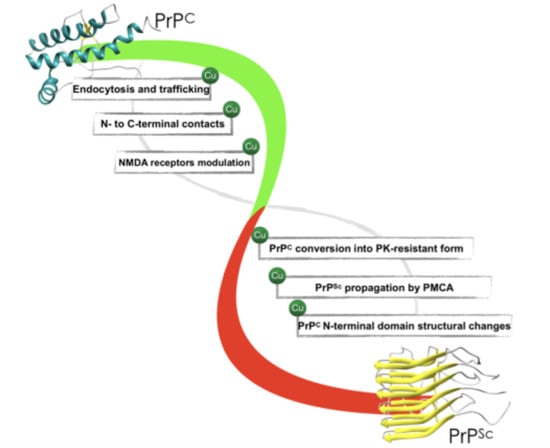
Structural Consequences of Copper Binding to the Prion Protein
Abstract
:1. Introduction
2. The Structure of PrPC and PrPSc
3. PrPC Functions Suggested by Copper Binding
4. Copper-Binding Models
5. Copper and Prion Diseases
6. Structural Consequences of Copper Binding
7. Conclusions
Funding
Conflicts of Interest
References
- Mead, S.; Stumpf, M.P.; Whitfield, J.; Beck, J.A.; Poulter, M.; Campbell, T.; Uphill, J.B.; Goldstein, D.; Alpers, M.; Fisher, E.M.; et al. Balancing selection at the prion protein gene consistent with prehistoric kurulike epidemics. Science 2003, 300, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, C.J., Jr.; Gajdusek, D.C.; Asher, D.M.; Alpers, M.P.; Beck, E.; Daniel, P.M.; Matthews, W.B. Creutzfeldt-Jakob disease (spongiform encephalopathy): Transmission to the chimpanzee. Science 1968, 161, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Notari, S.; Appleby, B.S.; Gambetti, P. Variably protease-sensitive prionopathy. Handb. Clin. Neurol. 2018, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Gajdusek, D.C.; Gibbs, C.J., Jr. Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Straussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain 1981, 104, 559–588. [Google Scholar] [CrossRef] [PubMed]
- Medori, R.; Tritschler, H.J.; LeBlanc, A.; Villare, F.; Manetto, V.; Chen, H.Y.; Xue, R.; Leal, S.; Montagna, P.; Cortelli, P.; et al. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N. Engl. J. Med. 1992, 326, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Revesz, T.; Holton, J.L.; Lashley, T.; Plant, G.; Frangione, B.; Rostagno, A.; Ghiso, J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009, 118, 115–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, M.W.; Ironside, J.W. Review: Creutzfeldt-Jakob disease: Prion protein type, disease phenotype and agent strain. Neuropathol. Appl. Neurobiol. 2012, 38, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Mahmood, S. An overview of animal prion diseases. Virol. J. 2011, 8, 493. [Google Scholar] [CrossRef]
- Benestad, S.L.; Telling, G.C. Chronic wasting disease: An evolving prion disease of cervids. Handb. Clin. Neurol. 2018, 153, 135–151. [Google Scholar]
- Hyun-Joo, S.; Jae-Hoon, K.; Jin-Ju, N.; Yi-Seok, J.; Young-Hwa, J.; Soo-Whan, A.; Ok-Kyung, K.; Dae-Yong, K.; BALACHANDRAN, A. A case of chronic wasting disease in an elk imported to Korea from Canada. J. Vet. Med. Sci. 2002, 64, 855–858. [Google Scholar]
- Benestad, S.L.; Mitchell, G.; Simmons, M.; Ytrehus, B.; Vikøren, T. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet. Res. 2016, 47, 88. [Google Scholar] [CrossRef] [PubMed]
- Stokstad, E. Norway seeks to stamp out prion disease. Science (New York, NY) 2017, 356, 12. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.; Roberts, H. Update on Chronic Wasting Disease in Europe. 2018. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/703368/sa-cwd-norway-20180425.pdf (accessed on 3 July 2019).
- Pirisinu, L.; Tran, L.; Chiappini, B.; Vanni, I.; Di, M.B.; Vaccari, G.; Vikøren, T.; Madslien, K.I.; Våge, J.; Spraker, T. Novel Type of Chronic Wasting Disease Detected in Moose (Alces alces), Norway. Emerg. Infect. Dis. 2018, 24, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Babelhadj, B.; Di Bari, M.A.; Pirisinu, L.; Chiappini, B.; Gaouar, S.B.S.; Riccardi, G.; Marcon, S.; Agrimi, U.; Nonno, R.; Vaccari, G. Prion Disease in Dromedary Camels, Algeria. Emerg. Infect. Dis. 2018, 24, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igel-Egalon, A.; Bohl, J.; Moudjou, M.; Herzog, L.; Reine, F.; Rezaei, H.; Beringue, V. Heterogeneity and Architecture of Pathological Prion Protein Assemblies: Time to Revisit the Molecular Basis of the Prion Replication Process? Viruses 2019, 11, 429. [Google Scholar] [CrossRef]
- Harris, D.A. Cellular biology of prion diseases. Clin. Microbiol. Rev. 1999, 12, 429–444. [Google Scholar] [CrossRef]
- Glatzel, M.; Aguzzi, A. PrPC expression in the peripheral nervous system is a determinant of prion neuroinvasion. J. Gen. Virol. 2000, 81, 2813–2821. [Google Scholar] [CrossRef]
- Wulf, M.A.; Senatore, A.; Aguzzi, A. The biological function of the cellular prion protein: An update. BMC Biol. 2017, 15. [Google Scholar] [CrossRef]
- Rapoport, T.A. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 2007, 450, 663–669. [Google Scholar] [CrossRef]
- Hebert, D.N.; Molinari, M. In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 2007, 87, 1377–1408. [Google Scholar] [CrossRef]
- Stahl, N.; Borchelt, D.R.; Hsiao, K.; Prusiner, S.B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 1987, 51, 229–240. [Google Scholar] [CrossRef]
