S100-EPISPOT: A New Tool to Detect Viable Circulating Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
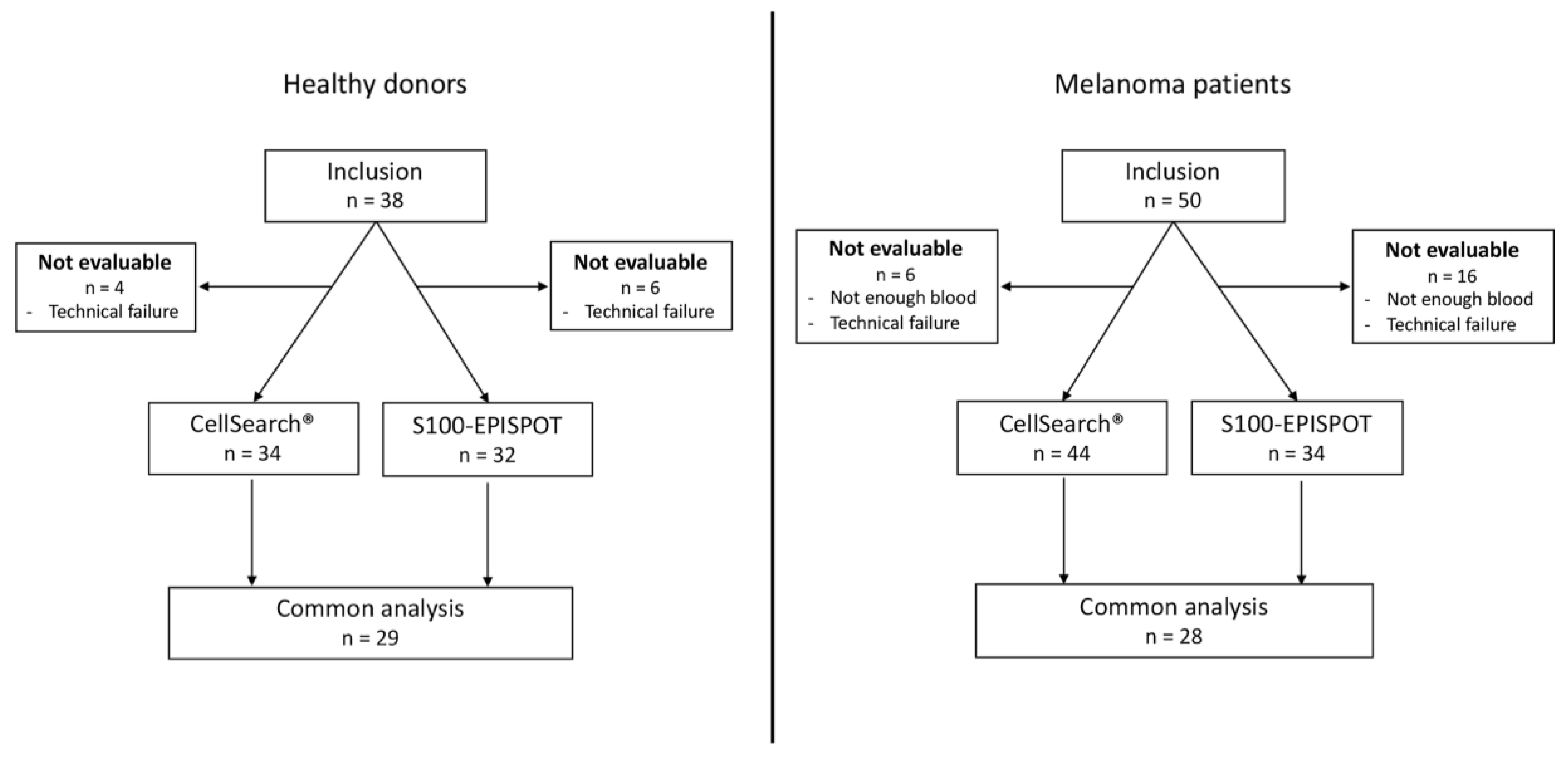
2.1. Patient Cohort
2.2. Melanoma Cell Lines
2.3. Flow Cytometry Experiments
2.4. Immunofluorescence Assay
2.5. S100-EPISPOT Assay
2.6. Cell Search® System
2.7. Statistical Analysis
3. Results
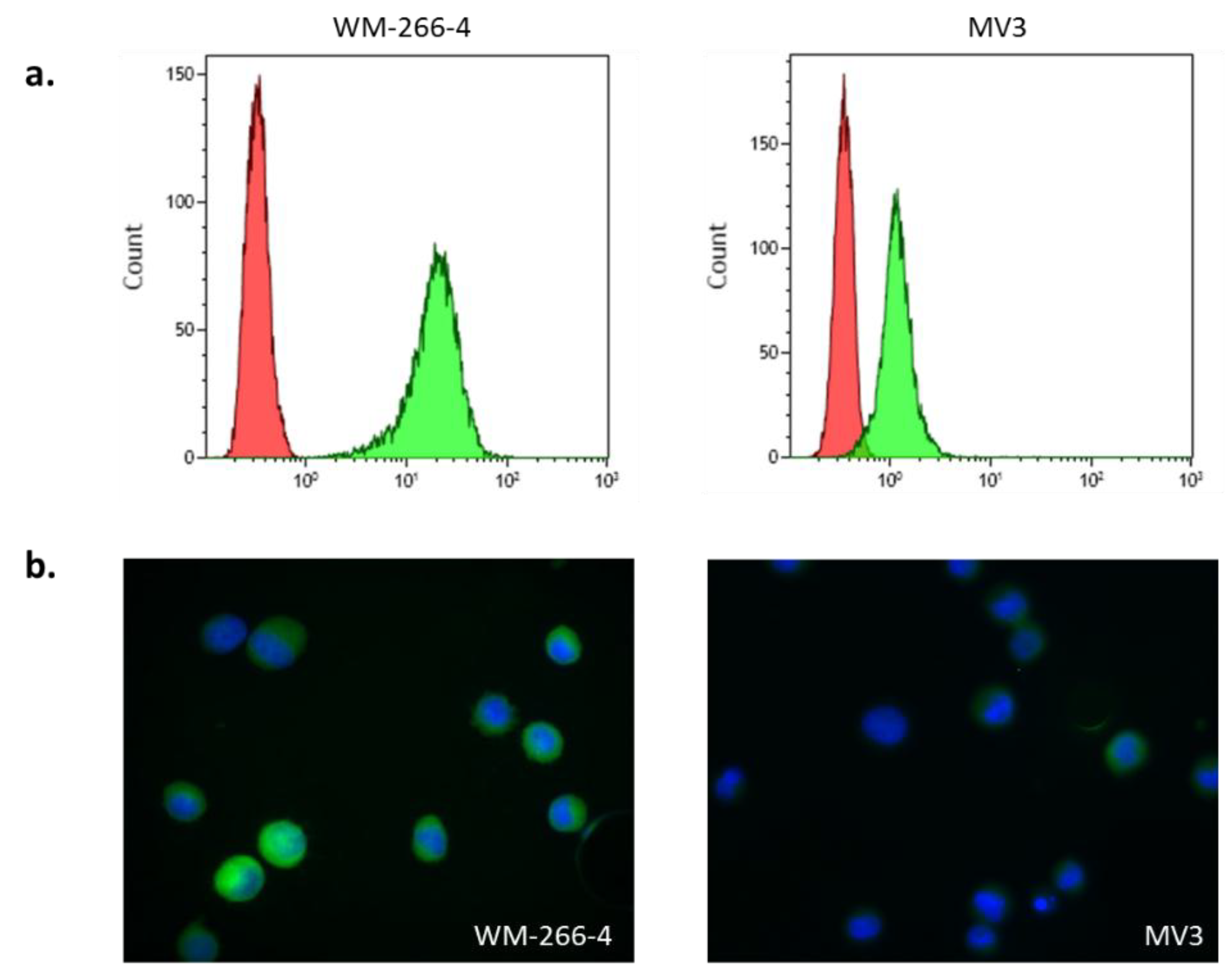
3.1. S100 Expression in Melanoma Cell Lines
3.2. S100-EPISPOT Assay
3.3. Specificity of the S100-EPISPOT Assay
3.4. Circulating Melanoma Cell Detection in Patients with Metastatic Melanoma
3.5. Clinical Relevance of CMC Detection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dummer, R.; Daud, A.; Puzanov, I.; Hamid, O.; Schadendorf, D.; Robert, C.; Schachter, J.; Pavlick, A.; Gonzalez, R.; Hodi, F.S.; et al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Technologies for detection of circulating tumor cells: Facts and vision. Lab Chip 2014, 14, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Nezos, A.; Msaouel, P.; Pissimissis, N.; Lembessis, P.; Sourla, A.; Armakolas, A.; Gogas, H.; Stratigos, A.J.; Katsambas, A.D.; Koutsilieris, M. Methods of detection of circulating melanoma cells: A comparative overview. Cancer Treat. Rev. 2011, 37, 284–290. [Google Scholar] [CrossRef] [PubMed]
- van der Toom, E.E.; Verdone, J.E.; Gorin, M.A.; Pienta, K.J. Technical challenges in the isolation and analysis of circulating tumor cells. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Schwarzenbach, H.; Pantel, K. Circulating tumor cells and circulating tumor DNA. Annu. Rev. Med. 2012, 63, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabieres, C. Liquid biopsy: Potential and challenges. Mol. Oncol. 2016, 10, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016. [Google Scholar] [CrossRef]
