Roscovitine Attenuates Microglia Activation and Monocyte Infiltration via p38 MAPK Inhibition in the Rat Frontoparietal Cortex Following Status Epilepticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Chemicals
2.2. Surgery and Drug Infusion
2.3. SE Induction
2.4. Tissue Processing and Immunohistochemistry
2.5. Cell Count and Measurement of Iba-1 Positive Area
2.6. Data Analysis
3. Results
3.1. The Effects of Roscovitine on Microglia Activation and Monocyte Infiltration Following SE
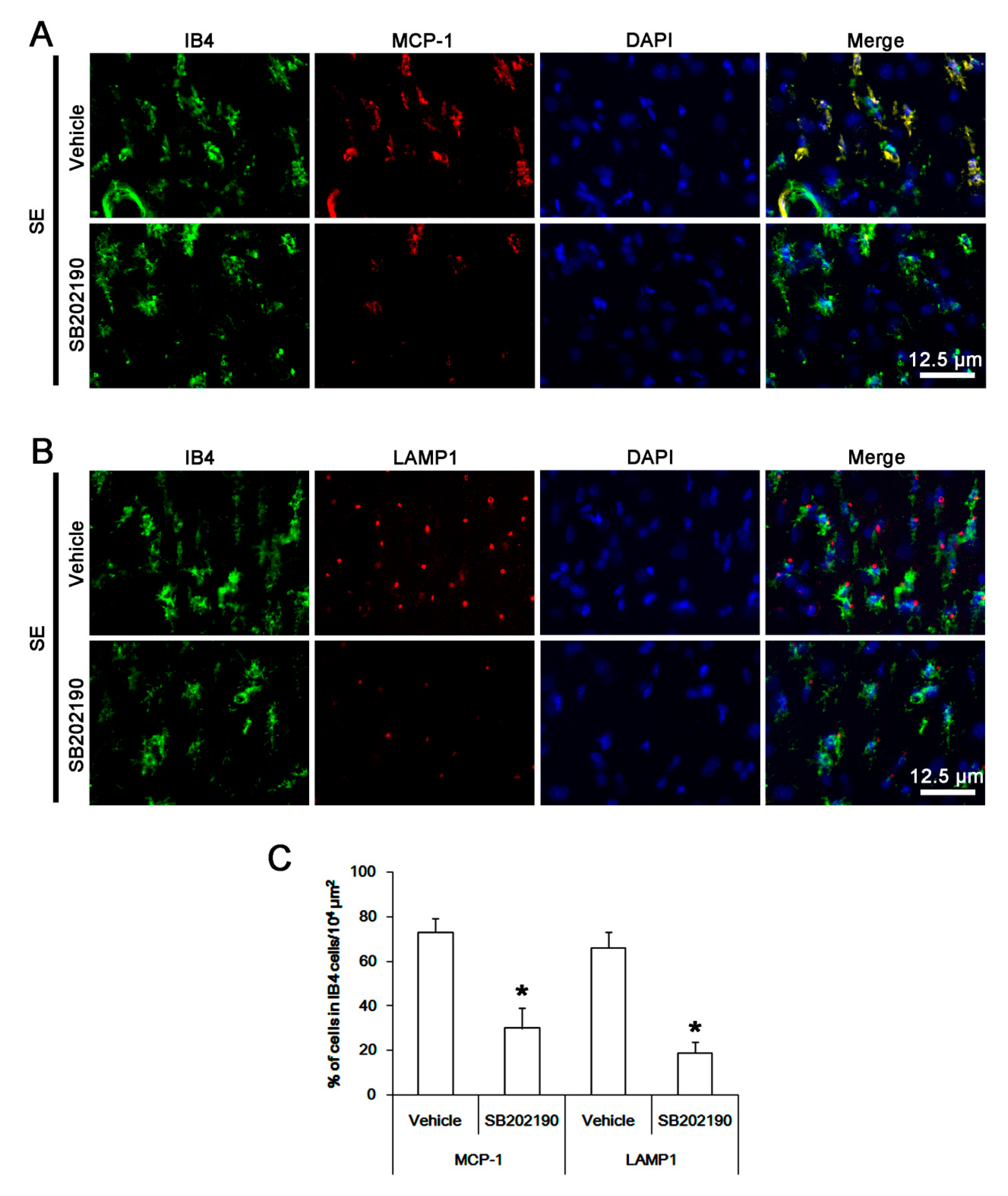
3.2. The Effect of Roscovitine on Microglial MCP-1 Induction After SE
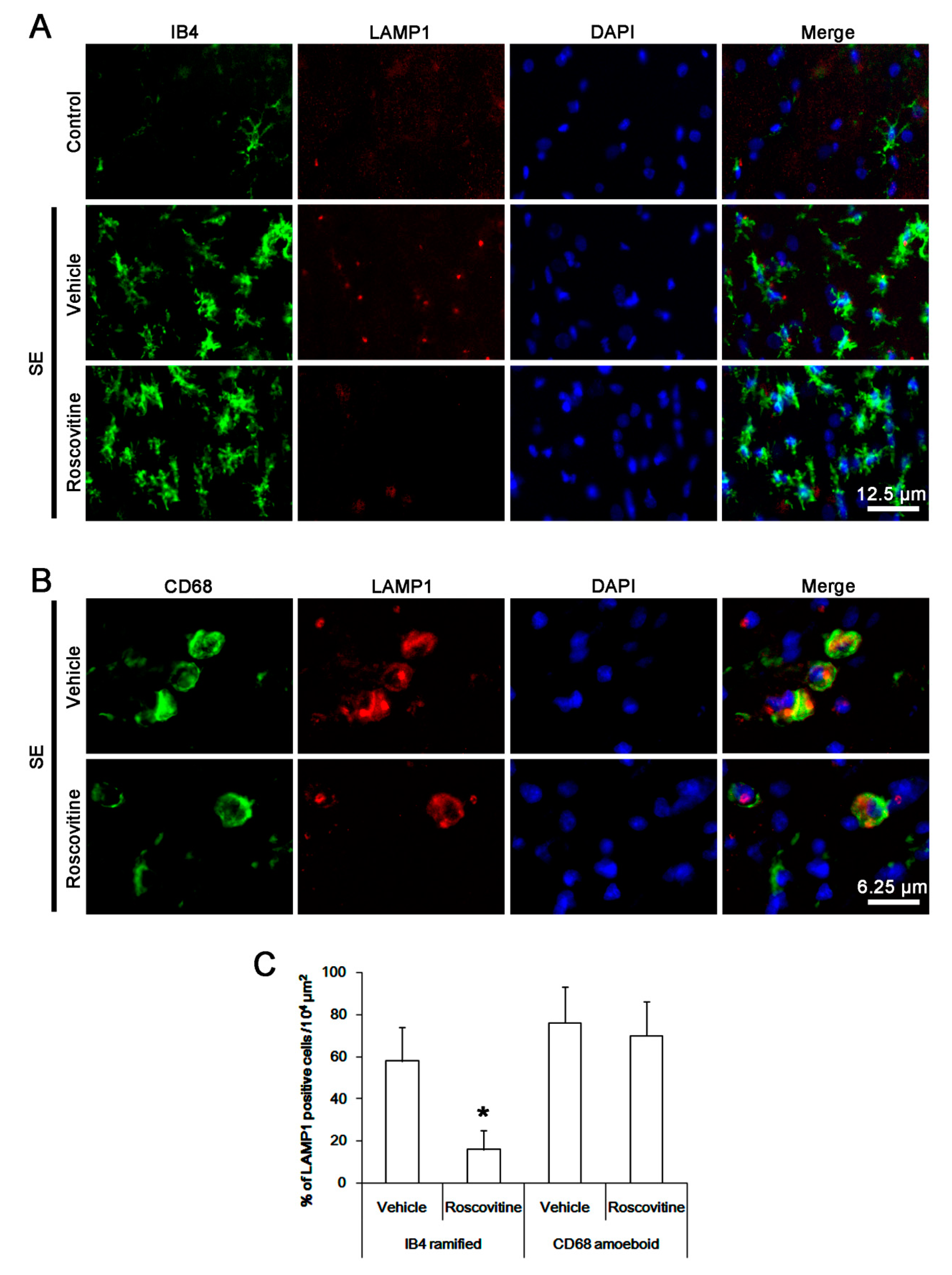
3.3. The Effects of Roscovitine on Phagocytosis of Activated Microglia and Monocytes Following SE
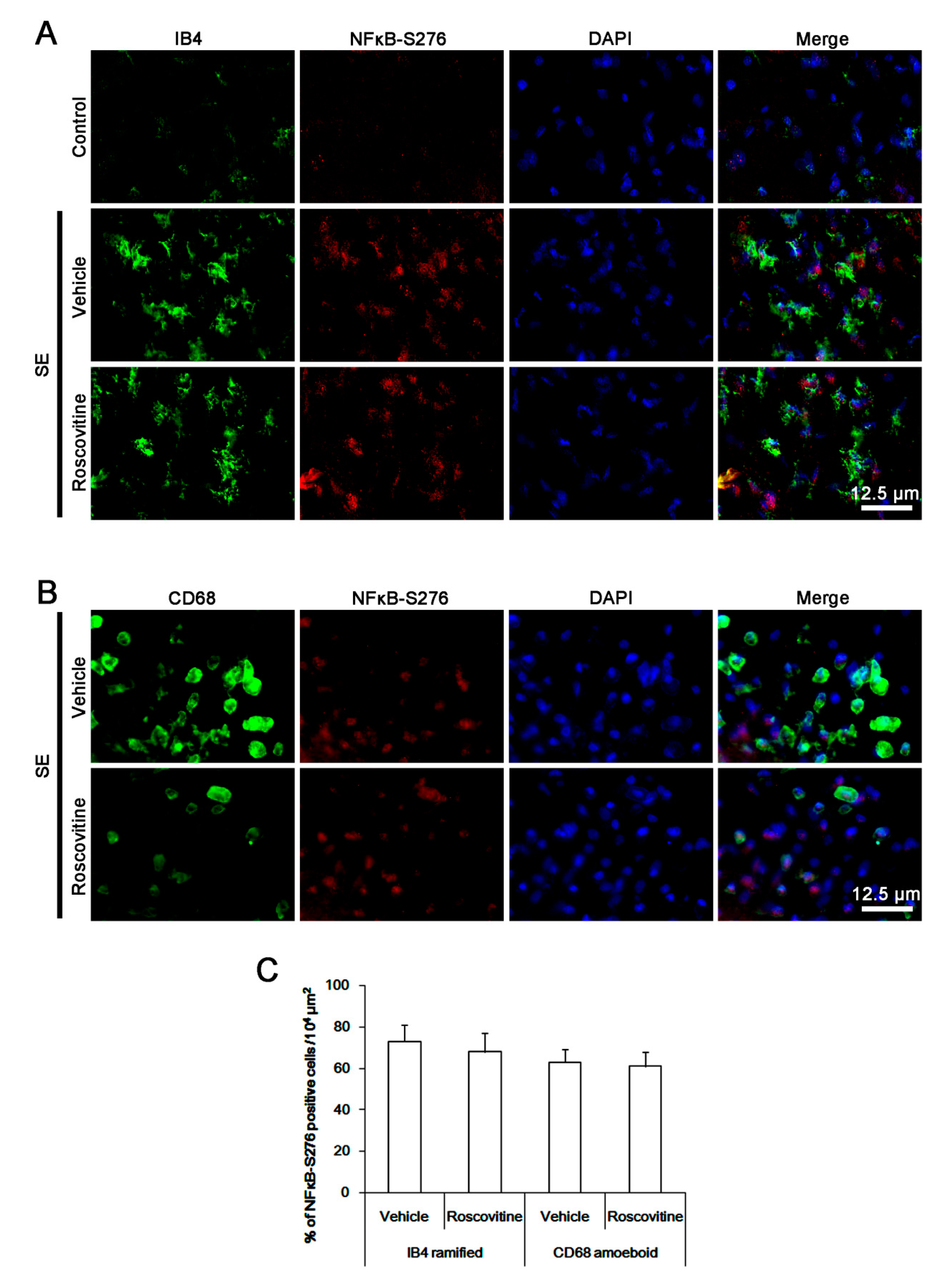
3.4. The Effects of Roscovitine on NFκB-S276 Phosphorylation in Microglia and Monocytes
3.5. The Effect of Roscovitine on p38 MAPK Activation in Activated Microglia and Monocytes
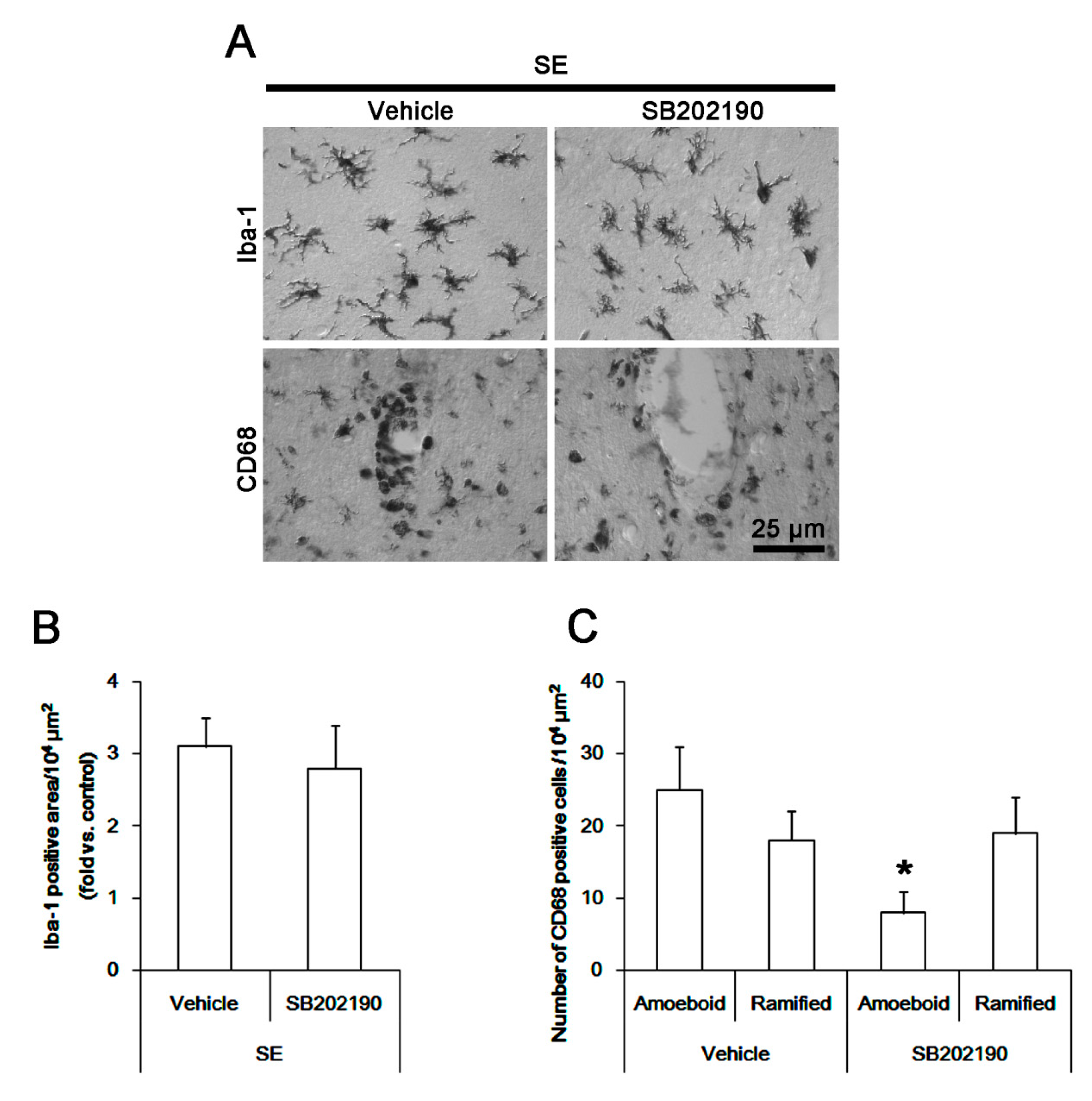
3.6. The Effect of SB202190 on Microglia Activation and Monocyte Infiltration Following SE
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Simoni, M.G.; Perego, C.; Ravizza, T.; Moneta, D.; Conti, M.; Marchesi, F.; De Luigi, A.; Garattini, S.; Vezzani, A. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur. J. Neurosci. 2000, 12, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Plata-Salamán, C.R.; Ilyin, S.E.; Turrin, N.P.; Gayle, D.; Flynn, M.C.; Romanovitch, A.E.; Kelly, M.E.; Bureau, Y.; Anisman, H.; McIntyre, D.C. Kindling modulates the IL-1β system, TNF-alpha, TGF-β1, and neuropeptide mRNAs in specific brain regions. Brain Res. Mol. Brain Res. 2000, 75, 248–258. [Google Scholar] [CrossRef]