- Stahl, N.; Baldwin, M.A.; Hecker, R.; Pan, K.M.; Burlingame, A.L.; Prusiner, S.B. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry 1992, 31, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Abid, K.; Morales, R.; Soto, C. Cellular factors implicated in prion replication. FEBS Lett. 2010, 584, 2409–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surewicz, W.K.; Apostol, M.I. Prion protein and its conformational conversion: A structural perspective. Top. Curr. Chem. 2011, 305, 135–167. [Google Scholar] [CrossRef] [PubMed]
- Abskharon, R.N.; Giachin, G.; Wohlkonig, A.; Soror, S.H.; Pardon, E.; Legname, G.; Steyaert, J. Probing the N-terminal beta-sheet conversion in the crystal structure of the human prion protein bound to a nanobody. J. Am. Chem. Soc. 2014, 136, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Liu, A.; Luhrs, T.; Riek, R.; von Schroetter, C.; Lopez Garcia, F.; Billeter, M.; Calzolai, L.; Wider, G.; Wuthrich, K. NMR solution structure of the human prion protein. Proc. Natl. Acad. Sci. USA 2000, 97, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, E.D.; Chattopadhyay, M.; Millhauser, G.L. The affinity of copper binding to the prion protein octarepeat domain: Evidence for negative cooperativity. Biochemistry 2006, 45, 13083–13092. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.D.; Stevens, D.J.; Spevacek, A.R.; Visconte, M.P.; Dei Rossi, A.; Millhauser, G.L. Copper binding extrinsic to the octarepeat region in the prion protein. Curr. Protein Pept. Sci. 2009, 10, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M.F.; Stewart, L.R.; White, A.R.; McLean, C.; Friedhuber, A.; Maher, F.; Beyreuther, K.; Masters, C.L.; Barrow, C.J.; Collins, S.J.; et al. The hydrophobic core sequence modulates the neurotoxic and secondary structure properties of the prion peptide 106–126. J. Neurochem. 1999, 73, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Dibble, J.P.; Evans, E.G.; Millhauser, G.L. A new paradigm for enzymatic control of alpha-cleavage and beta-cleavage of the prion protein. J. Biol. Chem. 2014, 289, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, A.R.; Watt, N.T.; Taylor, D.R.; Perera, W.S.S.; Hooper, N.M. alpha-cleavage of the prion protein occurs in a late compartment of the secretory pathway and is independent of lipid rafts. Mol. Cell. Neurosci. 2009, 40, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.W.; Prusiner, S.B. Prions. Cold Spring Harb. Perspect. Biol. 2011, 3, a006833. [Google Scholar] [CrossRef] [PubMed]
- Baskakov, I.V.; Caughey, B.; Requena, J.R.; Sevillano, A.M.; Surewicz, W.K.; Wille, H. The prion 2018 round tables (I): The structure of PrP(Sc). Prion 2019, 13, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Spagnolli, G.; Rigoli, M.; Orioli, S.; Sevillano, A.M.; Faccioli, P.; Wille, H.; Biasini, E.; Requena, J.R. Full atomistic model of prion structure and conversion. PLoS Pathog. 2019, 15, e1007864. [Google Scholar] [CrossRef] [PubMed]
- Wille, H.; Bian, W.; McDonald, M.; Kendall, A.; Colby, D.W.; Bloch, L.; Ollesch, J.; Borovinskiy, A.L.; Cohen, F.E.; Prusiner, S.B.; et al. Natural and synthetic prion structure from X-ray fiber diffraction. Proc. Natl. Acad. Sci. USA 2009, 106, 16990–16995. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Fernandez, E.; Vos, M.R.; Afanasyev, P.; Cebey, L.; Sevillano, A.M.; Vidal, E.; Rosa, I.; Renault, L.; Ramos, A.; Peters, P.J.; et al. The Structural Architecture of an Infectious Mammalian Prion Using Electron Cryomicroscopy. PLoS Pathog. 2016, 12, e1005835. [Google Scholar] [CrossRef]
- Baskakov, I.V.; Katorcha, E. Multifaceted Role of Sialylation in Prion Diseases. Front. Neurosci. 2016, 10, 358. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.K.; Cali, I.; Surewicz, K.; Kong, Q.; Gambetti, P.; Surewicz, W.K. Amyloid fibrils from the N-terminal prion protein fragment are infectious. Proc. Natl. Acad. Sci. USA 2016, 113, 13851–13856. [Google Scholar] [CrossRef] [Green Version]
- Theint, T.; Nadaud, P.S.; Aucoin, D.; Helmus, J.J.; Pondaven, S.P.; Surewicz, K.; Surewicz, W.K.; Jaroniec, C.P. Species-dependent structural polymorphism of Y145Stop prion protein amyloid revealed by solid-state NMR spectroscopy. Nat. Commun. 2017, 8, 753. [Google Scholar] [CrossRef]
- Theint, T.; Xia, Y.; Nadaud, P.S.; Mukhopadhyay, D.; Schwieters, C.D.; Surewicz, K.; Surewicz, W.K.; Jaroniec, C.P. Structural Studies of Amyloid Fibrils by Paramagnetic Solid-State Nuclear Magnetic Resonance Spectroscopy. J. Am. Chem. Soc. 2018, 140, 13161–13166. [Google Scholar] [CrossRef]