- Khoo, B.L.; Grenci, G.; Jing, T.; Lim, Y.B.; Lee, S.C.; Thiery, J.P.; Han, J.; Lim, C.T. Liquid biopsy and therapeutic response: Circulating tumor cell cultures for evaluation of anticancer treatment. Sci. Adv. 2016, 2, e1600274. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Khoja, L.; Lorigan, P.; Dive, C.; Keilholz, U.; Fusi, A. Circulating tumour cells as tumour biomarkers in melanoma: Detection methods and clinical relevance. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. ESMO 2015, 26, 33–39. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Functional Studies on Viable Circulating Tumor Cells. Clin. Chem. 2016, 62, 328–334. [Google Scholar] [CrossRef]
- Alix-Panabieres, C. EPISPOT assay: Detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 2012, 195, 69–76. [Google Scholar] [CrossRef]
- Deneve, E.; Riethdorf, S.; Ramos, J.; Nocca, D.; Coffy, A.; Daures, J.P.; Maudelonde, T.; Fabre, J.M.; Pantel, K.; Alix-Panabieres, C. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 2013, 59, 1384–1392. [Google Scholar] [CrossRef]
- Ramirez, J.M.; Fehm, T.; Orsini, M.; Cayrefourcq, L.; Maudelonde, T.; Pantel, K.; Alix-Panabieres, C. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. Clin. Chem. 2014, 60, 214–221. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Liquid biopsy in cancer patients: Advances in capturing viable CTCs for functional studies using the EPISPOT assay. Expert Rev. Mol. Diagn. 2015, 15, 1411–1417. [Google Scholar] [CrossRef]
- Kuske, A.; Gorges, T.M.; Tennstedt, P.; Tiebel, A.K.; Pompe, R.; Preisser, F.; Prues, S.; Mazel, M.; Markou, A.; Lianidou, E.; et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci. Rep. 2016, 6, 39736. [Google Scholar] [CrossRef]
- Soler, A.; Cayrefourcq, L.; Mazel, M.; Alix-Panabieres, C. EpCAM-Independent Enrichment and Detection of Viable Circulating Tumor Cells Using the EPISPOT Assay. Methods Mol. Biol. 2017, 1634, 263–276. [Google Scholar] [CrossRef]
- Alegre, E.; Sammamed, M.; Fernández-Landázuri, S.; Zubiri, L.; González, Á. Chapter Two-Circulating Biomarkers in Malignant Melanoma. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 69, pp. 47–89. [Google Scholar]