- Rizzi, M.; Perego, C.; Aliprandi, M.; Richichi, C.; Ravizza, T.; Colella, D.; Velískŏvá, J.; Moshé, S.L.; De Simoni, M.G.; Vezzani, A. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol. Dis. 2003, 14, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Conti, M.; De Luigi, A.; Ravizza, T.; Moneta, D.; Marchesi, F.; De Simoni, M.G. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: Functional evidence for enhancement of electrographic seizures. J. Neurosci. 1999, 19, 5054–5065. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Moneta, D.; Conti, M.; Richichi, C.; Ravizza, T.; De Luigi, A.; De Simoni, M.G.; Sperk, G.; Andell-Jonsson, S.; Lundkvist, J.; et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11534–11539. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, R.J.; Pellock, J.M.; Towne, A.R.; Boggs, J.G. Epidemiology of status epilepticus. J. Clin. Neurophysiol. 1995, 12, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Ryu, H.J.; Yeo, S.I.; Kang, T.C. P2X7 receptor regulates leukocyte infiltrations in rat frontoparietal cortex following status epilepticus. J. Neuroinflamm. 2010, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.M.; Ryu, H.J.; Kim, J.E.; Yeo, S.I.; Kim, M.J.; Choi, H.C.; Song, H.K.; Kang, T.C. Up-regulation of endothelial endothelin-1 expression prior to vasogenic edema formation in the rat piriform cortex following status epilepticus. Neurosci. Lett. 2011, 501, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, T.; Gagliardi, B.; Noé, F.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.W.; Roth, S.J.; Luther, E.; Rose, S.S.; Springer, T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA 1994, 91, 3652–3656. [Google Scholar] [CrossRef]