- Hornshaw, M.P.; McDermott, J.R.; Candy, J.M.; Lakey, J.H. Copper binding to the N-terminal tandem repeat region of mammalian and avian prion protein: Structural studies using synthetic peptides. Biochem. Biophys. Res. Commun. 1995, 214, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Das, D.; Singh, A.; Mohan, M.L. Prion protein and metal interaction: Physiological and pathological implications. Curr. Issues Mol. Biol. 2010, 12, 99–107. [Google Scholar] [PubMed]
- Pauly, P.C.; Harris, D.A. Copper stimulates endocytosis of the prion protein. J. Biol. Chem. 1998, 273, 33107–33110. [Google Scholar] [CrossRef] [PubMed]
- Herms, J.; Tings, T.; Gall, S.; Madlung, A.; Giese, A.; Siebert, H.; Schurmann, P.; Windl, O.; Brose, N.; Kretzschmar, H. Evidence of presynaptic location and function of the prion protein. J. Neurosci. 1999, 19, 8866–8875. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.S.; Hooper, N.M. Ablation of the metal ion-induced endocytosis of the prion protein by disease-associated mutation of the octarepeat region. Curr. Biol. 2001, 11, 519–523. [Google Scholar] [CrossRef] [Green Version]
- Kretzschmar, H.A.; Tings, T.; Madlung, A.; Giese, A.; Herms, J. Function of PrP(C) as a copper-binding protein at the synapse. Arch. Virol. Suppl. 2000, 239–249. [Google Scholar]
- Brown, L.R.; Harris, D.A. Copper and zinc cause delivery of the prion protein from the plasma membrane to a subset of early endosomes and the Golgi. J. Neurochem. 2003, 87, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.R.; Schulz-Schaeffer, W.J.; Schmidt, B.; Kretzschmar, H.A. Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp. Neurol. 1997, 146, 104–112. [Google Scholar] [CrossRef]
- Santuccione, A.; Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J. Cell Biol. 2005, 169, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, X.T.A.; Tran, T.H.; Cojoc, D.; Legname, G. Copper Binding Regulates Cellular Prion Protein Function. Mol. Neurobiol. 2019. [Google Scholar] [CrossRef]
- Linden, R.; Martins, V.R.; Prado, M.A.; Cammarota, M.; Izquierdo, I.; Brentani, R.R. Physiology of the prion protein. Physiol. Rev. 2008, 88, 673–728. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Meneghetti, E.; Pastore, B.; Benetti, F.; Legname, G. Prion protein and copper cooperatively protect neurons by modulating NMDA receptor through S-nitrosylation. Antioxid. Redox. Signal. 2015, 22, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, H.; Zhang, Y.; Tsutsui, S.; Hameed, S.; Altier, C.; Hamid, J.; Chen, L.; Villemaire, M.; Ali, Z.; Jirik, F.R.; et al. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J. Cell Biol. 2008, 181, 551–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stys, P.K.; You, H.; Zamponi, G.W. Copper-dependent regulation of NMDA receptors by cellular prion protein: Implications for neurodegenerative disorders. J. Physiol. 2012, 590, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Pushie, M.J.; Pickering, I.J.; Martin, G.R.; Tsutsui, S.; Jirik, F.R.; George, G.N. Prion protein expression level alters regional copper, iron and zinc content in the mouse brain. Metallomics 2011, 3, 206–214. [Google Scholar] [CrossRef] [PubMed]
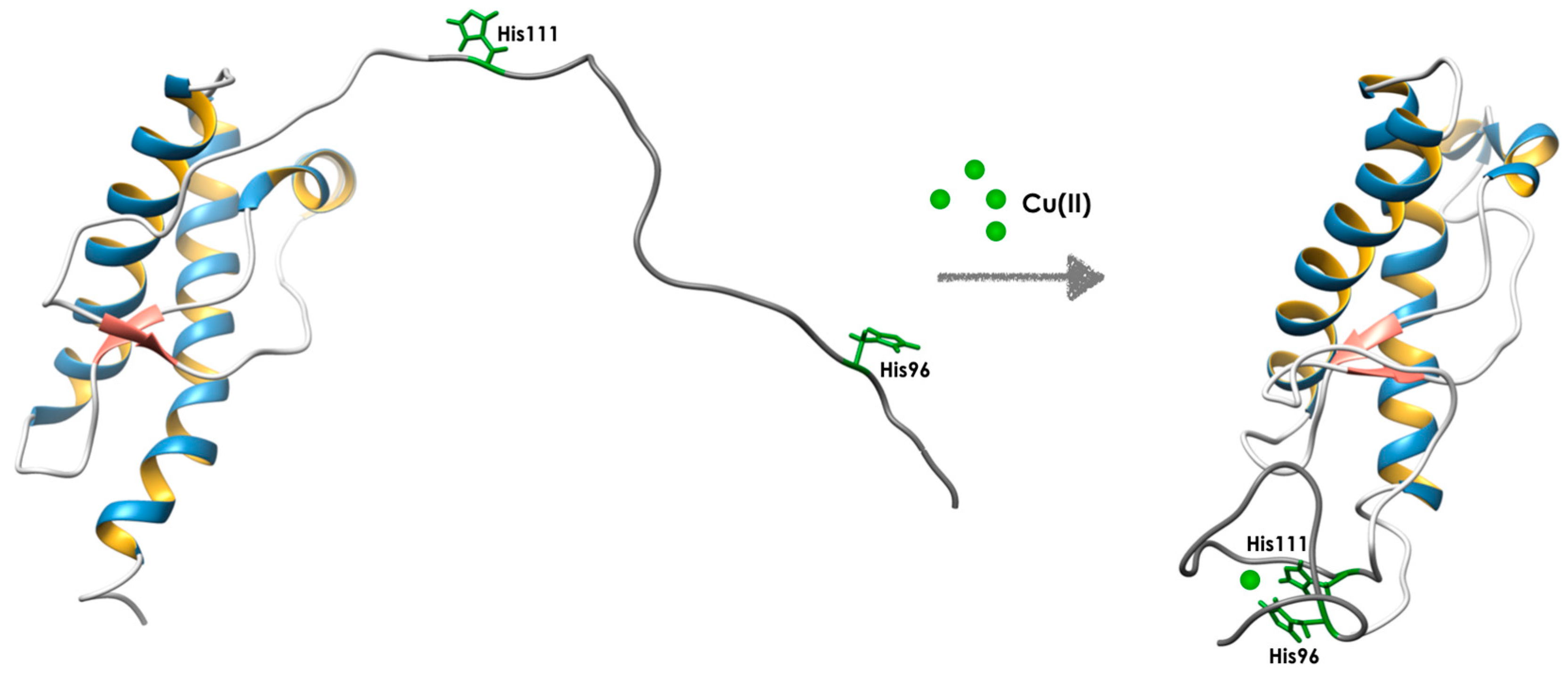
- Jones, C.E.; Abdelraheim, S.R.; Brown, D.R.; Viles, J.H. Preferential Cu2+ coordination by His96 and His111 induces beta-sheet formation in the unstructured amyloidogenic region of the prion protein. J. Biol. Chem. 2004, 279, 32018–32027. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Srivastava, A.K.; Srinivas, V.; Chary, K.V.; Rao, C.M. Copper alters aggregation behavior of prion protein and induces novel interactions between its N- and C-terminal regions. J. Biol. Chem. 2011, 286, 38533–38545. [Google Scholar] [CrossRef]
- Wells, M.A.; Jackson, G.S.; Jones, S.; Hosszu, L.L.; Craven, C.J.; Clarke, A.R.; Collinge, J.; Waltho, J.P. A reassessment of copper(II) binding in the full-length prion protein. Biochem. J. 2006, 399, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Wong, E.; Thackray, A.M.; Bujdoso, R. Copper induces increased beta-sheet content in the scrapie-susceptible ovine prion protein PrPVRQ compared with the resistant allelic variant PrPARR. Biochem. J. 2004, 380, 273–282. [Google Scholar] [CrossRef]
- Brown, D.R.; Qin, K.; Herms, J.W.; Madlung, A.; Manson, J.; Strome, R.; Fraser, P.E.; Kruck, T.; von Bohlen, A.; Schulz-Schaeffer, W.; et al. The cellular prion protein binds copper in vivo. Nature 1997, 390, 684–687. [Google Scholar] [CrossRef]
- Varela-Nallar, L.; Toledo, E.M.; Larrondo, L.F.; Cabral, A.L.; Martins, V.R.; Inestrosa, N.C. Induction of cellular prion protein gene expression by copper in neurons. Am. J. Physiol. Cell Physiol. 2006, 290, C271–C281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.R.; Watt, N.T.; Perera, W.S.; Hooper, N.M. Assigning functions to distinct regions of the N-terminus of the prion protein that are involved in its copper-stimulated, clathrin-dependent endocytosis. J. Cell Sci. 2005, 118, 5141–5153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt-Ulms, G.; Ehsani, S.; Watts, J.C.; Westaway, D.; Wille, H. Evolutionary descent of prion genes from the ZIP family of metal ion transporters. PLoS ONE 2009, 4, e7208. [Google Scholar] [CrossRef] [PubMed]