- Rao, C.; Bui, T.; Connelly, M.; Doyle, G.; Karydis, I.; Middleton, M.R.; Clack, G.; Malone, M.; Coumans, F.A.; Terstappen, L.W. Circulating melanoma cells and survival in metastatic melanoma. Int. J. Oncol. 2011, 38, 755–760. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Brouillet, J.P.; Fabbro, M.; Yssel, H.; Rousset, T.; Maudelonde, T.; Choquet-Kastylevsky, G.; Vendrell, J.P. Characterization and enumeration of cells secreting tumor markers in the peripheral blood of breast cancer patients. J. Immunol. Methods 2005, 299, 177–188. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Rebillard, X.; Brouillet, J.-P.; Barbotte, E.; Iborra, F.; Segui, B.; Maudelonde, T.; Jolivet-Reynaud, C.; Vendrell, J.-P. Detection of Circulating Prostate-Specific Antigen–Secreting Cells in Prostate Cancer Patients. Clin. Chem. 2005, 51, 1538–1541. [Google Scholar] [CrossRef]
- Khoja, L.; Lorigan, P.; Zhou, C.; Lancashire, M.; Booth, J.; Cummings, J.; Califano, R.; Clack, G.; Hughes, A.; Dive, C. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J. Investig. Dermatol. 2013, 133, 1582–1590. [Google Scholar] [CrossRef]
- Pantel, K.; Speicher, M.R. The biology of circulating tumor cells. Oncogene 2015. [Google Scholar] [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bauerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Hodgkinson, C.L.; Morrow, C.J.; Li, Y.; Metcalf, R.L.; Rothwell, D.G.; Trapani, F.; Polanski, R.; Burt, D.J.; Simpson, K.L.; Morris, K.; et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 2014, 20, 897–903. [Google Scholar] [CrossRef]
- Rodic, S.; Mihalcioiu, C.; Saleh, R.R. Detection methods of circulating tumor cells in cutaneous melanoma: A systematic review. Crit. Rev. Oncol. Hematol. 2014, 91, 74–92. [Google Scholar] [CrossRef]
- Xu, M.J.; Dorsey, J.F.; Amaravadi, R.; Karakousis, G.; Simone, C.B., 2nd; Xu, X.; Xu, W.; Carpenter, E.L.; Schuchter, L.; Kao, G.D. Circulating Tumor Cells, DNA, and mRNA: Potential for Clinical Utility in Patients With Melanoma. Oncology 2016, 21, 84–94. [Google Scholar] [CrossRef]
- Fusi, A.; Reichelt, U.; Busse, A.; Ochsenreither, S.; Rietz, A.; Maisel, M.; Keilholz, U. Expression of the stem cell markers nestin and CD133 on circulating melanoma cells. J. Investig. Dermatol. 2011, 131, 487–494. [Google Scholar] [CrossRef]
