [Google Scholar] [CrossRef]
- Kleinzeller, A.; Benos, D. Cell Lipids; Hoekstra, D., Ed.; Elsevier Ltd.: San Diego, CA, USA, 1994. [Google Scholar]
- Betti, M.; Pérez-Delgado, C.; García-Calderón, M.; Díaz, P.; Monza, J.; Márquez, A. Cellular Stress Following Water Deprivation in the Model Legume Lotus japonicus. Cells 2012, 1, 1089–1106. [Google Scholar] [CrossRef] [Green Version]
- Gérin, S.; Leprince, P.; Sluse, F.E.; Franck, F.; Mathy, G. New Features on the Environmental Regulation of Metabolism Revealed by Modeling the Cellular Proteomic Adaptations Induced by Light, Carbon, and Inorganic Nitrogen in Chlamydomonas reinhardtii. Front. Plant Sci. 2016, 7, 1158. [Google Scholar] [CrossRef]
- Deconinck, N.; Muylaert, K.; Ivens, W.; Vandamme, D. Innovative harvesting processes for microalgae biomass production: A perspective from patent literature. Algal Res. 2018, 31, 469–477. [Google Scholar] [CrossRef]
- Musa, M.; Rainey, T.; Thomas-hall, S. Optimization of Microalgae Dewatering using a Britt Dynamic Drainage Jar. In Proceedings of the 7th International Conference on Algal Biomass, Biofuels & Bioproducts, Miami, FL, USA, 18–21 June 2017. [Google Scholar]
- Zhu, L.D.; Hiltunen, E.; Li, Z. Using magnetic materials to harvest microalgal biomass: Evaluation of harvesting and detachment efficiency. Environ. Technol. 2019, 40, 1006–1012. [Google Scholar] [CrossRef]
- Munshi, F.M.; Church, J.; McLean, R.; Maier, N.; Sadmani, A.H.M.A.; Duranceau, S.J.; Lee, W.H. Dewatering algae using an aquaporin-based polyethersulfone forward osmosis membrane. Sep. Purif. Technol. 2018, 204, 154–161. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Musa, M.; Ward, A.; Ayoko, G.A.; Rösch, C.; Brown, R.; Rainey, T.J. New approach to single-step dynamic dewatering of microalgae from dilute suspensions. 2019; (unpublished). [Google Scholar]
- De Jesus, S.S.; Ferreira, G.F.; Moreira, L.S.; Wolf Maciel, M.R.; Maciel Filho, R. Comparison of several methods for effective lipid extraction from wet microalgae using green solvents. Renew. Energy 2019, 143, 130–141. [Google Scholar] [CrossRef]
- Murador, D.C.; de Souza Mesquita, L.M.; Vannuchi, N.; Braga, A.R.C.; de Rosso, V.V. Bioavailability and biological effects of bioactive compounds extracted with natural deep eutectic solvents and ionic liquids: Advantages over conventional organic solvents. Curr. Opin. Food Sci. 2019, 26, 25–34. [Google Scholar] [CrossRef]
- Ma, N.L.; Teh, K.Y.; Lam, S.S.; Kaben, A.M.; Cha, T.S. Optimization of cell disruption methods for efficient recovery of bioactive metabolites via NMR of three freshwater microalgae (chlorophyta). Bioresour. Technol. 2015, 190, 536–542. [Google Scholar] [CrossRef]
- Talukdar, J.; Kalita, M.C.; Goswami, B.C.; Hong, D.D.; Das, H.C. Liquid Hydrocarbon Production Potential of a Novel Strain of the Microalga Botryococcus braunii: Assessing the Reliability of in Situ Hydrocarbon Recovery by Wet Process Solvent Extraction. Energy Fuels 2014, 28, 3747–3758. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef]
- Madsen, R.B.; Lappa, E.; Christensen, P.S.; Jensen, M.M.; Klemmer, M.; Becker, J.; Iversen, B.B.; Glasius, M. Chemometric analysis of composition of bio-crude and aqueous phase from hydrothermal liquefaction of thermally and chemically pretreated Miscanthus x giganteus. Biomass Bioenergy 2016, 95, 137–145. [Google Scholar] [CrossRef]
- Chopra, J.; Mahesh, D.; Yerrayya, A.; Vinu, R.; Kumar, R.; Sen, R. Performance enhancement of hydrothermal liquefaction for strategic and sustainable valorization of de-oiled yeast biomass into green bio-crude. J. Clean. Prod. 2019, 227, 292–301. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; de Souza, C.O.; Vich, D.V.; de Carvalho, G.C.; Nascimento, M.A. Screening Microalgae Strains for Biodiesel Production: Lipid Productivity and Estimation of Fuel Quality Based on Fatty Acids Profiles as Selective Criteria. Bioenergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Islam, M.A.; Magnusson, M.; Brown, R.J.; Ayoko, G.A.; Nabi, M.N.; Heimann, K. Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 2013, 6, 5676–5702. [Google Scholar] [CrossRef]
- Havlik, I.; Lindner, P.; Scheper, T.; Reardon, K.F. On-line monitoring of large cultivations of microalgae and cyanobacteria. Trends Biotechnol. 2013, 31, 406–414. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, M.; Ayoko, G.A.; Ward, A.; Rösch, C.; Brown, R.J.; Rainey, T.J. Factors Affecting Microalgae Production for Biofuels and the Potentials of Chemometric Methods in Assessing and Optimizing Productivity. Cells 2019, 8, 851. https://doi.org/10.3390/cells8080851
Musa M, Ayoko GA, Ward A, Rösch C, Brown RJ, Rainey TJ. Factors Affecting Microalgae Production for Biofuels and the Potentials of Chemometric Methods in Assessing and Optimizing Productivity. Cells. 2019; 8(8):851. https://doi.org/10.3390/cells8080851
Chicago/Turabian StyleMusa, Mutah, Godwin A. Ayoko, Andrew Ward, Christine Rösch, Richard J. Brown, and Thomas J. Rainey. 2019. "Factors Affecting Microalgae Production for Biofuels and the Potentials of Chemometric Methods in Assessing and Optimizing Productivity" Cells 8, no. 8: 851. https://doi.org/10.3390/cells8080851
APA StyleMusa, M., Ayoko, G. A., Ward, A., Rösch, C., Brown, R. J., & Rainey, T. J. (2019). Factors Affecting Microalgae Production for Biofuels and the Potentials of Chemometric Methods in Assessing and Optimizing Productivity. Cells, 8(8), 851. https://doi.org/10.3390/cells8080851