- Pocanschi, C.L.; Ehsani, S.; Mehrabian, M.; Wille, H.; Reginold, W.; Trimble, W.S.; Wang, H.S.; Yee, A.; Arrowsmith, C.H.; Bozoky, Z.; et al. The ZIP5 Ectodomain Co-Localizes with PrP and May Acquire a PrP-Like Fold That Assembles into a Dimer. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Wong, B.S.; Hafiz, F.; Clive, C.; Haswell, S.J.; Jones, I.M. Normal prion protein has an activity like that of superoxide dismutase. Biochem. J. 1999, 344 Pt 1, 1–5. [Google Scholar] [CrossRef]
- Brown, D.R.; Besinger, A. Prion protein expression and superoxide dismutase activity. Biochem. J. 1998, 334 Pt 2, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.S.; Pan, T.; Liu, T.; Li, R.; Gambetti, P.; Sy, M.S. Differential contribution of superoxide dismutase activity by prion protein in vivo. Biochem. Biophys. Res. Commun. 2000, 273, 136–139. [Google Scholar] [CrossRef]
- Hutter, G.; Heppner, F.L.; Aguzzi, A. No superoxide dismutase activity of cellular prion protein in vivo. Biol. Chem. 2003, 384, 1279–1285. [Google Scholar] [CrossRef]
- Jones, S.; Batchelor, M.; Bhelt, D.; Clarke, A.R.; Collinge, J.; Jackson, G.S. Recombinant prion protein does not possess SOD-1 activity. Biochem. J. 2005, 392, 309–312. [Google Scholar] [CrossRef]
- Westergard, L.; Turnbaugh, J.A.; Harris, D.A. A nine amino acid domain is essential for mutant prion protein toxicity. J. Neurosci. 2011, 31, 14005–14017. [Google Scholar] [CrossRef]
- Li, A.; Christensen, H.M.; Stewart, L.R.; Roth, K.A.; Chiesa, R.; Harris, D.A. Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105-125. EMBO J. 2007, 26, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.; Tolnay, M.; Brabeck, C.; Pahnke, J.; Kloz, U.; Niemann, H.H.; Heikenwalder, M.; Rulicke, T.; Burkle, A.; Aguzzi, A. Lethal recessive myelin toxicity of prion protein lacking its central domain. EMBO J. 2007, 26, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, H.G.; Choi, J.K.; Kim, J.I.; Choi, E.K.; Carp, R.I.; Kim, Y.S. The cellular prion protein (PrPC) prevents apoptotic neuronal cell death and mitochondrial dysfunction induced by serum deprivation. Brain Res. Mol. Brain Res. 2004, 124, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Shyu, W.C.; Lin, S.Z.; Chiang, M.F.; Ding, D.C.; Li, K.W.; Chen, S.F.; Yang, H.I.; Li, H. Overexpression of PrPC by adenovirus-mediated gene targeting reduces ischemic injury in a stroke rat model. J. Neurosci. 2005, 25, 8967–8977. [Google Scholar] [CrossRef] [PubMed]
- Slapsak, U.; Salzano, G.; Amin, L.; Abskharon, R.N.N.; Ilc, G.; Zupancic, B.; Biljan, I.; Plavec, J.; Giachin, G.; Legname, G. The N Terminus of the Prion Protein Mediates Functional Interactions with the Neuronal Cell Adhesion Molecule (NCAM) Fibronectin Domain. J. Biol. Chem. 2016, 291, 21857–21868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, L.; Nguyen, X.T.; Rolle, I.G.; D’Este, E.; Giachin, G.; Tran, T.H.; Serbec, V.C.; Cojoc, D.; Legname, G. Characterization of prion protein function by focal neurite stimulation. J. Cell Sci. 2016, 129, 3878–3891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel, A.; Burgaya, F.; Gavin, R.; Soriano, E.; Aguzzi, A.; Del Rio, J.A. Enhanced susceptibility of Prnp-deficient mice to kainate-induced seizures, neuronal apoptosis, and death: Role of AMPA/kainate receptors. J. Neurosci. Res. 2007, 85, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Spudich, A.; Frigg, R.; Kilic, E.; Kilic, U.; Oesch, B.; Raeber, A.; Bassetti, C.L.; Hermann, D.M. Aggravation of ischemic brain injury by prion protein deficiency: Role of ERK-1/-2 and STAT-1. Neurobiol. Dis. 2005, 20, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, L.; Bladen, C.; Stys, P.K.; Zamponi, G.W. Differential modulation of NMDA and AMPA receptors by cellular prion protein and copper ions. Mol. Brain 2018, 11, 62. [Google Scholar] [CrossRef]
- Meneghetti, E.; Gasperini, L.; Virgilio, T.; Moda, F.; Tagliavini, F.; Benetti, F.; Legname, G. Prions Strongly Reduce NMDA Receptor S-Nitrosylation Levels at Pre-symptomatic and Terminal Stages of Prion Diseases. Mol. Neurobiol. 2019. [Google Scholar] [CrossRef]