- Kupas, V.; Weishaupt, C.; Siepmann, D.; Kaserer, M.L.; Eickelmann, M.; Metze, D.; Luger, T.A.; Beissert, S.; Loser, K. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J. Investig. Dermatol. 2011, 131, 944–955. [Google Scholar] [CrossRef]
- Schatton, T.; Murphy, G.F.; Frank, N.Y.; Yamaura, K.; Waaga-Gasser, A.M.; Gasser, M.; Zhan, Q.; Jordan, S.; Duncan, L.M.; Weishaupt, C.; et al. Identification of cells initiating human melanomas. Nature 2008, 451, 345–349. [Google Scholar] [CrossRef]
- Fang, D.; Nguyen, T.K.; Leishear, K.; Finko, R.; Kulp, A.N.; Hotz, S.; Van Belle, P.A.; Xu, X.; Elder, D.E.; Herlyn, M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005, 65, 9328–9337. [Google Scholar] [CrossRef]
- Boiko, A.D.; Razorenova, O.V.; van de Rijn, M.; Swetter, S.M.; Johnson, D.L.; Ly, D.P.; Butler, P.D.; Yang, G.P.; Joshua, B.; Kaplan, M.J.; et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010, 466, 133–137. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Bartkowiak, K.; Pantel, K. Functional studies on circulating and disseminated tumor cells in carcinoma patients. Mol. Oncol. 2016, 10, 443–449. [Google Scholar] [CrossRef]
- Palmer, S.R.; Erickson, L.A.; Ichetovkin, I.; Knauer, D.J.; Markovic, S.N. Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin. Proc. 2011, 86, 981–990. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Tsakiridis, K.; Karapantzou, C.; Lampaki, S.; Kioumis, I.; Pitsiou, G.; Papaiwannou, A.; Hohenforst-Schmidt, W.; Huang, H.; Kesisis, G.; et al. Use of proteins as biomarkers and their role in carcinogenesis. J. Cancer 2015, 6, 9–18. [Google Scholar] [CrossRef]
- Kruijff, S.; Hoekstra, H.J. The current status of S-100B as a biomarker in melanoma. Eur. J. Surg. Oncol. EJSO 2012, 38, 281–285. [Google Scholar] [CrossRef]
- Long, E.; Ilie, M.; Bence, C.; Butori, C.; Selva, E.; Lalvee, S.; Bonnetaud, C.; Poissonnet, G.; Lacour, J.P.; Bahadoran, P.; et al. High expression of TRF2, SOX10, and CD10 in circulating tumor microemboli detected in metastatic melanoma patients. A potential impact for the assessment of disease aggressiveness. Cancer Med. 2016. [Google Scholar] [CrossRef]