- Fuentes, M.E.; Durham, S.K.; Swerdel, M.R.; Lewin, A.C.; Barton, D.S.; Megill, J.R.; Bravo, R.; Lira, S.A. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J. Immunol. 1995, 155, 5769–5776. [Google Scholar] [PubMed]
- Leitch, A.E.; Riley, N.A.; Sheldrake, T.A.; Festa, M.; Fox, S.; Duffin, R.; Haslett, C.; Rossi, A.G. The cyclin-dependent kinase inhibitor R-roscovitine down-regulates Mcl-1 to override pro-inflammatory signalling and drive neutrophil apoptosis. Eur. J. Immunol. 2010, 40, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Hilton, G.D.; Stoica, B.A.; Byrnes, K.R.; Faden, A.I. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood Flow Metab. 2008, 28, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Tomov, N.; Surchev, L.; Wiedenmann, C.; Döbrössy, M.; Nikkhah, G. Roscovitine, an experimental CDK5 inhibitor, causes delayed suppression of microglial, but not astroglial recruitment around intracerebral dopaminergic grafts. Exp. Neurol. 2019, 318, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Vita, M.; Abdel-Rehim, M.; Olofsson, S.; Hassan, Z.; Meurling, L.; Sidén, A.; Sidén, M.; Pettersson, T.; Hassan, M. Tissue distribution, pharmacokinetics and identification of roscovitine metabolites in rat. Eur. J. Pharm. Sci. 2005, 25, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Sallam, H.; Jimenez, P.; Song, H.; Vita, M.; Cedazo-Minguez, A.; Hassan, M. Age-dependent pharmacokinetics and effect of roscovitine on Cdk5 and Erk1/2 in the rat brain. Pharmacol. Res. 2008, 58, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.W.; Min, S.J.; Kim, J.E. CDK5 inhibitors prevent astroglial apoptosis