- You, H.T.; Tsutsui, S.; Hameed, S.; Kannanayakal, T.J.; Chen, L.N.; Xia, P.; Engbers, J.D.T.; Lipton, S.A.; Stys, P.K.; Zamponi, G.W. A beta neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Marc, D.; Choiset, Y.; Takahashi, M.; Hui Bon Hoa, G.; Haertle, T.; Grosclaude, J.; Debey, P. High yield purification and physico-chemical properties of full-length recombinant allelic variants of sheep prion protein linked to scrapie susceptibility. Eur. J. Biochem. 2000, 267, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.M.; Zheng, Y.; Tien, P. On-column purification and refolding of recombinant bovine prion protein: Using its octarepeat sequences as a natural affinity tag. Protein Expr. Purif. 2003, 32, 104–109. [Google Scholar] [CrossRef]
- Hornemann, S.; Schorn, C.; Wuthrich, K. NMR structure of the bovine prion protein isolated from healthy calf brains. EMBO Rep. 2004, 5, 1159–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pergami, P.; Jaffe, H.; Safar, J. Semipreparative chromatographic method to purify the normal cellular isoform of the prion protein in nondenatured form. Anal. Biochem. 1996, 236, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y.; Rosenmann, H.; Hijazi, N.; Halimi, M.; Gabizon, R. Copper binding to the PrP isoforms: A putative marker of their conformation and function. J. Virol. 2001, 75, 7872–7874. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.; Strom, A.; Hunsmann, G.; Stuke, A.W. Separation of native prion protein (PrP) glycoforms by copper-binding using immobilized metal affinity chromatography (IMAC). Biochem. J. 2005, 388, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moudjou, M.; Bernard, J.; Sabuncu, E.; Langevin, C.; Laude, H. Glycan chains modulate prion protein binding to immobilized metal ions. Neurochem. Int. 2007, 50, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Dron, M.; Moudjou, M.; Chapuis, J.; Salamat, M.K.F.; Bernard, J.; Cronier, S.; Langevin, C.; Laude, H. Endogenous Proteolytic Cleavage of Disease-associated Prion Protein to Produce C2 Fragments Is Strongly Cell- and Tissue-dependent. J. Biol. Chem. 2010, 285, 10252–10264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, D.; Bartz, J.; Mirwald, J.; Olander, D.; Marsh, R.; Aiken, J. Reversibility of scrapie inactivation is enhanced by copper. J. Biol. Chem. 1998, 273, 25545–25547. [Google Scholar] [CrossRef] [PubMed]
- Igel-Egalon, A.; Moudjou, M.; Martin, D.; Busley, A.; Knapple, T.; Herzog, L.; Reine, F.; Lepejova, N.; Richard, C.A.; Beringue, V.; et al. Reversible unfolding of infectious prion assemblies reveals the existence of an oligomeric elementary brick. PLoS Pathog. 2017, 13, e1006557. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Choiset, Y.; Eghiaian, F.; Treguer, E.; Mentre, P.; Debey, P.; Grosclaude, J.; Haertle, T. Amyloidogenic unfolding intermediates differentiate sheep prion protein variants. J. Mol. Biol. 2002, 322, 799–814. [Google Scholar] [CrossRef]
- Wopfner, F.; Weidenhofer, G.; Schneider, R.; von Brunn, A.; Gilch, S.; Schwarz, T.F.; Werner, T.; Schatzl, H.M. Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. J. Mol. Biol. 1999, 289, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Millhauser, G.L. Copper and the prion protein: Methods, structures, function, and disease. Annu Rev. Phys. Chem. 2007, 58, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Aronoff-Spencer, E.; Burns, C.S.; Avdievich, N.I.; Gerfen, G.J.; Peisach, J.; Antholine, W.E.; Ball, H.L.; Cohen, F.E.; Prusiner, S.B.; Millhauser, G.L. Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry 2000, 39, 13760–13771. [Google Scholar] [CrossRef] [PubMed]
- Millhauser, G.L. Copper binding in the prion protein. Acc. Chem. Res. 2004, 37, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.S.; Aronoff-Spencer, E.; Dunham, C.M.; Lario, P.; Avdievich, N.I.; Antholine, W.E.; Olmstead, M.M.; Vrielink, A.; Gerfen, G.J.; Peisach, J.; et al. Molecular features of the copper binding sites in the octarepeat domain of the prion protein. Biochemistry 2002, 41, 3991–4001. [Google Scholar] [CrossRef]
- Pushie, M.J.; Rauk, A. Computational studies of Cu(II)[peptide] binding motifs: Cu[HGGG] and Cu[HG] as models for Cu(II) binding to the prion protein octarepeat region. J. Biol. Inorg. Chem 2003, 8, 53–65. [Google Scholar] [CrossRef]
- Zahn, R. The octapeptide repeats in mammalian prion protein constitute a pH-dependent folding and aggregation site. J. Mol. Biol 2003, 334, 477–488. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Walter, E.D.; Newell, D.J.; Jackson, P.J.; Aronoff-Spencer, E.; Peisach, J.; Gerfen, G.J.; Bennett, B.; Antholine, W.E.; Millhauser, G.L. The octarepeat domain of the prion protein binds Cu(II) with three distinct coordination modes at pH 7.4. J. Am. Chem. Soc. 2005, 127, 12647–12656. [Google Scholar] [CrossRef]