- Calapre, L.; Warburton, L.; Millward, M.; Ziman, M.; Gray, E.S. Circulating tumour DNA (ctDNA) as a liquid biopsy for melanoma. Cancer Lett. 2017, 404, 62–69. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019. [Google Scholar] [CrossRef]


| a. | ||||||
|---|---|---|---|---|---|---|
| S100-EPISPOT (n = 32) | CellSearch CMC (n = 34) | |||||
| <1 | 25 | (78%) | 32 | (94%) | ||
| ≥1 | 7 | (22%) | 2 | (6%) | ||
| <2 | 31 | (97%) | 33 | (97%) | ||
| ≥2 | 1 | (3%) | 1 | |||
| b. | ||||||
| S100 EPISPOT | CellSearch CMC | p value (Mac Nemar) | p value (corrected Mac Nemar) | |||
| Sensitivity (n = 29) | 48% | 21% | 0.0114 | 0.0269 | ||
| Specificity (n = 28) | 100% | 96% | 0.3173 | 1 | ||
| a. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nb Patients | Assay | Total | Failed | Mean | Standard Deviation | Median | Min | Max | Lower quartile | Upper quartile |
| 50 | S100-EPISPOT | 34 | 16 | 21.59 | 80.35 | 1.00 | 0.00 | 450.00 | 0.00 | 3.00 |
| CellSearch® | 44 | 6 | 142.57 | 752.01 | 0.00 | 0.00 | 4937.00 | 0.00 | 1.00 | |
| b. | ||||||||||
| Nb Patients | Assay | Total | Failed | Mean | Standard Deviation | Median | Min | Max | Lower quartile | Upper quartile |
| 29 | S100-EPISPOT | 29 | 0 | 25.24 | 86.68 | 1.00 | 0.00 | 450.00 | 0.00 | 4.00 |
| CellSearch® | 29 | 0 | 206.10 | 924.77 | 0.00 | 0.00 | 4937.00 | 0.00 | 1.00 | |
| EPISPOT | CellSearch® | |||||
|---|---|---|---|---|---|---|
| < 2 | ≥ 2 | p value (Fisher) | < 2 | ≥ 2 | p value (Fisher) | |
| n = 19 | n = 15 | n = 34 | n = 10 | |||
| Sex | ||||||
| Men | 8 | 9 | 0.4905 | 18 | 4 | 0.7205 |
| (42.11%) | (60.00%) | (52.94%) | (40.00%) | |||
| Women | 11 | 6 | 16 | 6 | ||
| (57.89%) | (40.00%) | (47.06%) | (60.00%) | |||
| BRAF mutation | ||||||
| No | 8 | 6 | 1.0000 | 15 | 2 | 0.4267 |
| (47.06%) | (54.55%) | (48.39%) | (28.57%) | |||
| Yes | 9 | 5 | 16 | 5 | ||
| (52.94%) | (45.45%) | (51.61%) | (71.43%) | |||
| Ulceration | ||||||
| Absence | 4 | 6 | 0.0656 | 11 | 4 | 0.6618 |
| (36.36%) | (85.71%) | (52.38%) | (66.67%) | |||
| Presence | 7 | 1 | 10 | 2 | ||
| (63.64%) | (14.29%) | (47.62%) | (33.33%) | |||
| Metastatic sites | ||||||