and reactive astrogliosis by regulating PKA and DRP1 phosphorylations in the rat hippocampus. Neurosci. Res. 2017, 119, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kang, T.C. Nucleocytoplasmic p27(Kip1) export is required for ERK1/2-mediated reactive astroglial proliferation following status epilepticus. Front. Cell. Neurosci. 2018, 12, 152. [Google Scholar] [CrossRef]
- Kim, D.S.; Min, S.J.; Kim, M.J.; Kim, J.E.; Kang, T.C. Leptomycin B ameliorates vasogenic edema formation induced by status epilepticus via inhibiting p38 MAPK/VEGF pathway. Brain Res. 2016, 1651, 27–35. [Google Scholar] [CrossRef]
- Streit, W.J.; Walter, S.A.; Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 1999, 57, 563–581. [Google Scholar] [CrossRef]
- Ramprasad, M.P.; Terpstra, V.; Kondratenko, N.; Quehenberger, O.; Steinberg, D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 1996, 93, 14833–14838. [Google Scholar] [CrossRef]
- Fu, H.; Liu, B.; Frost, J.L.; Hong, S.; Jin, M.; Ostaszewski, B.; Shankar, G.M.; Costantino, I.M.; Carroll, M.C.; Mayadas, T.N.; et al. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia. Glia 2012, 60, 993–1003. [Google Scholar] [CrossRef]
- Furusawa, J.; Funakoshi-Tago, M.; Tago, K.; Mashino, T.; Inoue, H.; Sonoda, Y.; Kasahara, T. Licochalcone A significantly suppresses LPS signaling pathway through the inhibition of NF-κB p65 phosphorylation at serine 276. Cell Signal. 2009, 21, 778–785. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, J.E.; Kim, Y.J.; Kim, M.J.; Kang, T.C. Hyperforin attenuates microglia activation and inhibits p65-Ser276 NFκB phosphorylation in the rat piriform cortex following status epilepticus. Neurosci. Res. 2014, 85, 39–50. [Google Scholar] [CrossRef]