- Fischer, M.; Rulicke, T.; Raeber, A.; Sailer, A.; Moser, M.; Oesch, B.; Brandner, S.; Aguzzi, A.; Weissmann, C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996, 15, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, E.; Chiesa, R.; Harris, D.A. Copper converts the cellular prion protein into a protease-resistant species that is distinct from the scrapie isoform. J. Biol. Chem. 2001, 276, 11432–11438. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Yang, Y.; Mastrangelo, P.; Westaway, D. Mapping Cu(II) binding sites in prion proteins by diethyl pyrocarbonate modification and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometric footprinting. J. Biol. Chem. 2002, 277, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.A.; Jelinska, C.; Hosszu, L.L.; Craven, C.J.; Clarke, A.R.; Collinge, J.; Waltho, J.P.; Jackson, G.S. Multiple forms of copper (II) co-ordination occur throughout the disordered N-terminal region of the prion protein at pH 7.4. Biochem. J. 2006, 400, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadal, R.C.; Davies, P.; Brown, D.R.; Viles, J.H. Evaluation of copper2+ affinities for the prion protein. Biochemistry 2009, 48, 8929–8931. [Google Scholar] [CrossRef] [PubMed]
- Treiber, C.; Thompsett, A.R.; Pipkorn, R.; Brown, D.R.; Multhaup, G. Real-time kinetics of discontinuous and highly conformational metal-ion binding sites of prion protein. J. Biol. Inorg. Chem. 2007, 12, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, C.; Rivillas-Acevedo, L.; Cruz-Vasquez, O.; Quintanar, L. Methionine 109 plays a key role in Cu(II) binding to His111 in the 92-115 fragment of the human prion protein. Inorg. Chim. Acta 2018, 481, 87–97. [Google Scholar] [CrossRef]
- Sanchez-Lopez, C.; Fernandez, C.O.; Quintanar, L. Neuroprotective alpha-cleavage of the human prion protein significantly impacts Cu(ii) coordination at its His111 site. Dalton Trans. 2018, 47, 9274–9282. [Google Scholar] [CrossRef]
- Sanchez-Lopez, C.; Rossetti, G.; Quintanar, L.; Carloni, P. Structural Determinants of the Prion Protein N-Terminus and Its Adducts with Copper Ions. Int. J. Mol. Sci. 2018, 20, 18. [Google Scholar] [CrossRef]
- Cereghetti, G.M.; Schweiger, A.; Glockshuber, R.; Van Doorslaer, S. Electron paramagnetic resonance evidence for binding of Cu(2+) to the C-terminal domain of the murine prion protein. Biophys. J. 2001, 81, 516–525. [Google Scholar] [CrossRef]
- Cereghetti, G.M.; Schweiger, A.; Glockshuber, R.; Van Doorslaer, S. Stability and Cu(II) binding of prion protein variants related to inherited human prion diseases. Biophys. J. 2003, 84, 1985–1997. [Google Scholar] [CrossRef]
- Brown, D.R.; Guantieri, V.; Grasso, G.; Impellizzeri, G.; Pappalardo, G.; Rizzarelli, E. Copper(II) complexes of peptide fragments of the prion protein. Conformation changes induced by copper(II) and the binding motif in C-terminal protein region. J. Inorg. Biochem. 2004, 98, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Cervenakova, L.; Buetefisch, C.; Lee, H.S.; Taller, I.; Stone, G.; Gibbs, C.J., Jr.; Brown, P.; Hallett, M.; Goldfarb, L.G. Novel PRNP sequence variant associated with familial encephalopathy. Am. J. Med. Genet. 1999, 88, 653–656. [Google Scholar] [CrossRef]
- Pattison, I.H.; Jebbett, J.N. Histopathological similarities between scrapie and cuprizone toxicity in mice. Nature 1971, 230, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, R.H.; Millson, G.C. The effects of cuprizone toxicity on the incubation period of scrapie in mice. J. Comp. Pathol. 1976, 86, 489–496. [Google Scholar] [CrossRef]
- Brown, D.R. Copper and prion disease. Brain Res. Bull. 2001, 55, 165–173. [Google Scholar] [CrossRef]
- Mitteregger, G.; Korte, S.; Shakarami, M.; Herms, J.; Kretzschmar, H.A. Role of copper and manganese in prion disease progression. Brain Res. 2009, 1292, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, N.; Shaked, Y.; Rosenmann, H.; Ben-Hur, T.; Gabizon, R. Copper binding to PrPC may inhibit prion disease propagation. Brain Res. 2003, 993, 192–200. [Google Scholar] [CrossRef]
- Bocharova, O.V.; Breydo, L.; Salnikov, V.V.; Baskakov, I.V. Copper(II) inhibits in vitro conversion of prion protein into amyloid fibrils. Biochemistry 2005, 44, 6776–6787. [Google Scholar] [CrossRef]
- Sigurdsson, E.M.; Brown, D.R.; Alim, M.A.; Scholtzova, H.; Carp, R.; Meeker, H.C.; Prelli, F.; Frangione, B.; Wisniewski, T. Copper chelation delays the onset of prion disease. J. Biol. Chem. 2003, 278, 46199–46202. [Google Scholar] [CrossRef]
- Yen, C.F.; Harischandra, D.S.; Kanthasamy, A.; Sivasankar, S. Copper-induced structural conversion templates prion protein oligomerization and neurotoxicity. Sci. Adv. 2016, 2, e1600014. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; McGuirl, M. Revisit the effect of fibrillization on functions of prion protein from the perspective of Cu(II) binding. Biochem. Biophys. Res. Commun. 2018, 503, 32–37. [Google Scholar] [CrossRef]