| Nb ≤ 2 | 10 | 7 | 1.0000 | 22 | 3 | 0.0738 |
| (52.63%) | (46.67%) | (64.71%) | (30.00%) | |||
| Nb > 2 | 9 | 8 | 12 | 7 | ||
| (47.37%) | (53.33%) | (35.29%) | (70.00%) | |||
| LDH value | ||||||
| Normal | 6 | 5 | 0.2909 | 10 | 1 | 0.0315 |
| (42.86%) | (35.71%) | (34.48%) | (10.00%) | |||
| > Normal | 7 | 4 | 16 | 4 | ||
| (50.00%) | (28.57%) | (55.17%) | (40.00%) | |||
| 2x > Normal | 1 | 5 | 3 | 5 | ||
| (7.14%) | (35.71%) | (10.34%) | (50.00%) | |||
| Primary tumor localization | ||||||
| Other | 1 | 6 | 0.0106 | 6 | 3 | 0.2017 |
| (2.94%) | (17.65%) | (13.95%) | (6.98%) | |||
| Face | 1 | 0 | 0 | 1 | ||
| (2.94%) | (0.00%) | (0.00%) | (2.33%) | |||
| Lower limb | 8 | 2 | 12 | 1 | ||
| (23.53%) | (5.88%) | (27.91%) | (2.33%) | |||
| Upper limb | 6 | 1 | 7 | 1 | ||
| (17.65%) | (2.94%) | (16.28%) | (2.33%) | |||
| Torso | 3 | 6 | 9 | 3 | ||
| (8.82%) | (17.65%) | (20.93%) | (6.98%) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cayrefourcq, L.; De Roeck, A.; Garcia, C.; Stoebner, P.-E.; Fichel, F.; Garima, F.; Perriard, F.; Daures, J.-P.; Meunier, L.; Alix-Panabières, C. S100-EPISPOT: A New Tool to Detect Viable Circulating Melanoma Cells. Cells 2019, 8, 755. https://doi.org/10.3390/cells8070755
Cayrefourcq L, De Roeck A, Garcia C, Stoebner P-E, Fichel F, Garima F, Perriard F, Daures J-P, Meunier L, Alix-Panabières C. S100-EPISPOT: A New Tool to Detect Viable Circulating Melanoma Cells. Cells. 2019; 8(7):755. https://doi.org/10.3390/cells8070755
Chicago/Turabian StyleCayrefourcq, Laure, Aurélie De Roeck, Caroline Garcia, Pierre-Emmanuel Stoebner, Fanny Fichel, Françoise Garima, Françoise Perriard, Jean-Pierre Daures, Laurent Meunier, and Catherine Alix-Panabières. 2019. "S100-EPISPOT: A New Tool to Detect Viable Circulating Melanoma Cells" Cells 8, no. 7: 755. https://doi.org/10.3390/cells8070755
APA StyleCayrefourcq, L., De Roeck, A., Garcia, C., Stoebner, P.-E., Fichel, F., Garima, F., Perriard, F., Daures, J.-P., Meunier, L., & Alix-Panabières, C. (2019). S100-EPISPOT: A New Tool to Detect Viable Circulating Melanoma Cells. Cells, 8(7), 755. https://doi.org/10.3390/cells8070755