- Morganti, J.M.; Goulding, D.S.; Van Eldik, L.J. Deletion of p38α MAPK in microglia blunts trauma-induced inflammatory responses in mice. J. Neuroinflamm. 2019, 16, 98. [Google Scholar] [CrossRef]
- Fang-Hu; Zhang, H.H.; Yang, B.X.; Huang, J.L.; Shun, J.L.; Kong, F.J.; Peng-Xu; Chen, Z.G.; Lu, J.M. Cdk5 contributes to inflammation-induced thermal hyperalgesia mediated by the p38 MAPK pathway in microglia. Brain Res. 2015, 1619, 166–175. [Google Scholar]
- Dinkel, K.; Dhabhar, F.S.; Sapolsky, R.M. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc. Natl. Acad. Sci. USA 2004, 101, 331–336. [Google Scholar] [CrossRef]
- Kielian, T.; Barry, B.; Hickey, W.F. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J. Immunol. 2001, 166, 4634–4643. [Google Scholar] [CrossRef]
- Streit, WJ. Microglial cells. In Neuroglia, 2nd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 60–71. [Google Scholar]
- Morioka, T.; Kalehua, A.N.; Streit, W.J. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2005, 11, 966–973. [Google Scholar] [CrossRef]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kumon, Y.; Watanabe, H.; Ohnishi, T.; Shudou, M.; Ii, C.; Takahashi, H.; Imai, Y.; Tanaka, J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J. Neurosci. Res. 2007, 85, 994–1009. [Google Scholar] [CrossRef]
- Rojanathammanee, L.; Murphy, E.J.; Combs, C.K. Expression of mutant alpha-synuclein modulates microglial phenotype In Vitro. J. Neuroinflamm. 2011, 8, 44. [Google Scholar] [CrossRef]
- Tanaka, Y.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M. Increased lysosomal biogenesis in activated microglia and exacerbated neuronal damage after traumatic brain injury in progranulin-deficient mice. Neuroscience 2013, 250, 8–19. [Google Scholar] [CrossRef]