- Treiber, C.; Simons, A.; Multhaup, G. Effect of copper and manganese on the de novo generation of protease-resistant prion protein in yeast cells. Biochemistry 2006, 45, 6674–6680. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, J.K.; Jeong, B.H.; Kim, J.I.; Kwon, M.S.; Carp, R.I.; Kim, Y.S. Effect of transition metals (Mn, Cu, Fe) and deoxycholic acid (DA) on the conversion of PrPC to PrPres. FASEB J. 2005, 19, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Orem, N.R.; Geoghegan, J.C.; Deleault, N.R.; Kascsak, R.; Supattapone, S. Copper (II) ions potently inhibit purified PrPres amplification. J. Neurochem. 2006, 96, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Samorodnitsky, D.; Nicholson, E.M. Differential effects of divalent cations on elk prion protein fibril formation and stability. Prion 2018, 12, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Wadsworth, J.D.; Hill, A.F.; Joiner, S.; Jackson, G.S.; Clarke, A.R.; Collinge, J. Strain-specific prion-protein conformation determined by metal ions. Nat. Cell Biol. 1999, 1, 55–59. [Google Scholar] [CrossRef]
- Flechsig, E.; Shmerling, D.; Hegyi, I.; Raeber, A.J.; Fischer, M.; Cozzio, A.; von Mering, C.; Aguzzi, A.; Weissmann, C. Prion protein devoid of the octapeptide repeat region restores susceptibility to scrapie in PrP knockout mice. Neuron 2000, 27, 399–408. [Google Scholar] [CrossRef]
- Shmerling, D.; Hegyi, I.; Fischer, M.; Blattler, T.; Brandner, S.; Gotz, J.; Rulicke, T.; Flechsig, E.; Cozzio, A.; von Mering, C.; et al. Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 1998, 93, 203–214. [Google Scholar] [CrossRef]
- Owen, F.; Poulter, M.; Lofthouse, R.; Collinge, J.; Crow, T.J.; Risby, D.; Baker, H.F.; Ridley, R.M.; Hsiao, K.; Prusiner, S.B. Insertion in prion protein gene in familial Creutzfeldt-Jakob disease. Lancet 1989, 1, 51–52. [Google Scholar] [CrossRef]
- Krasemann, S.; Zerr, I.; Weber, T.; Poser, S.; Kretzschmar, H.; Hunsmann, G.; Bodemer, W. Prion disease associated with a novel nine octapeptide repeat insertion in the PRNP gene. Brain Res. Mol. Brain Res. 1995, 34, 173–176. [Google Scholar] [CrossRef]
- Chiesa, R.; Piccardo, P.; Quaglio, E.; Drisaldi, B.; Si-Hoe, S.L.; Takao, M.; Ghetti, B.; Harris, D.A. Molecular distinction between pathogenic and infectious properties of the prion protein. J. Virol. 2003, 77, 7611–7622. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Leon, D.R.; Markham, K.A.; Wu, B.; Heckendorf, C.F.; Schilling, K.; Showalter, H.D.; Andrews, P.C.; McComb, M.E.; Pushie, M.J.; et al. Altered Domain Structure of the Prion Protein Caused by Cu(2+) Binding and Functionally Relevant Mutations: Analysis by Cross-Linking, MS/MS, and NMR. Structure 2019. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.G.; Pushie, M.J.; Markham, K.A.; Lee, H.W.; Millhauser, G.L. Interaction between Prion Protein’s Copper-Bound Octarepeat Domain and a Charged C-Terminal Pocket Suggests a Mechanism for N-Terminal Regulation. Structure 2016, 24, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.G.B.; Millhauser, G.L. Copper- and Zinc-Promoted Interdomain Structure in the Prion Protein: A Mechanism for Autoinhibition of the Neurotoxic N-Terminus. Prog. Mol. Biol. Transl. Sci. 2017, 150, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Hecel, A.; Valensin, D.; Kozlowski, H. How copper ions and membrane environment influence the structure of the human and chicken tandem repeats domain? J. Inorg. Biochem. 2019, 191, 143–153. [Google Scholar] [CrossRef]
- Hecel, A.; Draghi, S.; Valensin, D.; Kozlowski, H. The effect of a membrane-mimicking environment on the interactions of Cu2+ with an amyloidogenic fragment of chicken prion protein. Dalton Trans. 2017, 46, 7758–7769. [Google Scholar] [CrossRef]
- D’Angelo, P.; Della Longa, S.; Arcovito, A.; Mancini, G.; Zitolo, A.; Chillemi, G.; Giachin, G.; Legname, G.; Benetti, F. Effects of the pathological Q212P mutation on human prion protein non-octarepeat copper-binding site. Biochemistry 2012, 51, 6068–6079. [Google Scholar] [CrossRef]
- Giachin, G.; Mai, P.T.; Tran, T.H.; Salzano, G.; Benetti, F.; Migliorati, V.; Arcovito, A.; Della Longa, S.; Mancini, G.; D’Angelo, P.; et al. The non-octarepeat copper binding site of the prion protein is a key regulator of prion conversion. Sci. Rep. 2015, 5, 15253. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Yu, Z.; Yu, Y.; Liao, X.; Huang, P.; Guo, C.; Lin, D. Distinct effects of Cu2+-binding on oligomerization of human and rabbit prion proteins. Acta Biochim. Biophys. Sin. (Shanghai) 2015, 47, 842–850. [Google Scholar] [CrossRef]