- Roy, A.; Fung, Y.K.; Liu, X.; Pahan, K. Up-regulation of microglial CD11b expression by nitric oxide. J. Biol. Chem. 2006, 281, 14971–14980. [Google Scholar] [CrossRef]
- Zhong, L.M.; Zong, Y.; Sun, L.; Guo, J.Z.; Zhang, W.; He, Y.; Song, R.; Wang, W.M.; Xiao, C.J.; Lu, D. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS ONE 2012, 7, e32195. [Google Scholar] [CrossRef]
- Berberich, N.; Uhl, B.; Joore, J.; Schmerwitz, U.K.; Mayer, B.A.; Reichel, C.A.; Krombach, F.; Zahler, S.; Vollmar, A.M.; Fürst, R. Roscovitine blocks leukocyte extravasation by inhibition of cyclin-dependent kinases 5 and 9. Br. J. Pharmacol. 2011, 163, 1086–1098. [Google Scholar] [CrossRef]
- Meijer, L.; Borgne, A.; Mulner, O.; Chong, J.P.; Blow, J.J.; Inagaki, N.; Inagaki, M.; Delcros, J.G.; Moulinoux, J.P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997, 243, 527–536. [Google Scholar] [CrossRef]
- Katayama, T.; Kobayashi, H.; Okamura, T.; Yamasaki-Katayama, Y.; Kibayashi, T.; Kimura, H.; Ohsawa, K.; Kohsaka, S.; Minami, M. Accumulating microglia phagocytose injured neurons in hippocampal slice cultures: Involvement of p38 MAP kinase. PLoS ONE 2012, 7, e40813. [Google Scholar] [CrossRef]
- Zhou, Y.; Ling, E.A.; Dheen, S.T. Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. J. Neurochem. 2007, 102, 667–678. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Lü, J.; Xie, M.; Pan, D.; Luo, X.; Yu, Z.; Dong, Q.; Wang, W. Cell cycle inhibition attenuates microglial proliferation and production of IL-1beta, MIP-1alpha, and NO after focal cerebral ischemia in the rat. Glia 2009, 57, 908–920. [Google Scholar] [CrossRef]