- Eigenbrod, S.; Frick, P.; Bertsch, U.; Mitteregger-Kretzschmar, G.; Mielke, J.; Maringer, M.; Piening, N.; Hepp, A.; Daude, N.; Windl, O.; et al. Substitutions of PrP N-terminal histidine residues modulate scrapie disease pathogenesis and incubation time in transgenic mice. PLoS ONE 2017, 12, e0188989. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhao, L.; Qin, K. Copper induces structural changes in N-terminus of human prion protein. Biochem. Biophys. Res. Commun. 2018, 499, 470–474. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, N.; Rossi, L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int. 2015, 90, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kiachopoulos, S.; Heske, J.; Tatzelt, J.; Winklhofer, K.F. Misfolding of the prion protein at the plasma membrane induces endocytosis, intracellular retention and degradation. Traffic 2004, 5, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Pani, A.; Mandas, A.; Dessi, S. Cholesterol, Alzheimer’s disease, prion disorders: A menage a trois? Curr. Drug Targets 2010, 11, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.L.; Guo, B.; Scicluna, B.; Coleman, B.M.; Lawson, V.A.; Ellett, L.; Meikle, P.J.; Bukrinsky, M.; Mukhamedova, N.; Sviridov, D.; et al. Prion infection impairs cholesterol metabolism in neuronal cells. J. Biol. Chem. 2014, 289, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Dotti, C.G.; Perez-Canamas, A.; Ledesma, M.D.; Benetti, F.; Legname, G. Prion protein accumulation in lipid rafts of mouse aging brain. PLoS ONE 2013, 8, e74244. [Google Scholar] [CrossRef] [PubMed]
- Toni, M.; Massimino, M.L.; De Mario, A.; Angiulli, E.; Spisni, E. Metal Dyshomeostasis and Their Pathological Role in Prion and Prion-Like Diseases: The Basis for a Nutritional Approach. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Singh, A.; Isaac, A.O.; Luo, X.; Mohan, M.L.; Cohen, M.L.; Chen, F.S.; Kong, Q.Z.; Bartz, J.; Singh, N. Abnormal Brain Iron Homeostasis in Human and Animal Prion Disorders. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef]
- Watt, N.T.; Griffiths, H.H.; Hooper, N.M. Neuronal zinc regulation and the prion protein. Prion 2013, 7, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Gasperini, L.; Meneghetti, E.; Legname, G.; Benetti, F. In Absence of the Cellular Prion Protein, Alterations in Copper Metabolism and Copper-Dependent Oxidase Activity Affect Iron Distribution. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaler, S.G. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol. 2011, 7, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Siggs, O.M.; Cruite, J.T.; Du, X.; Rutschmann, S.; Masliah, E.; Beutler, B.; Oldstone, M.B.A. Disruption of copper homeostasis due to a mutation of Atp7a delays the onset of prion disease. Proc. Natl. Acad. Sci. USA 2012, 109, 13733–13738. [Google Scholar] [CrossRef] [PubMed]
- Vonk, W.I.M.; Wijmenga, C.; van de Sluis, B. Relevance of animal models for understanding mammalian copper homeostasis. Am. J. Clin. Nutr. 2008, 88, 840s–845s. [Google Scholar] [CrossRef]

| Cu(II)-Mediated Function | Experimental System | Reference |
|---|---|---|
| Endocytosis and trafficking | Cell culture, mice | [44,45,46,47,48,63] |
| Superoxide dismutase-like activity | Cell culture | [49] |
| Neuritogenesis | Primary hippocampal cultures | [51] |
| N-methyl-d-aspartate (NMDA) receptors modulation | Organotypic hippocampal culture, primary cell culture, mice | [53,54,55,80,81,82] |
| Brain metal homeostasis | Cell culture, mice | [56] |
| Inducing or inhibiting β-sheet conversion and amyloidal aggregation | Recombinant mouse prion protein, recombinant human prion protein | [57,58,59,60] |
| Increasing expression of Prnp | Cell culture, primary cell culture | [62] |
| One-step purification by using Cu-loaded IMAC column | Recombinant prion protein, brain tissues | [85,86,87,93] |
| Enhanced reversibility of scrapie inactivation | Mice | [91] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzano, G.; Giachin, G.; Legname, G. Structural Consequences of Copper Binding to the Prion Protein. Cells 2019, 8, 770. https://doi.org/10.3390/cells8080770
Salzano G, Giachin G, Legname G. Structural Consequences of Copper Binding to the Prion Protein. Cells. 2019; 8(8):770. https://doi.org/10.3390/cells8080770
Chicago/Turabian StyleSalzano, Giulia, Gabriele Giachin, and Giuseppe Legname. 2019. "Structural Consequences of Copper Binding to the Prion Protein" Cells 8, no. 8: 770. https://doi.org/10.3390/cells8080770
APA StyleSalzano, G., Giachin, G., & Legname, G. (2019). Structural Consequences of Copper Binding to the Prion Protein. Cells, 8(8), 770. https://doi.org/10.3390/cells8080770