| Antigen | Host | Manufacturer (Catalog Number) | Dilution Used |
|---|---|---|---|
| Iba-1 | Rabbit | Biocare Medical (CP 290) | 1:500 |
| IB4 | Vector (B-1205) | 1:200 | |
| CD68 | Mouse | Abcam (ab31630) | 1:100 |
| p-p38 MAPK | Rabbit | Abbiotec (# 251256) | 1:200 |
| NFκB-S276 | Rabbit | Abcam (ab106129) | 1:100 |
| LAMP1 | Rabbit | Abcam (ab24170) | 1:100 |
| MCP-1 | Mouse | Abcam (ab25124) | 1:100 |
| CCR2 | Rabbit | Abcam (ab227015) | 1:100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Park, H.; Choi, S.-H.; Kong, M.-J.; Kang, T.-C. Roscovitine Attenuates Microglia Activation and Monocyte Infiltration via p38 MAPK Inhibition in the Rat Frontoparietal Cortex Following Status Epilepticus. Cells 2019, 8, 746. https://doi.org/10.3390/cells8070746
Kim J-E, Park H, Choi S-H, Kong M-J, Kang T-C. Roscovitine Attenuates Microglia Activation and Monocyte Infiltration via p38 MAPK Inhibition in the Rat Frontoparietal Cortex Following Status Epilepticus. Cells. 2019; 8(7):746. https://doi.org/10.3390/cells8070746
Chicago/Turabian StyleKim, Ji-Eun, Hana Park, Seo-Hyeon Choi, Min-Jeong Kong, and Tae-Cheon Kang. 2019. "Roscovitine Attenuates Microglia Activation and Monocyte Infiltration via p38 MAPK Inhibition in the Rat Frontoparietal Cortex Following Status Epilepticus" Cells 8, no. 7: 746. https://doi.org/10.3390/cells8070746
APA StyleKim, J.-E., Park, H., Choi, S.-H., Kong, M.-J., & Kang, T.-C. (2019). Roscovitine Attenuates Microglia Activation and Monocyte Infiltration via p38 MAPK Inhibition in the Rat Frontoparietal Cortex Following Status Epilepticus. Cells, 8(7), 746. https://doi.org/10.3390/cells8070746




